The effect of dietary factors on Ca absorption is poorly understood and there is a need for detailed studies to define the ways in which food components and functional food ingredients influence Ca absorption in order to determine how Ca bioavailability from foods can be optimised (Kennefick & Cashman, Reference Kennefick and Cashman2000). Evidence principally from animal studies suggests that n-3 fatty acids, especially those from marine oils, could stimulate intestinal Ca absorption and promote bone health (for reviews, see Kruger & Horrobin, Reference Kruger and Horrobin1997; Watkins et al. Reference Watkins, Lippman, Le Bouteiller, Li and Seifert2001), and thus may be potential functional food ingredients. However, there have been no studies in human subjects of the effect of marine oil-based n-3 fatty acids on Ca absorption. Such fatty acids may also be of potential use for promoting Ca absorption in ‘at-risk’ individuals.
Osteopenia and osteoporosis are common conditions among patients with Crohn's disease. Osteopenia is seen in about 30 % of Crohn's disease patients, while 10–12 % of patients have osteoporosis (Bjarnason et al. Reference Bjarnason, Macpherson, Mackintosh, Buxton-Thomas, Forgacs and Moniz1997; Szulc & Meunier, Reference Szulc and Meunier2001). The pathogenesis of osteopenia and osteoporosis in Crohn's disease is likely to be multifactorial, and has been attributed to an adverse effect of sex hormone deficiency, reduced physical activity, prolonged corticosteroid therapy, bowel surgery, smoking, and the potential deleterious effects of circulating cytokines and other mediators (for example, IL-1 and IL-6 and TNF-α) released by the inflamed intestines on bone metabolism (Pollak et al. Reference Pollak, Karmeli, Eliakim, Ackerman, Tabb and Rachmilewitz1998). Another pathogenic mechanism which has been implicated in the low bone mineral density in Crohn's disease is the existence of one or more nutritional inadequacies in these patients. For example, several studies have reported an association between low bone mineral density and a reduced intake or malabsorption of Ca (Bernstein et al. Reference Bernstein, Seeger, Anton, Artinian, Geffrey, Goodman, Belin and Shanahan1996; Silvennoinen et al. Reference Silvennoinen, Lamberg-Allardt, Karkkainen, Niemela and Lehtola1996; Scott et al. Reference Scott, Gaywood and Scott2000), as well as a deficiency of vitamins D and/or K (Arnaud et al. Reference Arnaud, Goldsmith, Lambert and Go1975; Compston & Creamer, Reference Compston and Creamer1977; Schoon et al. Reference Schoon, Muller, Vermeer, Schurgers, Brummer and Stockbrugger2001; Haderslev et al. Reference Haderslev, Jeppesen, Sorensen, Mortensen and Staun2003; Duggan et al. Reference Duggan, O'Brien, Kiely, McCarthy, Shanahan and Cashman2004). Therefore, improvement of nutritional status must form part of the overall strategy for prevention of osteoporosis in Crohn's disease patients.
In addition to possible beneficial effects on intestinal Ca absorption in Crohn's disease patients, n-3 fatty acids have also been shown to alleviate intestinal inflammation in Crohn's disease patients, and thus are considered possible preventative and/or therapeutic agents for the disease itself (Endres et al. Reference Endres, Lorenz and Loeschke1999; Belluzzi, Reference Belluzzi2002; Cashman & Shanahan, Reference Cashman and Shanahan2003). In this way, n-3 fatty acids may indirectly benefit bone, by lessening the deleterious effects of circulating cytokines and other mediators (for example, IL-1, IL-6 and TNF-α) released by the inflamed intestines, on bone. Thus, the use of n-3 fatty acids could benefit intestinal Ca absorption, while calming intestinal inflammation in Crohn's disease patients, both of which may help reduce the risk of osteoporosis and osteopenia in these patients. However, this has not been investigated.
Therefore, the objective of the present study was to investigate the effect of two n-3 fatty acids, namely 20 : 5n-3 and 22 : 6n-3, on Ca transport across normal and inflamed polarised human intestinal epithelial (Caco-2) cell monolayers. Caco-2 cells have been suggested to be a suitable model for predicting Ca absorption in man (Giuliano & Wood, Reference Giuliano and Wood1991; Fleet & Wood, Reference Fleet and Wood1999). Recent work has shown that these cells respond, in terms of Ca absorption, to fatty acid intervention (Jewell & Cashman, Reference Jewell and Cashman2003; Jewell et al. Reference Jewell, Cusack and Cashman2005). In addition, Caco-2 cells secrete inflammatory cytokines when treated with TNF-α (Lang et al. Reference Lang, Lahav, Sakhnini, Barshack, Fidder, Avidan, Bardan, Hershkoviz, Bar-Meir and Chowers2004). Therefore, this relatively simple in vitro method appears to be a good model for predicting the effect of n-3 fatty acids on Ca absorption in healthy human subjects as well as in Crohn's disease patients.
Materials and methods
Materials
Tissue culture materials, including Dulbecco's modified Eagle's medium with l-glutamine and sodium bicarbonate, fetal bovine serum, minimum essential medium, non-essential amino acids and PBS were purchased from Sigma-Aldrich Ireland Ltd (Dublin, Republic of Ireland). 45Ca (as 45Ca in an aqueous solution of CaCl2, with a specific activity of 1·85 MBq/mg Ca) was purchased from Nensure™ (Boston, MA, USA). Fluorescein Na salt, 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT), 20 : 5n-3, 22 : 6n-3 and TNF-α were purchased from Sigma-Aldrich Ireland Ltd.
Conditions of cell culture
The human colon adenocarcinoma cell line, Caco-2, was purchased from the European Collection of Animal Cell Cultures (Salisbury, Wiltshire, UK). Cells were routinely grown in 75 cm2 plastic culture flasks (Costar, Cambridge, MA, USA) in Dulbecco's modified Eagle's medium supplemented with non-essential amino acids (10 ml/l) and fetal bovine serum (100 ml/l). Caco-2 cells were maintained at 37°C in a CO2–air (5:95, v/v) atmosphere. Cells were seeded at 3 × 104/cm2 and passaged when reaching 90 % confluency. Cells used in transepithelial Ca transport experiments were seeded at a density of 3 × 104/cm2 onto permeable Transwell® filter inserts (24 mm diameter, 0·4 μm pore size; Costar). Cell culture media was changed on alternate days for 15 d. For IL-8 studies, cells (3 × 104/cm2) were seeded onto Transwell® filter inserts (Costar). For viability studies, cells (3 × 104/cm2) were seeded into forty-eight-well culture plates (Costar).
Cell viability assays
The effect of TNF-α, 20 : 5n-3, 22 : 6n-3 and 20 : 5n-3+22 : 6n-3 on Caco-2 cell viability was investigated using the MTT assay in forty-eight-well culture plates (Costar), as described by Mossman (Reference Mossman1983) and Edmonson et al. (Reference Edmonson, Armstrong and Martinez1988). Results were expressed as a percentage of the control value, representing the surviving fraction relative to control samples. A value < 85 % of the control value was taken as a benchmark for toxicity.
Transepithelial electrical resistance
For all transport experiments, the transepithelial electrical resistance (TEER; a measure of the integrity of polarised epithelial cell monolayers) was checked before the experiment by a Millicell® ERS meter (Millipore Corporation, Bedford, MA, USA) connected to a pair of thin side-by-side electrodes as described by Tanaka et al. (Reference Tanaka, Taki, Sakane, Nadai, Sezaki and Yamashita1995). TEER readings were taken at 37°C. A TEER value ≥ 1500 Ω × cm2 was used as an indicator that the epithelial layer was intact and ready to use for Ca transport studies.
Cell treatments
Two different approaches were used in the present study: (1) pre-treatment of Caco-2 cells with n-3 fatty acids before co-treatment with pro-inflammatory TNF-α (nominally referred to as preventative studies); (2) treatment of Caco-2 cells with TNF-α followed by co-treatment of Caco-2 cells with n-3 fatty acids (nominally referred to as therapeutic studies).
Preventative studies
For Ca transport experiments, cells grown in the Transwell® inserts were treated with vehicle only (for control), 80 μm-20 : 5n-3, 80 μm-22 : 6n-3, or 40 μm-20 : 5n-3+40 μm-22 : 6n-3 for 6 d followed by co-treatment with TNF-α (10 ng/ml) or vehicle for a further 48 h (which was shown in a pilot study to be sufficient to induce biomarkers of inflammation in Caco-2 cells, including IL-8). We have previously found that 6 d is sufficient to allow fatty acid uptake into Caco-2 cells (Ryan, Reference Ryan2004). All compounds were added to complete culture medium before their addition to the cells. The vehicle never exceeded 1 ml/l. TEER measurements were taken immediately before treatment with test compounds and after treatment.
Therapeutic studies
For Ca transport experiments, cells grown in the Transwell® inserts were treated with TNF-α (10 ng/ml) or vehicle only (for control) for 48 h followed by co-treatment with vehicle only, 80 μm-20 : 5n-3, 80 μm-22 : 6n-3, or 40 μm-20 : 5n-3+40 μm-22 : 6n-3 for a further 6 d. All compounds were added to complete culture medium before their addition to the cells. The vehicle never exceeded 1 ml/l. TEER measurements were taken immediately before treatment with test compounds and after treatment.
Transepithelial calcium transport studies
The method used for determining Ca transport across the Caco-2 membrane in the present study is a modification of the methods of Giuliano & Wood (Reference Giuliano and Wood1991) and Fleet & Wood (Reference Fleet and Wood1994). Transepithelial transport of Ca was studied with Caco-2 cells grown on permeable membrane supports for 15 d, by which time the cells are fully differentiated. On the day of an experiment, the medium containing test compounds was removed and the inserts rinsed with buffer. The buffer was prepared fresh before use and consisted of 140 mm-NaCl, 5·8 mm-KCl, 1·2 mm-CaCl2, 0·8 mm-MgSO4, 0·44 mm-KH2PO4, 0·34 mm-Na2HPO4, 4 mm-glutamine, 25 mm-glucose and 20 mm-HEPES (pH 7·4). After rinsing, 2·6 ml of this buffer was added to the lower chamber of the Transwell® (Costar, Cambridge, MA, USA) inserts. At time zero, 1·5 ml of transport buffer was added to the upper chamber of the Transwell® (Costar) inserts. This consisted of the same buffer as the lower chamber (see composition of buffer earlier) except it also contained 45Ca (with an activity of 148 kBq/ml) and 5·3 mm-fluorescein (as the Na salt). Fluorescein was included in the transport buffer, in place of phenol red, as used by Fleet & Wood (Reference Fleet and Wood1994), as a means of measuring paracellular (diffusional) transport across the Caco-2 monolayer (Lindmark et al. Reference Lindmark, Kimura and Artursson1998). Following the addition of the transport buffer to the Transwell® (Costar) inserts, the plates were covered and incubated at 37°C in a shaking water-bath (set to 48 oscillations per min) for 60 min. At 30 and 60 min after addition of 45Ca-labelled transport buffer, duplicate samples (10 μl) of the buffer from the lower chamber (basolateral buffer) were taken from each well and placed in wells of a blackened ninety-six-well plate (Costar). When samples had been taken at both time points, 200 μl 50 mm-n-ethylmorpholine buffer (pH 8·0) was added to each of the ninety-six wells and fluorescence (excitation, 485 nm; emission, 535 nm) was measured in a Spectrafluor+ Tecan fluorescence plate reader (Tecan AG, Hombrechtikon, Switzerland). In addition, at the same time points duplicate samples (50 μl) of the basolateral buffer were taken for determination of 45Ca content. Samples of the basolateral buffer were placed in scintillation vials and 5 ml of liquid scintillation cocktail (biodegradable counting scintillant; Amersham International plc, Little Chalfont, Bucks, UK) was added to each vial. Counts were measured on a Beckman LS 6500 multipurpose liquid scintillation counter (Beckman Instruments, Inc., Fullerton, CA, USA). An equal volume of fresh basolateral buffer was added back to the lower chamber following each sampling point. The concentration of fluorescein appearing in the lower buffer after 60 min was determined using a standard curve of fluorescein and this value was expressed as a percentage of the total fluorescein added to the upper chamber of the Transwell® inserts (Costar). This represented the paracellular route of Ca transport. The amount of 45Ca appearing in the basolateral buffer was expressed as a percentage of the total 45Ca applied to the upper chamber. This represented total transepithelial 45Ca transport (i.e. by both the paracellular and transcellular transport routes) and was expressed as nmol transported/min per well during the 30–60 min time interval. The amount of 45Ca crossing the Caco-2 cell monolayer by the transcellular (active) route was calculated by subtracting the paracellular contribution from total transepithelial Ca transport, and both paracellular and transcellular Ca transport were expressed as nmol/well per min. In all studies, at least three wells were examined per treatment. Experiments were repeated at least three times.
Secretion of interleukin-8 by Caco-2 monolayers
The concentration of IL-8 secreted from Caco-2 monolayers (as a biomarker of intestinal cell inflammation) was determined using a recently developed ELISA (Human IL-8 DuoSet ELISA Development System; R&D Systems Europe Ltd, Abingdon, Oxon, UK), as described elsewhere (O'Hara et al. Reference O'Hara, O'Regan, Fanning, O'Mahony, Macsharry, Lyons, Bienenstock, O'Mahony and Shanahan2006).
Statistical methods
The Ca transport (total and paracellular) data from preventative studies, paracellular Ca transport data from therapeutic studies, and IL-8 data from both sets of studies were not normally distributed and, therefore, values were logarithmically (natural log; Ln) transformed before statistical analysis, to achieve near-normal distributions. Transcellular Ca transport data, and total and transcellular Ca transport data, from the preventative and therapeutic studies, respectively, and TEER data from both sets of studies were normally distributed. All data were subjected to two-way ANOVA, with variation attributed to fish oil treatment and TNF-α treatment (Snedecor & Cochran, Reference Snedecor and Cochran1967). In some cases, where applicable, data were subjected to one-way ANOVA, with variation attributed to fish oil treatment (Snedecor & Cochran, Reference Snedecor and Cochran1967). Results are presented as mean values with their pooled standard errors. Where applicable, when data were Ln-transformed, for ease of interpretation, the data were back-transformed to geometric means. To follow up the ANOVA, all pairs of means were compared by the method of least significant difference (Snedecor & Cochran, Reference Snedecor and Cochran1967).
Results
There was no effect of 80 μm-20 : 5n-3, 80 μm-22 : 6n-3 or 40 μm-20 : 5n-3+40 μm-22 : 6n-3 on Caco-2 cell survival and viability as determined using the MTT assay, which is based on mitochondrial dehydrogenase activity (data not shown). Higher concentrations of 20 : 5n-3 and 22 : 6n-3 produced some evidence of reduced cell viability, as did the combination of 80 μm-20 : 5n-3+80 μm-22 : 6n-3 (data not shown). In addition, exposure of Caco-2 cells to TNF-α (10 ng/ml) for 8 d had no effect on cell viability (data not shown).
Preventative studies
Total transepithelial Ca transport across fully differentiated Caco-2 cell monolayers was significantly increased by lipid treatment (P < 0·0001) and TNF-α treatment (P = 0·0003), with a significant (P < 0·05) interaction between the two factors (Table 1). Treatment of Caco-2 cell monolayers with 80 μm-20 : 5n-3 and 80 μm-22 : 6n-3 significantly (P < 0·005) increased total transepithelial Ca transport compared with control, irrespective of whether the cells were untreated or treated with TNF-α (Table 1). Treatment of Caco-2 cell monolayers with 40 μm-20 : 5n-3+40 μm-22 : 6n-3 also significantly (P < 0·01) increased total transepithelial Ca transport compared with control, with no significant differences between 20 : 5n-3+22 : 6n-3 and either 20 : 5n-3 or 22 : 6n-3 alone, irrespective of whether the cells were untreated or treated with TNF-α (Table 1). Total transepithelial Ca transport across TNF-α-treated Caco-2 cells was significantly higher in control (non-lipid-treated) cells and those treated with 22 : 6n-3, but not in cells treated with 20 : 5n-3 or 20 : 5n-3+22 : 6n-3, compared with the equivalent groups not treated with TNF-α (Table 1).
Table 1 The effect of n-3 fatty acids on transepithelial calcium transport in healthy and inflamed Caco-2 cell monolayers grown in culture: preventative studies (Mean values and pooled standard errors of the mean)
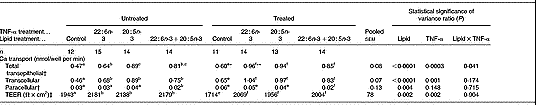
TEER, transepithelial electrical resistance.
a,b,c Mean values within a row within TNF-α-untreated groups with unlike superscript letters were significantly different (P < 0·05).
d,e Mean values within a row within TNF-α-treated groups with unlike superscript letters were significantly different (P < 0·05).
Mean value was significantly different from that of equivalent cells not treated with TNF-α: *P < 0·05, **P < 0·0001.
† Values represent data back-transformed (anti-Ln) to geometric means.
‡ TEER value across monolayer after transport study.
Transcellular Ca transport across fully differentiated Caco-2 cell monolayers was significantly increased by lipid treatment (P < 0·0001) and TNF-α treatment (P = 0·001), but there was no significant (P>0·1) interaction between the two factors (Table 1). Treatment of Caco-2 cell monolayers with 80 μm-20 : 5n-3, 80 μm-22 : 6n-3 and 40 μm-20 : 5n-3+40 μm-22 : 6n-3 significantly (P < 0·001) increased transcellular Ca transport compared with control, irrespective of whether the cells were untreated or treated with TNF-α (Table 1).
Paracellular Ca transport across fully differentiated Caco-2 cell monolayers (representing only 4–6 % of total transepithelial Ca transport) was significantly influenced by lipid treatment (P ≤ 0·004), but was unaffected by TNF-α treatment and there was no significant (P>0·1) interaction between the treatments (Table 1).
TEER across fully differentiated Caco-2 cell monolayers was significantly (P = 0·002) increased by lipid treatment and significantly (P = 0·002) decreased by TNF-α treatment, but there was no significant (P>0·05) interaction between the two factors. Treatment of Caco-2 cell monolayers with 80 μm-20 : 5n-3, 80 μm-22 : 6n-3 and 40 μm-20 : 5n-3+40 μm-22 : 6n-3 significantly (P < 0·001) increased TEER compared with control, irrespective of whether the cells were untreated or treated with TNF-α (Table 1).
IL-8 secretion from fully differentiated Caco-2 cell monolayers was significantly increased by TNF-α treatment (P ≤ 0·0001), but was unaffected by lipid treatment (P>0·05) and there was no significant (P>0·05) interaction between the treatments (Fig. 1(A)).

Fig. 1 The effect of n-3 fatty acid treatment on IL-8 secretion from fully differentiated Caco-2 cell monolayers in (A) preventative studies and (B) therapeutic studies (for details, see p. 283). Data are mean values (n 11–13), with standard errors represented by vertical bars. Mean values for TNF-α-treated (10 ng/ml) cells were significantly different from those of controls in both preventative and therapeutic studies (P ≤ 0·0001). There was no significant effect of n-3 fatty acid treatment and no significant interactions with TNF-α treatment in either preventative or therapeutic studies (P>0·1).
Therapeutic studies
Total transepithelial and transcellular Ca transport across fully differentiated Caco-2 cell monolayers was significantly increased by TNF-α treatment (P < 0·001), but was unaffected by lipid treatment (P>0·1). There were trends for a significant (P = 0·062–0·064) interaction between the two factors (Table 2). One-way ANOVA showed that treatment of non-TNF-α-treated Caco-2 cell monolayers with 80 μm-22 : 6n-3, but not 80 μm-20 : 5n-3 or 40 μm-20 : 5n-3+40 μm-22 : 6n-3, significantly (P < 0·005) increased total transepithelial and transcellular Ca transport compared with control (Table 2). Exposure of TNF-α-treated Caco-2 cell monolayers to 80 μm-20 : 5n-3, 80 μm-22 : 6n-3 or 40 μm-20 : 5n-3+40 μm-22 : 6n-3 had no significant effect on total transepithelial or transcellular Ca transport relative to the control (Table 2).
Table 2 The effect of n-3 fatty acids on transepithelial calcium transport in healthy and inflamed Caco-2 cell monolayers grown in culture: therapeutic studies (Mean values and pooled standard errors of the mean)
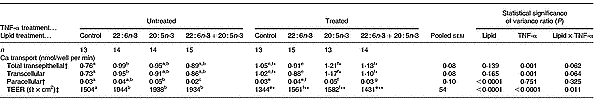
TEER, transepithelial electrical resistance.
a,b,c Mean values within a row within TNF-α-untreated groups with unlike superscript letters were significantly different (P < 0·05).
d,e,f Mean values within a row within TNF-α-treated groups with unlike superscript letters were significantly different (P < 0·05).
Mean value was significantly different from that of equivalent cells not treated with TNF-α: *P < 0·05, **P < 0·0001.
† Values represent data back-transformed (anti-Ln) to geometric means.
‡ TEER value across monolayer after transport study.
Paracellular Ca transport across fully differentiated Caco-2 cell monolayers was significantly influenced by lipid treatment (P ≤ 0·0001), but was unaffected by TNF-α treatment and there was no significant (P>0·1) interaction between the treatments (Table 2).
TEER across fully differentiated Caco-2 cell monolayers was significantly (P < 0·0001) increased by lipid treatment and significantly (P < 0·0001) decreased by TNF-α treatment, and there was a significant (P = 0·01) interaction between the two factors. Treatment of non-TNF-α-treated Caco-2 cell monolayers with 80 μm-20 : 5n-3, 80 μm-22 : 6n-3 and 40 μm-20 : 5n-3+40 μm-22 : 6n-3 significantly (P < 0·001) increased TEER compared with control (Table 2). Treatment of TNF-α-treated Caco-2 cell monolayers with 80 μm-20 : 5n-3 and 80 μm-22 : 6n-3, but not 40 μm-20 : 5n-3+40 μm-22 : 6n-3 significantly (P < 0·001) increased TEER compared with control (Table 2). TNF-α-treated cells had significantly (P ≤ 0·0001) lower TEER compared with untreated cells, irrespective of the lipid treatment (Table 2).
IL-8 secretion from fully differentiated Caco-2 cell monolayers was significantly increased by TNF-α treatment (P ≤ 0·0001), but was unaffected by lipid treatment (P>0·05) and there was no significant (P>0·05) interaction between the treatments (Fig. 1(B)).
Discussion
Caco-2 cells have been suggested to be a suitable model for predicting intestinal Ca absorption in man (Giuliano & Wood, Reference Giuliano and Wood1991; Fleet & Wood, Reference Fleet and Wood1999). While Caco-2 cells are colonocytes in origin, in culture, they spontaneously differentiate and form a polarised epithelial monolayer with tight junctions and express a differentiated cell phenotype consistent with absorptive small intestine-like enterocytes (Pinto et al. Reference Pinto, Robine-Leon and Appay1983; Yee, Reference Yee1997). In particular, these cells have a functional vitamin D receptor (Giuliano et al. Reference Giuliano, Franceschi and Wood1991) and have Ca transport kinetics that suggest the presence of both a saturable and non-saturable Ca transport pathway, similar to what has been observed in human and animal intestine (Fleet & Wood, Reference Fleet and Wood1999). 1,25 Dihydroxyvitamin D3 treatment induces the saturable, but not diffusional, component of Ca transport (Giuliano & Wood, Reference Giuliano and Wood1991) and induces accumulation of calbindin D9K mRNA in these cells (Fleet & Wood, Reference Fleet and Wood1994).
In the present study, treatment with 20 : 5n-3 and 22 : 6n-3, separately, and in combination, over 8 d (preventative studies) significantly enhanced total transepithelial Ca transport in non-inflamed Caco-2 cell monolayers. This enhancement was related to an increased rate of transcellular Ca transport. The stimulatory effect of the 20 : 5n-3 and 20 : 5n-3+22 : 6n-3 on total and transcellular Ca transport was less evident, just failing to reach statistical significance, in cells treated in the therapeutic studies, and even though the stimulatory effects of 22 : 6n-3 on these parameters were statistically significant, they were of less magnitude than those seen in the preventative studies. However, the treatment with fatty acids in the therapeutic studies was for 6 d as opposed to the 8 d in the preventative studies, which may have lessened their effect on Ca transport. The duration of exposure was chosen because 6 d has been shown to be sufficient to allow fatty acid uptake into Caco-2 cells. The present findings in human intestinal-like Caco-2 cells, a good in vitro model for Ca absorption in man (Giuliano & Wood, Reference Giuliano and Wood1991; Fleet & Wood, Reference Fleet and Wood1999), support the findings from animal studies. For example, Haag & Kruger (Reference Haag and Kruger2001) reported that n-3 fatty acids, 20 : 5n-3 and 22 : 6n-3, enhanced duodenal Ca uptake in rats. Coetzer et al. (Reference Coetzer, Claassen, van Papendorp and Kruger1994) found that fish oil (rich in 20 : 5n-3 and 22 : 6n-3) significantly enhanced Ca transport across brush-border membranes of rat intestinal cells, while oils such as evening primrose and sunflower-seed (both particularly high in 18 : 1n-9 and 18 : 2n-6) or coconut oil (particularly rich in 12 : 0, 14 : 0 and 16 : 0) had no effects on Ca transport. While not investigated in the present study, the mechanism by which the n-3 fatty acids stimulated transcellular Ca transport may be related to effects at the basolateral membrane (i.e. Ca extrusion); their modulation of Ca2+-ATPase and Na+,K+-ATPase activity is either by a direct action on the enzyme or by phosphorylation processes via protein kinases A and C (Haag & Kruger, Reference Haag and Kruger2001; Haag et al. Reference Haag, Vermeulen, Magada and Kruger1999, Reference Haag, Magada, Claassen, Bohmer and Kruger2003) and/or influencing Ca uptake at the brush border by changing the phospholipids composition and therefore, possibly permeability (Coetzer et al. Reference Coetzer, Claassen, van Papendorp and Kruger1994). In the present study, only a very low proportion of overall Ca transport in our Caco-2 cells occurred by the paracellular route (about 4–6 %). Furthermore, in general, treatment with n-3 fatty acids had little, if any, effects on this mode of Ca transport.
In the present study, a model of inflamed intestinal cells, typical of that in Crohn's disease patients, was achieved by treatment of Caco-2 cells with TNF-α. TNF-α treatment of Caco-2 cells led to significantly higher levels of the chemokine, IL-8, which has a central role in attracting neutrophils to the inflamed mucosa. However, n-3 fatty acid treatment did not appear to prevent or lower the induction of this biomarker of inflammation upon treatment of Caco-2 cells with TNF-α, or therapeutically lessen the production of IL-8 in already induced cells. It must be emphasised, however, that effects on the inflammatory process per se were not included as a primary outcome measure in our studies, because we were cognisant of the fact that Caco-2 cells, as an in vitro mono-culture model, lack the complex interactions and communications with immune cells that exist in vivo. The present study also investigated the effect of n-3 fatty acids on intestinal inflammation from a luminal perspective, as opposed to the more systemic effect. Interestingly, a recent study in Caco-2 cells showed that allicin, a bioactive substance present in freshly crushed garlic, inhibited the TNF-α-induced secretion of a number of cytokines, including IL-8, suggesting an attenuation of intestinal inflammation by a luminal food bioactive compound (Lang et al. Reference Lang, Lahav, Sakhnini, Barshack, Fidder, Avidan, Bardan, Hershkoviz, Bar-Meir and Chowers2004). Notwithstanding this lack of effect on the biomarker of inflammation in the present study, treatment with n-3 fatty acids for 8 d (preventative studies) appeared to promote total transepithelial and transcellular Ca transport in TNF-α-treated Caco-2 cell monolayers. On the other hand, treatment of Caco-2 cells with 22 : 6n-3 for 6 d following TNF-α treatment for 48 h (therapeutic studies) failed to stimulate total and transcellular Ca transport, while 20 : 5n-3 and 20 : 5n-3+22 : 6n-3 did promote these modes of Ca transport. As with case of non-TNF-α-treated Caco-2 cells, the slightly shorter duration of treatment in the therapeutic studies may have lessened their effect on Ca transport, with the magnitude of stimulation less than that seen in the preventative studies. The reason for the differential effects of 22 : 6n-3 on total and transcellular Ca transport between preventative and therapeutic studies is unclear, but, while not assessed in the present study, it may be related to possible different uptake efficiencies of the fatty acid or effects on gene transcription between cells in which inflammation was acute (over 48 h; preventative studies) compared with longer-term (over 8 d; therapeutic studies).
The findings of the present study also showed that treatment of Caco-2 cells with acute (48 h) and longer-term (8 d) treatment with TNF-α, independent of lipid treatment, increased total transepithelial and transcellular Ca transport, lowered TEER but had no effect on paracellular Ca transport. While there are no reports in the literature of the effect of TNF-α on Ca transport, these effects on both transcellular and paracellular transport were somewhat unexpected. In relation to paracellular transport, there are several reports of an enhanced paracellular permeability (and reduced TEER) across Caco-2 cell monolayers treated with TNF-α (Marano et al. Reference Marano, Lewis, Garulacan, Soler and Mullin1998; Ma et al. Reference Ma, Iwamoto, Hoa, Akotia, Pedram, Boivin and Said2004, Reference Ma, Boivin, Ye, Pedram and Said2005; Lang et al. Reference Lang, Lahav, Sakhnini, Barshack, Fidder, Avidan, Bardan, Hershkoviz, Bar-Meir and Chowers2004). However, as mentioned already, only a very low proportion of overall Ca transport in our Caco-2 cells occurred by the paracellular route, possibly making it difficult to detect effects of TNF-α (or lipid) treatments. In fact, TEER across Caco-2 cell monolayers was significantly reduced by treatment with TNF-α, possibly suggesting a loosening of tight junctions. While not providing evidence of enhanced Ca uptake, Schmidt et al. (Reference Schmidt, Kosche, Baumeister and Vetter1995) showed that patients with active Crohn's disease (or ulcerative colitis) (and in whom TNF-α would be expected to be elevated) had moderately higher Ca2+ levels in colon biopsies samples compared with that in healthy controls. Furthermore, there was a significant decrease in intracellular Ca2+ concentration in patients with quiescent disease who were receiving maintenance therapy (and in whom TNF-α would be expected to be largely normalised). The effect of TNF-α on intestinal Ca handling requires further investigation to understand its physiological and pathophysiological relevance.
Further research in intestinal cells in culture may help improve our understanding of the mechanistic aspects of the effects of marine oil-derived fatty acids on Ca absorption. However, as a better understanding of the effect of these fatty acids on Ca absorption in human subjects is the ultimate goal, research is urgently required on the influence of marine oil-based n-3 fatty acids on Ca absorption in vivo in studies of healthy human subjects as well as of Crohn's disease patients, an area which to date has not received much research emphasis.







