Antibiotic-associated diarrhoea (AAD) is a frequent complication of antibiotic use( Reference Bartlett 1 ). Broad-spectrum antibiotics, such as ampicillin, cefixime and amoxicillin–clavulanic acid, can disrupt the balance of intestinal microbiota, resulting in the clinical symptoms of diarrhoea( Reference Bartlett 1 – Reference Forssten, Evans and Wilson 3 ). Various mechanisms of AAD have been proposed, including overgrowth of toxigenic bacteria leading to infectious diarrhoea and/or the loss of beneficial metabolic activities of intestinal microbes leading to excessive carbohydrates in the colonic lumen and osmotic diarrhoea( Reference Dunne 4 – Reference Young and Schmidt 6 ). Clostridium difficile accounts for 10–20 % of cases, leaving the majority of AAD resulting from other enteric pathogens or noninfectious mechanisms( Reference Hogenauer, Hammer and Krejs 5 ).
Amoxicillin–clavulanic acid is a commonly prescribed antibiotic that combines the β-lactam antibiotic, amoxicillin trihydrate, with a β-lactamase inhibitor, potassium clavulanate. This results in an antibiotic with potent bactericidal effects, and a broader range of action with efficacy against amoxicillin-resistant bacteria that produce β-lactamase( Reference White, Stokes and Slocombe 7 ). However, this can also increase the chance of AAD, with amoxicillin–clavulanic acid treatment having significantly greater occurrence of AAD than amoxicillin alone( Reference Gillies, Ranakusuma and Hoffmann 8 ). Studies have reported a 10–25 % rate of AAD in patients receiving amoxicillin–clavulanic acid for bacterial infections( Reference Bartlett 1 ). Furthermore, it has been shown to induce diarrhoea and microbial disturbances in healthy adult subjects( Reference Forssten, Evans and Wilson 3 , Reference Engelbrektson, Korzenik and Pittler 9 ). In addition to the proposed AAD mechanisms above, amoxicillin–clavulanic acid can increase motility in the small intestine, leading to diarrhoea( Reference Caron, Ducrotte and Lerebours 10 ).
Probiotics have been shown to inhibit pathogens, a trait that could be advantageous in counteracting AAD( Reference Coconnier, Lievin and Bernet-Camard 11 , Reference Hudault, Lievin and Bernet-Camard 12 ). Because of the strength of available evidence for probiotics, primary healthcare practitioners will advise that patients take a probiotic when taking antibiotics to help prevent AAD( Reference Rodgers, Kirley and Mounsey 13 ).
Meta-analyses on the use of probiotics for AAD in paediatric and adult populations show that probiotic intake may reduce the risk of AAD( Reference Goldenberg, Lytvyn and Steurich 14 , Reference Hempel, Newberry and Maher 15 ). However, the presence of confounding factors such as heterogeneity in the type, duration and dose of antibiotic use and the presence of different types of infections in adult populations( Reference Hempel, Newberry and Maher 15 ) has made it challenging to determine probiotic strain(s) that offer the greatest clinical efficacy in reducing AAD. In the present study, a commercially available multi-strain probiotic product (Lacidofil® STRONG), containing Lactobacillus helveticus R0052 and Lactobacillus rhamnosus R0011, was administered to investigate its effects on amoxicillin–clavulanic acid-induced AAD. Our study design mitigated confounders by standardising the antibiotic type and duration, as well as using healthy subjects, thereby controlling for variables related to infection.
The primary outcomes for this study were to determine the effect of probiotic supplementation with antibiotic use on consistency (as measured by the weekly mean of the daily Bristol Stool Scale (BSS) score value) and frequency of bowel movements (captured from the daily bowel habits diary (DBHD)). The secondary outcomes included the assessment of the proportion of participants reporting diarrhoea-like defecations (DLD), gastrointestinal symptoms using the Gastrointestinal Symptom Rating Scale (GSRS), safety (including biometric, vital signs and blood parameters) and adverse events.
Methods
This study was reviewed by the Therapeutic Products Directorate (TPD) and the Natural and Non-prescription Health Products Directorate of Health Canada, and approvals were obtained on 1 August 2013 from the TPD, Ottawa, Ontario. Research ethics board approval was obtained on 13 August 2013 from Institutional Review Board (IRB) Services, Aurora, Ontario. This trial was conducted in accordance with the ethical principles that have their origins in the Declaration of Helsinki and its subsequent amendments (Clinicaltrials.gov identifier NCT01941160).
Study design
This was a randomised, double-blind, placebo-controlled parallel study conducted at a single centre, KGK Synergize Inc., London, ON, Canada, between August 2013 and April 2014. Recruitment occurred from August 2013 to January 2014, and follow-ups occurred from October 2013 to April 2014. The duration of this study was 10 weeks with five distinct periods, as outlined in Fig. 1. These periods were run-in (day –7 to baseline), antibiotic (amoxicillin–clavulanic acid) plus probiotic or placebo (days 1–7), probiotic or placebo only (days 8–14), no probiotic or placebo (days 15–21) and follow-up (days 22–63). During the run-in period, subjects were instructed to begin completing weekly 3-d food records and a DBHD. After eligibility was confirmed during baseline assessments, all volunteers received amoxicillin–clavulanic acid (875 mg amoxicillin/125 mg clavulanic acid) for 1 week (days 1–7) plus probiotic or placebo intervention for 2 weeks (days 1–14). Weekly in-clinic visits were scheduled to collect outcome and compliance data for the initial 4 weeks (days –7 to 21) of the trial. During the subsequent 6-week follow-up period, subjects were required to continue completing their DBHD, complete weekly GSRS questionnaires and participate in two scheduled telephone calls (week 5 and week 8) to review study requirements.
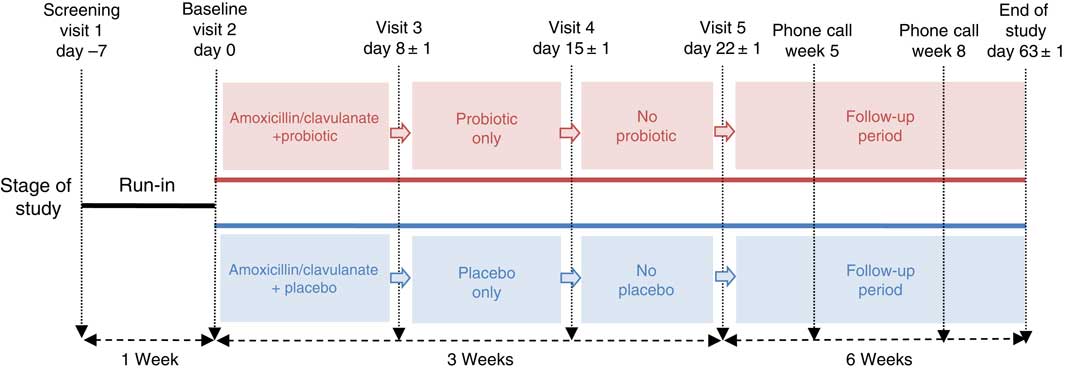
Fig. 1 Schematic representation of the study design. This 10-week study consisted of five periods: run-in (day –7 to baseline), antibiotic (amoxicillin–clavulanic acid) plus treatment (day 1–7), treatment only (day 8–14), no treatment (days 15–21) and follow-up (days 22–63).
Participants
Study participants were free-living, healthy individuals recruited from the region of Southwestern Ontario, Canada. Recruitment for this study was performed using KGK Synergize Inc.’s internal participant database along with local electronic and physical advertisement, with no sex or racial bias. The inclusion criteria were as follows: males and females between the ages of 18 and 50 years (inclusive); if female, either not of child-bearing potential or using a medically approved method of birth control; healthy individuals as determined by laboratory results, medical history and physical examination; a BMI of 18·0–29·9 kg/m2; agreement to maintain their regular diet (with the exception of avoiding probiotics and prebiotics) and exercise; and voluntary written and informed consent to participate in the study. Exclusion criteria were as follows: women who were pregnant, breast-feeding or planning to become pregnant during the course of the trial; BMI≥30·0 kg/m2; abnormal number of bowel movements (>3/d or <3/week); record of chronic gastrointestinal disorders; immune-compromised conditions; vegetarian or vegan diet; abuse of alcohol or drugs within 1 year of study; participation in a clinical research trial within 30 d of commencement of study; allergy or sensitivity to any substances used within the study; use of antibiotics within 60 d of randomisation; consumption of foods or supplements containing probiotics and/or prebiotics within 3 weeks of study randomisation; exposure to laxatives, enemas or suppositories within 1 week of randomisation; and any other condition that, in the investigator’s opinion, may have affected the subject’s ability to complete the study or its measures, or may have posed significant risk to the subject. The protocol was amended from the original to remove the requirement for enrolling equal numbers of male and female subjects (IRB amendment date of 13 December 2013).
Interventions
Participants were instructed to take one capsule of amoxicillin–clavulanic acid 30 min before breakfast and one capsule 30 min before dinner, whereas one capsule of investigational product or placebo was taken with each of those meals. Each probiotic capsule (Lacidofil® STRONG, Lot No. FD 0580) contained freeze-dried L. helveticus R0052 at 0·2 billion colony-forming units (CFU) and L. rhamnosus R0011 at 3·8 billion CFU with excipients of ascorbic acid, hydroxypropyl methylcellulose, magnesium stearate, potato starch and titanium dioxide. The placebo (Lot No. EK 1537) mimicked the size, shape, colour and taste of the probiotic capsules and contained ascorbic acid, hydroxypropyl methylcellulose, magnesium stearate, potato starch and titanium dioxide. The probiotic and placebo investigational products were manufactured by Lallemand Health Solutions. The study products were stored at 4±3°C. Each APO-AMOXI-CLAV capsule contained 875 mg of amoxicillin trihydrate and 125 mg of potassium clavulanate with excipients of magnesium stearate, croscarmellose sodium, colloidal silicon dioxide, hydroxypropyl methylcellulose, polyethylene glycol, ethylcellulose and titanium dioxide. The broad-spectrum semisynthetic antibiotic administered during this study, amoxicillin–clavulanic acid (APO-AMOXI-CLAV; DIN 02245623), was stored at or <25°C.
Outcome measures
The primary outcome measures were the between-group difference in the weekly mean BSS scores (consistency), and the between-group difference in the weekly mean number of bowel movements (frequency). The BSS describes and depicts the form of the faeces on a seven-point scale, from ‘separate hard lumps, like nuts’ (1) to ‘watery, no solid pieces’ (7)( Reference Lewis and Heaton 16 ). Each participant was required to record personal BSS scores in the DBHD for the duration of the study.
The secondary outcomes were the proportion of subjects having DLD, quality of life as reflected by a composite of GSRS questionnaire scores, biometric readings (weight, BMI, waist circumference), vital signs (resting heart rate and blood pressure) and blood safety parameters (complete blood count, electrolytes, glucose, creatinine, aspartate aminotransferase, alanine aminotransferase, γ-glutamyltransferase, bilirubin), as well as any incidence of adverse events as reported by participants. A DLD event, as defined by Koning et al.( Reference Koning, Jonkers and Stobberingh 17 ), corresponded to a stool frequency ≥3/d and/or stool consistency ≥5 (on the BSS) for at least 2 consecutive days.
The GSRS questionnaire was administered during the scheduled clinic visits and completed by the participants independently during the follow-up weeks. The GSRS provided a clinical rating score from 1 to 7 (1 being no discomfort to 7 being very severe discomfort) for gastrointestinal-related syndromes, including diarrhoea, constipation, abdominal pain, indigestion and reflux. The bowel habits questionnaire was completed by participants in their DBHD for all bowel movements. This questionnaire surveyed the strain to initiate or terminate the bowel movement, as well as the feeling of completeness of defecation. Blood samples were analysed at LifeLabs (London, ON, Canada) using standardised procedures.
Sample size
The proposed sample size for this study was 160 enrolled subjects, with eighty subjects randomised to each of the two study arms in a double-blinded manner. The sample size calculation was based on a sd of 1, a significance level of 5 % (two-sided α), 80 % power (β=0·20), 20 % attrition rate and a 0·5-point detectable difference in BSS scores between groups. This was based on a previous study that examined DLD events in healthy individuals following antibiotic administration( Reference Koning, Jonkers and Stobberingh 17 ).
Compliance
Compliance was assessed by counting the returned study product and antibiotics at each visit. Per cent compliance was calculated by determining the number of dosage units consumed divided by the number expected to have been taken and multiplied by 100. In the event of a discrepancy between the information in the subject diary and the amount of the study product returned, calculations were based on the product returned unless an explanation for lost product was provided. Subjects found to have a compliance of <80 % or >120 % at any visit were counselled. Compliance of <70 or >130 % was considered as noncompliant, and any subject demonstrating noncompliance for two consecutive visits was withdrawn from the study.
Randomisation and blinding
A randomisation schedule was prepared using block randomisation by an unblinded person at the study site who was not involved in study assessment. Within each block of four consecutively enrolled subjects, two subjects received placebo and two subjects received probiotic in a randomly permuted order generated using www.randomization.com. Upon enrolment into the study, every eligible participant was assigned a randomisation number based on the randomisation schedule.
The investigational products were labelled according to the requirements of International Conference on Harmonisation Good Clinical Practice guidelines, applicable local regulatory guidelines and included the applicable randomisation number. All clinic staff involved in product dispensing, visit assessments, conduct of the study, monitoring charts and analysis of outcomes remained blinded for the duration of the study. Treatment allocation was implemented using six-digit randomisation codes, with the list generated by an unblinded individual not involved in conducting the study. In case a serious adverse event would require the randomisation code to be broken for a given participant, sealed opaque envelopes labelled with the randomisation number and containing the associated treatment were prepared by the same unblinded individual, and kept at the coordinating centre. No premature unblinding occurred during the course of this study.
Statistical analysis
All hypotheses were conducted using two-sided tests and a type I error rate of α=0·05. All outcomes were tested by comparing treatments within each time period, as well as testing within treatments across time periods. For each end-point variable, a full general or generalised linear mixed model contained BMI, sex, time, treatment and the interaction of each with treatment as covariates. Backward selection was used to remove non-significant covariates with the final model always including time, treatment and their interaction. Some end points (some blood parameters and GSRS syndromes) required natural-logarithm transformation to ensure approximate normality and homogeneous variance. Other end points (bowel habits questionnaires and report of DLD events) were modelled using binary distributions.
Bowel consistency and frequency were calculated by combining each participant’s DBHD, and tallying the total number of bowel movements and average BSS each day. From this score, the weekly averages of the daily records were calculated such that each participant had ten observations, one for each week.
The proportion of participants who experienced a DLD event was identified using an indicator variable to mark each discrete event. The reporting of DLD events was modelled using binary distributions. Post hoc analysis of the duration of DLD was performed using a generalised linear mixed model to account for variability at baseline and between participants.
Individual questions in the GSRS were averaged into their respective syndrome scores. The GSRS reflux syndrome was modelled as a binary variable (reflux score >1 v. reflux score=1) because of the large proportion of participants reporting scores of 1 at all times during the study (84 % of all records).
A random effect for subject was used to account for the repeated observations, and the denominator degrees of freedom were adjusted using the Kenward–Roger method. Family-wise error rates within each analysis were controlled using the Tukey–Kramer or Dunnett’s method, as appropriate. If treatments differed at baseline, the changes from baseline week to post-baseline weeks were compared between the placebo and probiotic groups. All baseline characteristics were tested for differences among groups using tests of two proportions for per cent compliance to treatment, to antibiotic and to sex. A χ 2 test of a 2×r contingency table was performed for ethnicity and race. Two-sample t tests were performed for all continuous variables measured at baseline.
Results
Participant flow and baseline characteristics
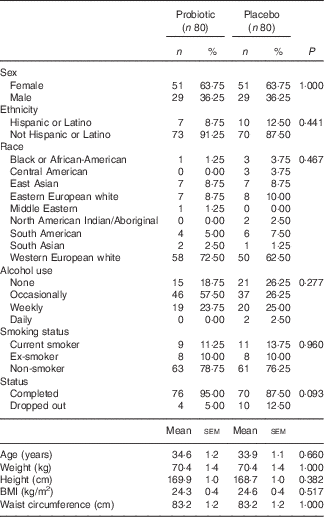
A flow diagram showing the participant disposition through the study is presented in Fig. 2. In the probiotic group, four study participants withdrew (5 %): one because of personal reasons, and three were lost to follow-up. In the placebo group, ten study participants withdrew (12·5 %): three because of personal reasons, two because of adverse events and five were lost to follow-up. There were no participants withdrawn because of failure of compliance to the study protocol. In total, 146 participants completed the study. The baseline characteristics for all participants are summarised in Table 1.
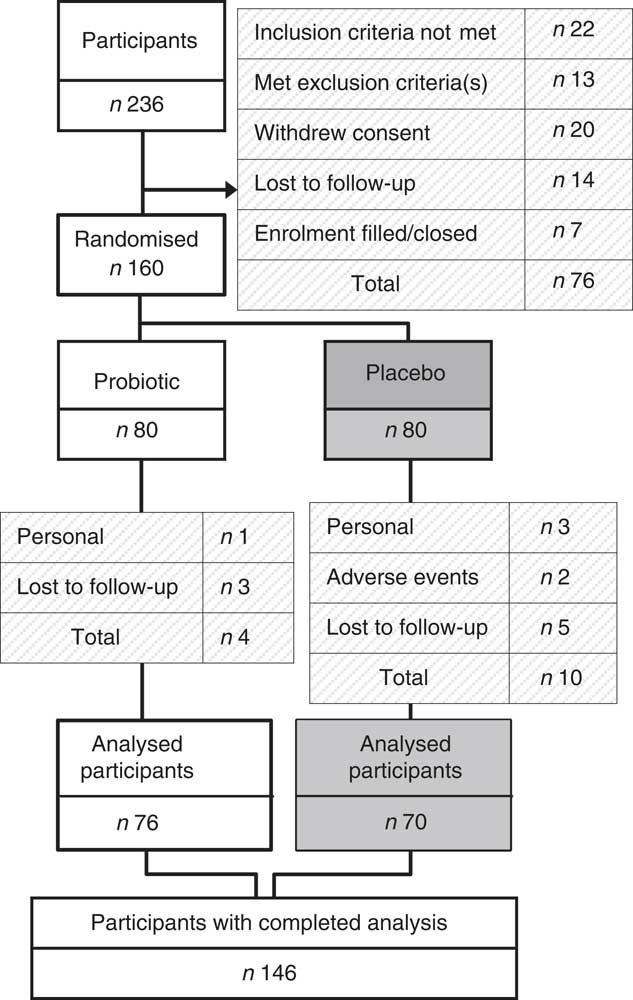
Fig. 2 Participant flow through the study. Subjects were recruited using KGK Synergize Inc.’s electronic clinic subject database and advertisements. A total of 236 subjects were screened and 160 eligible subjects were randomised. Of the 160 enrolled subjects, seventy-six in the probiotic group and seventy in the placebo group completed the study.
Table 1 Demographics and characteristics of all randomised participants at screening (Numbers and percentages; mean values with their standard errors)
Primary outcomes
Consistency of bowel movements: both probiotic and placebo groups showed an increase in BSS score during the amoxicillin–clavulanic acid plus treatment period compared with the run-in period (probiotic, P<0·001; placebo, P<0·001). There were no significant differences observed in the weekly mean of daily BSS values between the probiotic and placebo groups at any time point during the study (Table 2).
Table 2 Weekly average of daily Bristol Stool Scale (BSS) scores and bowel movement frequency (Mean values and standard deviations)Footnote *
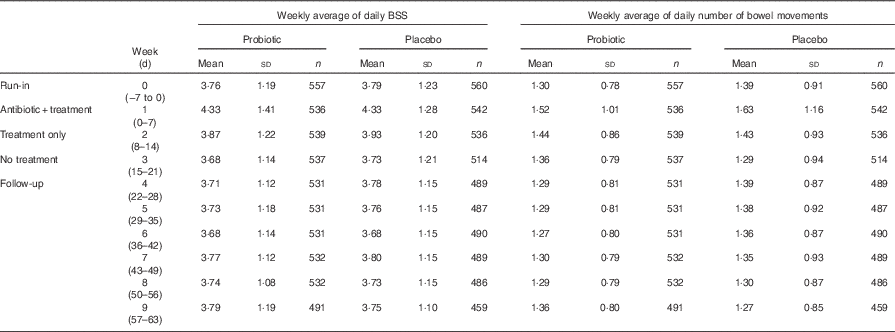
n, Number of bowel movements.
* Between-group comparisons of probiotic v. placebo did not reach statistical significance.
Frequency of bowel movements: there was a significant within-group increase in the frequency of bowel movements during amoxicillin–clavulanic acid plus treatment period compared with the run-in period in both the probiotic (P=0·036) and placebo (P=0·038) groups. This increase returned to baseline values by the follow-up period. There were no significant differences in bowel movement frequency between treatments (Table 2).
Post hoc analysis
The length of DLD events was significantly reduced with probiotic supplementation compared with placebo (P=0·037; effect size=0·52). The average length of DLD for the participants in the placebo group was 3·71 (sem 0·36) d, whereas participants taking the probiotic reported an average length of 2·70 (sem 0·36) d (Fig. 3(a)).
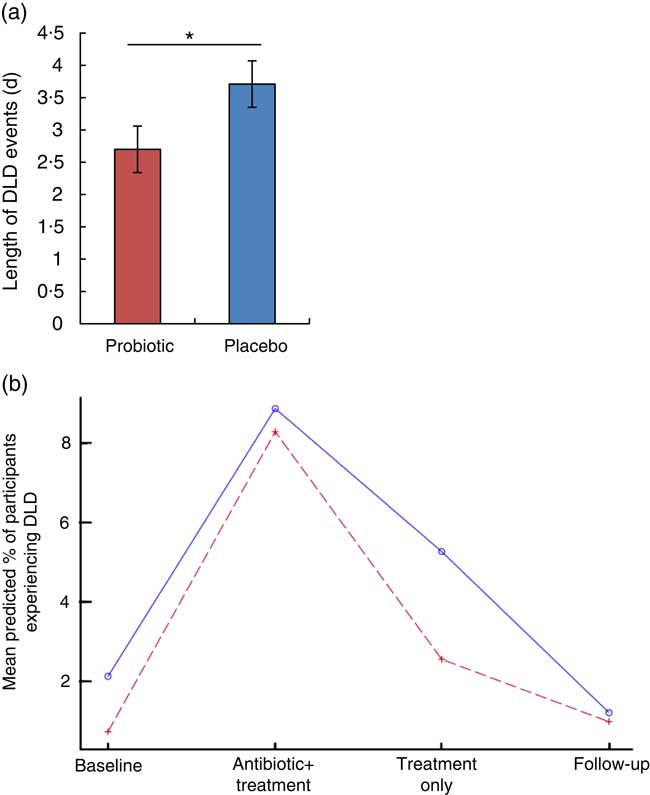
Fig. 3 Diarrhoea-like defecation (DLD) events following antibiotic treatment. (a) The mean length of DLD events over the course of the study was significantly reduced by the probiotic therapy during and following antibiotic treatment (probiotic (![]() ), n 21; placebo (
), n 21; placebo (![]() ), n 29). Values are means, with their standard errors. * P<0·05 using t test. (b) Regression analysis showing the per cent probability that a participant will experience a DLD event.
), n 29). Values are means, with their standard errors. * P<0·05 using t test. (b) Regression analysis showing the per cent probability that a participant will experience a DLD event.
Secondary outcomes
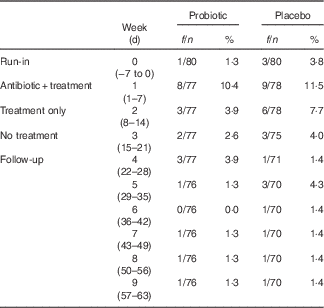
Proportion of participants reporting DLD: the proportions of participants who reported at least one DLD are presented in Table 3. There were a total of forty-six subjects who experienced at least one DLD event during the study: 33 % of the participants in the placebo group and 25 % of participants in the probiotic group reported at least one DLD event. Although not significant at any period, the probiotic group had a lower model-predicted proportion of participants experiencing DLD events resulting from amoxicillin–clavulanic acid administration. The greatest difference in the predicted proportion of participants who are likely to experience at least one DLD event occurred during the probiotic- or placebo-only period (probiotic, 2·54 % v. placebo, 5·26 %) (Fig. 3(b)). These mean predicted percentage values were obtained from the back transformation of the estimated log odds, which were the results of the logistic regression used to model the incidence of DLD.
Table 3 Proportion of participants who reported at least one diarrhoea-like defecation (Frequency/number of participants and percentages)
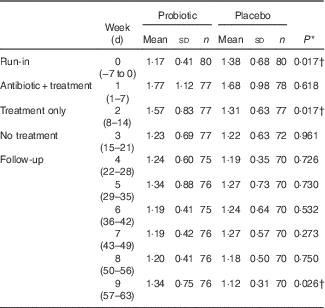
GSRS: significant differences in GSRS scores were not observed between groups for the constipation, abdominal pain and indigestion syndromes. However, the diarrhoea and reflux syndrome showed differences between groups. Significant differences in diarrhoea syndrome scores were seen in participants in the probiotic v. placebo groups during the run-in period (probiotic, 1·17 (sd 0·41); placebo, 1·38 (sd 0·68), P=0·017), treatment-only period (probiotic, 1·57 (sd 0·83); placebo, 1·31(sd 0·63), P=0·017) and week 9 of the follow-up period (probiotic, 1·34 (sd 0·75); placebo, 1·12 (sd 0·31), P=0·026) (Table 4), with the score slightly higher in the probiotic group. A test for treatment effect on the change from baseline to the probiotic or placebo-only periods showed significant differences (P=0·0001). The diarrhoea syndrome score increased by 0·4 in the probiotic group v. 0·07 in the placebo group. These weekly mean GSRS scores were <2, out of a possible 1–7, denoting slight to no discomfort. For the reflux syndrome, the proportion of participants reporting a score >1 in the placebo group was significantly lower compared with the probiotic group during the 1st week of follow-up (probiotic, 3·9 (sem 1·5) %; placebo, 1·13 (sem 0·54) %, P=0·044); however, this does not represent a clinically significant change, as the scores were below 2 in both groups (probiotic: 1·10 (sem 0·32); placebo: 1·05 (sem 0·25)).
Table 4 Average weekly Gastrointestinal Symptom Rating Scale diarrhoea syndrome scores (Mean values and standard deviations)
n, Number of participants.
* P value represents differences between probiotic and placebo groups.
† Significant difference (P<0·05) using t test.
Bowel habits questionnaire: the bowel habits questionnaire did not show a treatment effect between the probiotic and placebo groups. There was no significant difference between groups for the proportion of participants reporting straining to start or stop defecations, or for the feeling of incomplete evacuation (data not shown).
Safety parameters
There were no significant changes in the biometric and vital parameters between screening and study-end visits. Haematological parameters did not show significant interaction between treatment and visit, or in treatment main effect, from the screening to the final clinic visit. All biometric, vital and haematological parameters remained within normal/healthy physiological laboratory ranges and were not clinically significant (data not shown).
Adverse events
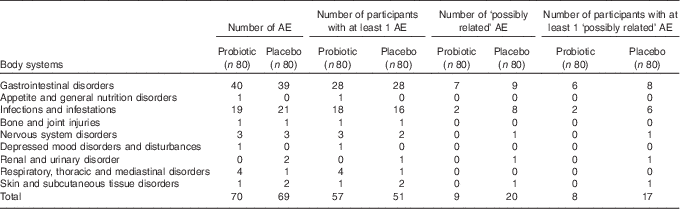
The causal relationship between adverse events and the treatments was assessed by the qualified investigator. Reporting of adverse events in this study used the standardised terminology as set out by the Medical Dictionary for Regulatory Activities. A total of 139 adverse events was reported during this trial, with 52 % of participants experiencing at least one adverse event. Of these events, twenty-nine (nine probiotic, and twenty placebo) were categorised as ‘possibly related’ to the investigational product. The distribution by body/organ systems of ‘possibly related’ adverse events was primarily gastrointestinal and infectious disorders (Table 5). Two study participants from the placebo group withdrew from the study because of adverse events: one withdrew during the antibiotic plus placebo period, whereas the other reported mild to moderate gastrointestinal symptoms related to bloating and gas experienced during the placebo-only period.
Table 5 Adverse events (AE) distributed by body system
Discussion
Statement of principal findings
The current study was designed to test the safety and efficacy of a two-strain lactobacillus probiotic in healthy subjects with a standardised antibiotic administration. This design controlled for confounding factors present in other probiotic studies of AAD.
The primary end points of the consistency and frequency of bowel movements, and the secondary end points of the proportion of participants reporting DLD events, GSRS Syndrome Scores and Bowel Habits did not show statistical significance between the probiotic intervention and placebo. A post hoc analysis found that participants supplemented with probiotic experienced significantly shorter duration of DLD events compared with those taking the placebo. This is a notable and clinically relevant improvement, with an effect size of 0·52. This is the first study to demonstrate this beneficial effect with probiotics on AAD in a population of healthy adults, and it provides a role for the probiotic product containing L. helveticus R0052 and L. rhamnosus R0011 for the attenuation of AAD duration in a population in which prolonged DLD events may lead to serious consequences.
Occurrence of antibiotic-associated diarrhoea
The occurrence of diarrhoea resulting from antibiotic treatment has been reported to be 5–39 % depending on the type of antibiotic used and other factors influencing the vulnerability of the population( Reference McFarland 18 ). Within a healthy population, the critical risk factors are the age of the subject and the specific antibiotic used. The susceptibility to AAD increases under the age of 6 years and over the age of 50 years( Reference McFarland 19 , Reference McFarland, Surawicz and Greenberg 20 ). The antibiotic used in this study, amoxicillin–clavulanic acid, is a potent bactericide( Reference White, Stokes and Slocombe 7 ) and among the higher inducers of AAD( Reference Bartlett 1 , Reference McFarland 18 ). A meta-analysis of seventeen randomised clinical trials examining amoxicillin–clavulanic acid therapy for infections showed a correlation between diarrhoea events and antibiotic treatment( Reference Gillies, Ranakusuma and Hoffmann 8 ). This meta-analysis also showed that the incidence of AAD occurred with every ten antibiotic courses. Furthermore, when amoxicillin–clavulanic acid was given to healthy subjects in a clinical trial, in the same dosage used in this study, AAD occurred in four of the fifty-one participants (7·8 %), all within 7 d of starting the antibiotic( Reference Engelbrektson, Korzenik and Pittler 9 ). The AAD occurrence in this current study was 10·4 % in the probiotic group and 11·5 % in the placebo group during the antibiotic plus treatment period. This rate of diarrhoea with amoxicillin–clavulanic acid is in accordance with published research. However, the relatively low prevalence of AAD in healthy individuals between 18 and 50 years of age made statistical inference in some study end points challenging.
Possible mechanism
Administration of amoxicillin and clavulanic acid (825 and 125 mg, respectively) once or twice daily, similar to that used in this study, showed microbiota disturbance in healthy subjects and significant increases in faecal bacterial counts for Enterobacteriaceae, which cause diarrhoea( Reference Forssten, Evans and Wilson 3 , Reference Engelbrektson, Korzenik and Pittler 9 ). The lactobacillus strains used in this study have been shown to survive passage through the gastrointestinal tract when given to healthy volunteers( Reference Firmesse, Mogenet and Bresson 21 ). In in vitro studies, these strains demonstrated the ability to adhere to human epithelial cells( Reference Sherman, Johnson-Henry and Yeung 22 ), maintain the gut barrier( Reference Sherman, Johnson-Henry and Yeung 22 ), block pathogen adhesion( Reference Alemka, Clyne and Shanahan 23 – Reference Wine, Gareau and Johnson-Henry 25 ) and stimulate an anti-inflammatory response( Reference Wallace, Bradley and Buckley 26 ). It is possible that these mechanisms have a role in reducing the duration of DLD events.
Rationale for probiotic concentration
Several clinical studies have investigated the use of this specific combination of lactobacillus strains in AAD. Most studies examined the probiotic intervention in children experiencing an infection requiring antibiotic therapy.
Maydannik et al.( Reference Maydannik, Khaytovych and Boyarskaya 27 ) investigated probiotic dosages ranging from 2×109 CFU for children under 1 year old to 6–12×109 CFU for children over 12 years old receiving antibiotic treatment for an infection (i.e. respiratory or urinary). The authors reported that a daily dose of 8×109 CFU of Lacidofil® resulted in a significant decrease in the occurrence of AAD, duration of diarrhoea and decrease in C. difficile carriage as compared with antibiotic treatment alone( Reference Maydannik, Khaytovych and Boyarskaya 27 ). A study in adults with a lower dose of 4×109 CFU/d did not show a significant difference for AAD occurrence compared with placebo( Reference Song, Kim and Jung 28 ). The significant decrease in AAD and duration of diarrhoea reported by Maydannik et al. with an 8×109 CFU probiotic concentration, and the absence of improvement in the adult study with a concentration of 4×109 CFU/d, provided the rationale for using a probiotic concentration of 8×109 CFU in this trial.
Reduction in the duration of diarrhoea-like defecation events
The critical finding of this study was the reduction in the average duration of DLD events from 3·71 d in the placebo group to 2·70 d with the supplementation with L. helveticus R0052 and L. rhamnosus R0011. Probiotics have been previously shown to affect the length of DLD events. A meta-analysis, conducted by Huang et al.( Reference Huang, Bousvaros and Lee 29 ), examined eighteen studies that administered probiotic treatment along with standard hydration therapy during acute diarrhoea in children and reported a 0·8-d reduction in the duration of diarrhoea compared with standard therapy alone. Another meta-analysis assessed the effectiveness of Saccharomyces boulardii in treating acute infectious diarrhoea in children by examining four randomized clinical trials that contained data on the duration of diarrhoea( Reference Szajewska, Skorka and Dylag 30 ). Szajewska et al. reported a 1·1-d reduction in the duration of diarrhoea with probiotics compared with placebo. Furthermore, a 1-d reduction in diarrhoea duration was shown in response to probiotic treatment in a geriatric population admitted to hospital and receiving antibiotics( Reference Wright, Wright and Murray 31 ). In that multi-centre randomised controlled trial, the mean duration of diarrhoea was 4 d for the probiotic group compared with 5 d in the placebo group. These studies corroborate and support our finding that probiotic treatment can decrease DLD events by a full day.
Risk/benefit
There are risks associated with any treatment intervention; however, with regard to probiotics, this risk is low. Randomised clinical trials have examined the safety of probiotics, and shown them to be well-tolerated and associated with few adverse events in healthy individuals in dosages exceeding those used in the current study( Reference Hanifi, Culpepper and Mai 32 – Reference Wind, Tolboom and Klare 34 ). Given the significant clinical relevance of reducing the length of DLD events arising from AAD, one could affirm that the benefits of probiotic supplementation greatly outweigh the risks. The adverse effects of prolonged AAD include dehydration, malnutrition, hypokalaemia, renal failure, rare cases of toxic megacolon, perforated colon and shock( Reference Kale-Pradhan, Jassal and Wilhelm 35 ). Furthermore, it can represent a substantial burden to the healthcare system. The use of this multi-strain probiotic reduced the duration of DLD events by a full day. If this 27 % reduction in DLD length can be translated to a reduction of utilisation of hospital resources and/or duration of hospital stays, the economic savings would be substantial. The economic impact can be extended to non-hospitalised populations by decreasing the loss of productivity that may be incurred as a result of AAD. Last, the effect of shortening DLD events with antibiotic treatment could have positive effects on regimen compliance, thereby reducing the decreased efficacy and adverse effects caused by improper antibiotic use. Given the likelihood and possible severity of AAD among both healthy and high-risk populations, reducing the length of DLD with probiotic co-treatment is likely to have a significant impact on individual outcomes, as well as the healthcare system as a whole.
Conclusions
Antibiotic treatment causes a dysregulation of gut microbiota, which frequently leads to intestinal colonisation of harmful bacteria. In this study, both probiotic and placebo groups showed a significant increase in BSS score and frequency of bowel movements during the antibiotic plus probiotic or antibiotic plus placebo period, as compared with the run-in period. Although no significant differences between groups were observed in the primary or secondary end points, the duration of DLD events from amoxicillin–clavulanic acid administration was significantly reduced by 1 d (24 h) for participants who received supplementation with probiotics. Decreasing the number of days of diarrhoea after antibiotic treatment has clinical relevance, as it may reduce complications related to AAD. This is particularly important among patients who are more susceptible to severe AAD, as well as in attenuating the symptoms of AAD in individuals with a healthy digestive system receiving antibiotics for infections outside the gut.
Acknowledgements
The authors thank Ronan Gire, from Lallemand Health Solutions, who helped with various aspects of this study.
The study funding and study supplementation were provided by Lallemand Health Solutions Inc. Mirabel, Quebec, Canada.
T. A. T, S.-A. G. and M. E. contributed to the design of the study, and drafting and revision of the manuscript. M. C. C. wrote the statistical plan for the study and analysed the data. M. E. was involved in the conduct of the study. R. P. S. contributed by interpretation of data, drafting and revising the manuscript. All authors reviewed and approved the final version of the manuscript.
This study was fully funded by Lallemand Health Solutions Inc., Mirabel, Quebec, Canada. Lallemand Health Solutions Inc. contributed to the study design and preparation of the manuscript.













