Maintaining a healthy lifestyle including a healthy diet is important to support good health in humans. Digestive functions play a key role in maintaining or improving health status. In its guidance on health claims related to gut and immune function, the European Food and Safety Agency indicates that reducing gastrointestinal (GI) discomfort is considered as an indicator of improved GI function, and is a beneficial physiological effect( 1 ). The efficacy of probiotics or prebiotics/fibres on GI discomfort or well-being has been assessed during the last decade primarily in studies of patients with irritable bowel syndrome (IBS)( Reference Clarke, Cryan and Dinan 2 – Reference Moayyedi, Ford and Talley 4 ). In the absence of validated biomarkers, patient-reported outcomes (PRO) based on the patient's self-report are the standard used to determine the efficacy of these products on GI discomfort as well as on digestive symptoms.
The primary population targeted for dietary change and potentially beneficial food products is the general population, and, consequently, this population is a good and relevant study group for efficacy trials aimed at changing GI discomfort or well-being. Designing such trials is challenging because almost all clinical trials to date have been performed in IBS patients defined according to the Rome criteria( Reference Longstreth, Thompson and Chey 5 ). Patients with IBS have generally been considered as an appropriate study group to substantiate claims on GI discomfort intended for the general population( 1 ). Although some digestive symptoms as well as alterations of bowel function are common in the general population( Reference van Kerkhoven, Eikendal and Laheij 6 – Reference Heaton, O'Donnell and Braddon 10 ), there are significant differences in the type, severity and presentation of GI problems between those with IBS and the general population. For example, while IBS is characterised by abdominal pain and/or discomfort, digestive symptoms in the general population are less frequent and severe, and rarely include pain. On the other hand, predominant symptoms in non-patients include non-painful symptoms such as bloating, flatulence and borborygmi( Reference van Kerkhoven, Eikendal and Laheij 6 , Reference Leibbrand, Cuntz and Hiller 7 ). Given these differences, it is unlikely that a PRO developed for the assessment of symptom improvement in IBS is appropriate for the assessment of improvement in mild GI discomfort and of GI well-being improvement in the general population. However, valid PRO are needed given the growing interest in testing products for the general population targeting GI discomfort or wellness.
In the general population with less frequent and less severe symptoms, the target of a food intervention is to improve the overall sensation of GI comfort or well-being. An overall assessment may allow individuals to use their own weighting of the importance of various aspects of GI comfort or well-being in determining the degree of change (improved, unchanged or worse). Due to the absence of a predominant symptom in this broad population, we hypothesised that people within the general population are more able to summarise adequately their symptom experience in a global single item than IBS patients, making the content validity of the global assessment more likely. A PRO measuring this concept must capture both improvement and worsening to ensure the absence of side effects of the food intervention that may occur with some fibres, for example. Overall binary assessment (e.g. adequate relief with yes/no modalities) that was extensively used in IBS trials was criticised for the absence of measuring worsening( Reference Trentacosti, He and Burke 11 ).
There are no specific guidelines for developing PRO for food-related studies. In the absence of available valid and reliable PRO for measuring GI well-being or comfort in the general population, an overall assessment of GI well-being was developed using a three-point scale (improved, unchanged or worse)( Reference Guyonnet, Schlumberger and Mhamdi 12 ). This instrument was shown to be able to capture the beneficial effect of dairy products with probiotics in the non-IBS general population( Reference Guyonnet, Schlumberger and Mhamdi 12 ).
In the present study, we explored the properties of this PRO end point in a non-IBS general population and compared changes in GI well-being with the change in a composite score of frequency of digestive symptoms, bowel function and health-related quality of life (HRQoL).
Materials and methods
Study protocol and study subjects
The study was single-centre (Harrison Clinical Centre, Munich, Germany), randomised, double-blind, controlled, parallel-group design assessing the effect of a 4-week daily consumption of a fermented dairy product with probiotic v. a non-fermented dairy product with no probiotic (control group).
The study design, and inclusion and exclusion criteria of subjects have been described in detail in Guyonnet et al. ( Reference Guyonnet, Schlumberger and Mhamdi 12 ). All subjects were women, aged 18–60 years, with normal weight or overweight (BMI 18–30 kg/m2) without a diagnosis of any digestive disease or systematic disease. Patients with IBS or any functional GI disorder were excluded as well as individuals under medications for digestive symptoms or with lactose intolerance. Subjects who took antibiotics within the month before the inclusion visit were not included. Subjects must have bowel frequency within the normal range (3–21 bowel movements per week).
During a 2-week baseline period, baseline values were obtained for the outcome parameters with a weekly assessment of frequency of digestive symptoms (abdominal pain/discomfort, bloating, flatulence/passage of gas and borborygmi/rumbling stomach), bowel function (bowel movement and stool consistency) and HRQoL. Only subjects having a mean composite score for the frequency of digestive symptoms between 2 and 12 (score ranging from 0 (none of the four symptoms) to 16 (all symptoms every day)) during this baseline period were randomised. Subjects had bowel movement frequency within the normal range (3–21 per week). This allowed recruiting subjects among the general population experiencing a minimal level of digestive symptoms and who could benefit from the food intervention.
Randomised subjects consumed either two 125 g servings of a fermented dairy product with probiotic or a control dairy product daily during 4 weeks. The fermented dairy product with probiotic contained Bifidobacterium lactis (strain no. I-2494 in the French National Collection of Cultures of Micro-organisms (CNCM, Paris, France)), referred to as DN-173 010 in previous publications such as in the Guyonnet et al. ( Reference Guyonnet, Schlumberger and Mhamdi 12 ) paper, together with the two classical yogurt starters, Streptococcus thermophilus (CNCM strain no. I-1630) and Lactobacillus delbrueckii subsp. bulgaricus (CNCM strain no. I-1632 and I-1519), and Lactococcus lactis ssp. lactis (CNCM strain no. I-1631). The test product contains 1·25 × 1010 colony-forming units/cup of B. lactis CNCM I-2494/DN-173 010 and 1·2 × 109 colony-forming units/cup of S. thermophilus and Lactobacillus delbrueckii subsp. bulgaricus. The control dairy product was a milk-based non-fermented dairy product without probiotics and with a lactose content of < 4 g/cup, which is similar to the content of lactose in the test product. These two products had a similar appearance, texture and taste.
Subjects were not allowed to consume any probiotic food or supplement and fermented dairy product other than those provided for the intervention period.
The study protocol was conducted in accordance with the Declaration of Helsinki and approved by the Ethics Committee Bayerische Landesaerztekammer (Munich, Germany). All volunteers gave written informed consent before inclusion in the study.
Overall gastrointestinal well-being
GI well-being was self-evaluated by subjects weekly during the 4 weeks of product consumption. This scale was adapted from a scale developed by Guyatt et al. ( Reference Guyatt, Deyo and Charlson 13 ). Subjects assessed the changes in their GI well-being with the following question: ‘How do you consider in the past 7 d, your GI well-being (intestinal transit, stool frequency and consistency, abdominal pain/discomfort, bloating, flatulence/passage of gas, borborygmi/rumbling stomach) compared to the period before beginning the consumption of the study product?’. In order to have a simple instrument and decrease ambiguity in the subject's assessment, a three-point Likert scale was used to report changes (improved, unchanged or worse) in GI well-being. The fifteen-point answer option of the original Guyatt global assessment was considered as inappropriate to a population with mild digestive symptoms, i.e. seven-point ( − 7 to − 1) for subjects answering ‘worse’ and seven-point (+1 to +7) for subjects answering ‘improved’. Specifically, the concern was that subjects with and infrequent symptoms would not be able to reliably discriminate GI well-being using such a fine-grained scale.
Overall GI well-being improvement for the study period was calculated as the number of weeks rated as ‘improved’ during the 4-week period of intervention (range 0–4).
As recommended in guidelines for the design of treatment trials for functional GI disorders( Reference Irvine, Whitehead and Chey 14 ), each subject was classified as a responder or a non-responder for GI well-being that was used as the primary end point in the intervention trial( Reference Guyonnet, Schlumberger and Mhamdi 12 ). The definition of a responder must reflect a clinically meaningful improvement for each participant. In the absence of consensus on what constitutes a clinically meaningful improvement, the pre-specified definition of a responder was based on the recommendations for the global assessment of symptom relief in IBS trials( Reference Irvine, Whitehead and Chey 14 ).
A responder was defined as a subject having an improvement in their GI well-being, i.e. answering ‘improved’ on the three-point Likert scale, on at least 2 weeks over the 4-week period of product consumption. Recent guidelines for defining responders for abdominal pain in IBS suggest possible use of a ‘no worsening’ condition( 15 ). In the present analysis, an alternative responder definition, another definition of a responder including no rating of ‘worsening’ at any week during the 4-week period of product consumption, was therefore used to perform exploratory sensitivity analysis.
Digestive symptoms
The frequency of four individual digestive symptoms (abdominal pain/discomfort, bloating, flatulence/passage of gas and borborygmi/rumbling stomach) was evaluated weekly with five-point Likert scales that range from 0 (never) to 4 (every day of the week) throughout the study. A composite score of all symptoms was calculated ranging from 0 to 16.
Stool frequency and consistency
Bowel movements were reported daily throughout the study as well as stool consistency for each stool passed according to the Bristol Stool Form Scale( Reference O'Donnell, Virjee and Heaton 16 ). In order to assess the normalisation of stool consistency, scores of stool consistency were recoded as follow: 0 = type 4 (like a sausage or snake, smooth and soft); 1 = types 3 (like a sausage but with cracks on surface) and 5 (soft blobs with clear-cut edges); 2 = types 2 (sausage shaped but lumpy) and 6 (fluffy pieces with ragged edges, a mushy stool); 3 = types 1 (separate hard lumps like nuts, difficult to pass) and 7 (watery, no solid pieces, entirely liquid).
Health-related quality of life questionnaires
HRQoL of subjects was assessed by self-administration of two questionnaires: the Food and Benefits Assessment (FBA)( Reference Guyonnet, Chassany and Picard 17 ) and the Psychological General Well-Being Index (PGWBI)( 18 ). The questionnaires were completed at baseline and after 4 weeks of product consumption.
The FBA questionnaire comprises forty-one items, making it possible to calculate scores for seven dimensions (snacking, vitality, well-being, physical appearance, aesthetics, digestive comfort and disease prevention). The scores range from 0 to 100 (best).
The generic questionnaire PGWBI measures psychological well-being and distress, and is composed of twenty-two items that constitute six dimensions (anxiety, depression, self-control, positive well-being, general health and vitality). The scores of all dimensions can be summarised to provide a global score( 18 ). The scores range from 0 to 100 (best).
The FBA digestive comfort dimension score and the PGWBI global score were defined as the main scores for HRQoL analysis. Other dimensions of both questionnaires were considered as secondary HRQoL criteria.
Statistical analyses
All the analyses were carried out on the intention-to-treat population. At baseline, descriptive statistics were done (mean, standard deviation and CI with a risk fixed at 5 %) on digestive symptoms, composite score, stool frequency and consistency, digestive dimension of the FBA questionnaire and the PGWBI, age and BMI.
The change from baseline, at each week, was calculated for digestive symptoms, composite score, stool consistency and frequency.
For each week and by the degree of GI well-being (improved, unchanged or worse), the mean change of each previous outcome was calculated.
By week and over the 4 weeks of product consumption, a comparison of the mean change of the outcomes was carried out between degrees of GI well-being. An ANOVA followed by a Tukey–Kramer adjustment test, to take into account the multiplicity of tests, was used to test for statistical differences.
For each subject, the number of weeks rated as ‘improved’ for overall GI well-being during the 4-week period of intervention was counted, allowing classification of subjects in five groups corresponding to 0, 1, 2, 3 or 4 weeks with ‘improved’ overall GI well-being. For each group, the mean and standard deviation of digestive symptom, composite score, the four individual digestive symptoms, stool consistency and frequency, and HRQoL responses were calculated at baseline. The correlation between the number of weeks of improvements and the other outcomes was evaluated with a Spearman correlation coefficient (non-parametric correlation test).
A similar analysis was carried out on the mean change from baseline of the different outcomes and the number of weeks of GI well-being improvement over the 4 weeks (0–4 weeks) and the overall GI well-being responder status (yes/no).
In addition to the aforementioned primary analyses, two exploratory analyses were also performed to further examine potential clinical utility of the instrument.
The first one consists to cross the overall GI well-being responder status and digestive symptom responder status.
A digestive symptom responder was defined on the following definition: (1) a subject having a decrease of at least 30 % from baseline in the weekly mean score of the composite score of digestive symptoms for at least 2 of the 4 weeks of product intervention; (2) a subject having no worsening from baseline at any weekly composite score of GI symptoms. The 30 % threshold decrease was chosen according to the last Food and Drug Administration's IBS guideline describing this threshold for abdominal pain.
A contingency analysis followed by Fisher's exact test and κ coefficient was carried out to evaluate the concordance between overall GI well-being responders and digestive symptom responders.
A discriminate partial least square regression was done to assess the second exploratory aim: determination of the contribution of the different symptoms (abdominal pain, bloating, flatulence and borborygmi), stool frequency and stool consistency to the improvement of GI well-being (responder status: yes/no).
The software used to manage data was the SAS System package (SAS Institute, Inc.) version 9.2 and to carry out the statistical analysis was JMP (SAS Institute, Inc.) version 8.
Results
Subject characteristics
From the 217 subjects included in the run-in period, 202 were randomised in the 4-week daily consumption of a fermented dairy product with probiotic v. control product. Table 1 describes the baseline data of the 197 randomised subjects retained for the analyses (five subjects had a premature withdrawal with no evaluable data). All subjects were female with a mean age of 32·2 (18–59) years. The highest score (i.e. more frequent) for digestive symptoms was flatulence (2·48 (sd 0·84)) and the lowest abdominal pain/discomfort (1·02 (sd 0·75)). Subjects reported normal bowel movement frequency of approximately once per d (7·28 (sd 3·01)).
Table 1 Baseline characteristics of the subjects (Mean values and standard deviations, n 197)
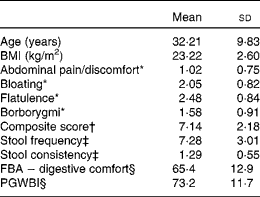
FBA, Food and Benefits Assessment; PGWBI, Psychological General Well-Being Index.
* Frequency of symptom, score 0 (never) to 4 (every day of the week). Values represent the two weekly assessments during baseline.
† Sum of individual symptoms, score 0–16.
‡ Adapted from the Bristol Stool Form Scale: 0 (type 4); 1 (types 3 and 5); 2 (types 2 and 6); 3 (types 1 and 7).
§ Score from 0 to 100 (best).
Gastrointestinal well-being changes and relationship to the other outcomes
The associations between weekly changes in the overall assessment of GI well-being and changes in the other outcomes are shown in Table 2. Regardless of the time point in the study, subjects reporting improved GI well-being had significantly larger decreases in the composite score of digestive symptoms than subjects with unchanged or worse GI well-being.
Table 2 Association between different changes in gastrointestinal well-being (improved, unchanged or worse) and other parameters (Mean values and standard deviations)
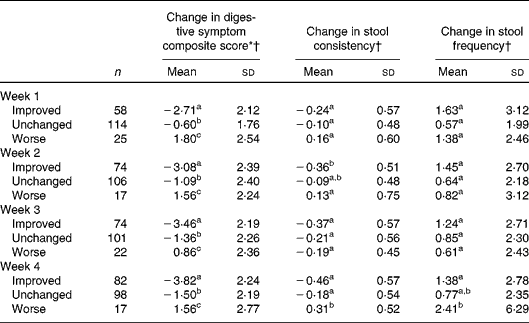
a,b,cMean values with unlike superscript letters were significantly different (P< 0·05; pair comparison, Tukey–Kramer test).
* Digestive symptom composite score is the sum of the weekly assessment of the four individual intestinal symptoms (abdominal pain, bloating, flatulence and borborygmi). Score from 0 (never) to 16 (every day of the week).
† Changes in digestive symptom composite score, stool consistency and stool frequency at each week are calculated v. baseline values.
The unchanged group had also decreases in composite score, whereas the worsened group experienced increased composite score, these two groups also being significantly different.
For stool frequency and consistency, differences were only found between those rating their GI well-being as improved and worsened at week 2 for consistency and at week 4 for both consistency and frequency.
There was a significant (P< 0·0001) correlation between the number of weeks of GI well-being improvement and mean changes in the composite score of digestive symptoms ( − 0·58) and scores on the digestive comfort dimension of the FBA questionnaire ( − 0·46) (Table 3). Correlations with stool consistency and frequency were also significant, although modest ( − 0·28 and 0·25, respectively). The mean changes over the 4-week period of intervention for each individual digestive symptom were also significantly (P< 0·0001) correlated with the number of weeks rated as ‘improved’ for GI well-being (Table 4). These correlations were all inferior to the correlation of the composite score, with flatulence showing the highest correlation ( − 0·50).
Table 3 Mean values and correlations between the number of weeks with improved gastrointestinal (GI) well-being and the average change in the other outcome measures (Mean values and standard deviations, n 197)
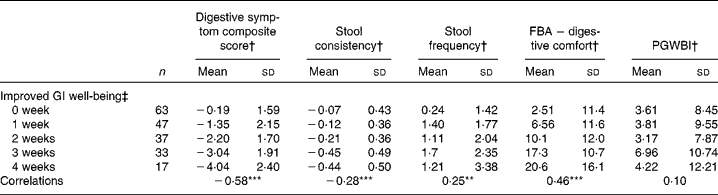
FBA, Food and Benefits Assessment; PGWBI, Psychological General Well-Being Index.
Correlations between the number of weeks of GI well-being improvement and the other parameters were significant (Spearman's non-parametric test): ** P< 0·001, *** P< 0·0001.
† Outcome measure changes are calculated using the mean change over the 4-week period of intervention v. baseline values.
‡ Number of weeks with improved GI well-being during the 4 weeks of intervention.
Table 4 Mean values and correlations between the number of weeks with improved gastrointestinal (GI) well-being and the average change in individual digestive symptoms (Mean values and standard deviations, n 197)
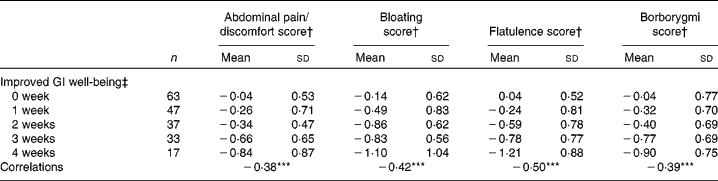
Correlations between the number of weeks of GI well-being improvement and the other parameters were significant (Spearman's non-parametric test): *** P< 0·0001.
† Outcome measure changes are calculated using the mean change over the 4-week period of intervention v. baseline values.
‡ Number of weeks with improved GI well-being during the 4 weeks of intervention.
Comparison of gastrointestinal well-being scores between responders and non-responders
Subjects who were responders to GI well-being assessment when compared with non-responders had clearly better GI health status as shown by a significantly greater improvement in the composite score of digestive symptoms (P< 0·0001, − 38·5 % in the responder group v. − 9·9 % in the non-responder group; Table 5). A significantly greater improvement in bowel function (stool frequency, P< 0·0001 and consistency, P< 0·05) was also observed for the responder group compared with non-responders as well as a greater improvement in HRQoL from the digestive comfort dimension of the FBA questionnaire (P< 0·0001). Sensitivity analysis with a more strict definition of responders (i.e. without worsening at any week) showed similar data (decrease in digestive symptoms: − 41·8 % in responders v. − 10·9 % in non-responders), except for stool frequency that was no longer significant (Table 6).
Table 5 Comparison of outcome measures in gastrointestinal (GI) well-being responders and non-responders (Mean values and standard deviations)
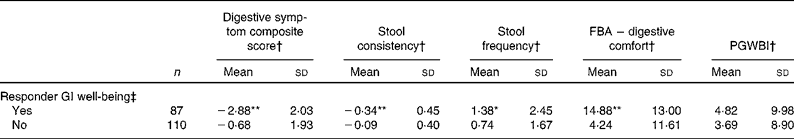
FBA, Food and Benefits Assessment; PGWBI, Psychological General Well-Being Index.
Mean values were significantly different compared with the non-responder group (t test): * P< 0·05, ** P< 0·0001.
† Changes are calculated using the mean change over the 4-week period of intervention v. baseline values.
‡ A responder is defined as a subject reporting an improvement of its GI well-being during at least 2 weeks of the 4 weeks of intervention.
Table 6 Sensitivity analysis for comparison of outcome measures in gastrointestinal (GI) well-being responders and non-responders (Mean values and standard deviations)

FBA, Food and Benefits Assessment; PGWBI, Psychological General Well-Being Index.
Mean values were significantly different compared with the non-responder group (t test): * P< 0·05, * * P< 0·0001.
† Changes are calculated using the mean change over the 4-week period of intervention v. baseline values.
‡ A responder is defined as a subject reporting an improvement of its GI well-being during at least 2 weeks of the 4 weeks of intervention without worsening at any time.
Effect of baseline status on gastrointestinal well-being improvement and responder status
Improvement in GI well-being during the 4 weeks of intervention was not correlated (r − 0·09 to 0·17) with the baseline value of any outcome (composite score of digestive symptoms, stool frequency and consistency, and HRQoL).
Responder status was also evaluated for its relationship with baseline symptom measures. Scores for individual digestive symptoms and composite score were generally higher in responders v. non-responders (Table 7), with abdominal pain (P= 0·02) and stool consistency (P= 0·02) being the only symptoms statistically significantly higher in responders when compared with non-responders. Similar results were found with the alternative ‘no worsening’ definition of a responder with significant differences for abdominal pain (P= 0·03), stool consistency (P= 0·02) and the composite score (P= 0·01) (Table 8).
Table 7 Baseline characteristics of gastrointestinal (GI) well-being responders and non-responders (Mean values and standard deviations, n 197)
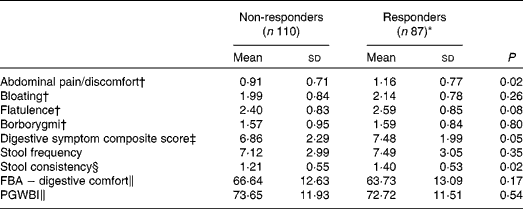
FBA, Food and Benefits Assessment; PGWBI, Psychological General Well-Being Index.
* A responder is defined as a subject reporting an improvement of its GI well-being during at least 2 weeks of the 4 weeks of intervention.
† Frequency of symptom, score 0 (never) to 4 (every day of the week). Values represent the two weekly assessments during baseline.
‡ Adapted from the Bristol Stool Form Scale: 0 (type 4); 1 (types 3 and 5); 2 (types 2 and 6); 3 (types 1 and 7).
§ Sum of individual symptoms, score 0–16.
∥ Score from 0 to 100 (best).
Table 8 Baseline characteristics of gastrointestinal (GI) well-being responders and non-responders with a modified definition (Mean values and standard deviations, n 197)
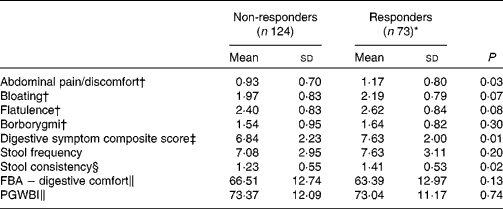
FBA, Food and Benefits Assessment; PGWBI, Psychological General Well-Being Index.
* A responder is defined as a subject reporting an improvement of its GI well-being during at least 2 weeks of the 4 weeks of intervention without worsening at any time.
† Frequency of symptom, score 0 (never) to 4 (every day of the week). Values represent the two weekly assessments during baseline.
‡ Adapted from the Bristol Stool Form Scale: 0 (type 4); 1 (types 3 and 5); 2 (types 2 and 6); 3 (types 1 and 7).
§ Sum of individual symptoms, score 0–16.
∥ Score from 0 to 100 (best).
Exploratory analyses
The distribution of the two modalities (yes/no) was comparable for GI well-being responders and for the decrease in the composite score of digestive symptom responders (Fisher's exact test, P< 0·0001, κ = 0·44). The true positive rate (yes for both parameters) was 65·1 % (fifty-six/eighty-six responders for digestive symptoms) and the true negative rate (no for both parameters) is 79·3 % (eighty-four/106 non-responders for digestive symptoms). The corresponding negative and positive predictive values for GI well-being are 73·7 % (eighty-four/114 non-responders for GI well-being) and 71·8 % (fifty-six/seventy-eight responders for GI well-being), respectively.
The partial least square analysis investigating the contribution of the different symptoms (abdominal pain, bloating, flatulence and borborygmi) and stool frequency and consistency to the improvement of GI well-being shows that the four symptoms are the major contributors. These symptoms accounted for 73·2 %, whereas 26·8 % of the model was explained by stool consistency (21·2 %) and stool frequency (5·6 %). Among the digestive symptoms, flatulence had the higher impact (30·9 %) followed by bloating (16·9 %) and abdominal pain (15·8 %), with borborygmi having the lower impact (9·6 %).
Discussion
The present study provides preliminary construct validity for a single-item PRO as a measure of GI well-being in a non-IBS population, by demonstrating that this measure is reliably associated with a broad range of digestive symptom changes. These data support the use of this PRO as an end point for clinical trials in the general healthy population with mild GI non-painful discomfort, aiming at demonstrating the effect of specific foods on GI (dis)comfort and common GI symptoms.
In the absence of validated biomarkers, it is necessary that the primary outcome measure in studies targeting overall GI well-being or (dis)comfort be based on patient report, i.e. a PRO. The use of a validated questionnaire is required to demonstrate a beneficial physiological effect of a food on GI discomfort for health claim in Europe( 1 ). Important research efforts have been made in the past 10 years to improve PRO used in IBS trials( 15 , Reference Chang and Drossman 19 ), but despite the high interest in making a claim on GI discomfort for foods, there has been little emphasis to develop PRO to evaluate this construct in the general population. Developing an instrument measuring GI comfort or well-being outside the IBS population is challenging due to the absence of pain, and the lower level of GI discomfort and symptoms reported by healthy subjects. However, subjects among the general population frequently experience non-painful digestive symptoms( Reference van Kerkhoven, Eikendal and Laheij 6 – Reference Frexinos, Denis and Allemand 8 ) and the development of food aiming at improving their GI heath status requires appropriate tools.
The results obtained in the present study demonstrate that the simple weekly global rating of change in GI well-being is a good representation of multidimensional change in digestive symptoms and is also significantly, but to a lesser extent, related to changes in bowel function. This is supported by the similar significant correlation values between each of the four digestive symptoms (abdominal pain/discomfort, bloating, borborygmi/rumbling stomach and flatulence) and the number of weeks with improved GI well-being. This single-item PRO seems to be able to capture important signs and symptoms that can have an impact of GI (dis)comfort, and to summarise adequately the mild digestive symptoms commonly reported by the general population. In contrast, such a single-item global assessment may not be suitable for clinical trials in the IBS population, where patients suffer from a greater number and severity of symptoms, including abdominal pain, and treatments are more targeted for specific symptoms( Reference Trentacosti, He and Burke 11 ).
This PRO is an improved construct compared with the binary outcome used in the past in IBS clinical trials that did not include an answer option capturing worsening of condition( Reference Trentacosti, He and Burke 11 , Reference Passos, Lembo and Conboy 20 ). At least for the composite measure of digestive symptoms, a rating of worse GI well-being was clearly associated with a negative change in digestive symptoms. Although valid assessment of worsening symptoms may not be relevant for measures of responder status in all trials, for some it may be important to separate out those with a positive or no change from those with worse outcomes. The present data clearly showed the limitations and weaknesses of the use of a binary response (improved or unchanged) that may mask potential negative effects.
Beyond the statistical significance, the nature and size of biological relevance of an effect is a key criterion when assessing the efficacy of a food/nutritional intervention( 21 ). When using PRO, a responder definition is usually needed to identify participants who achieve a predefined clinically meaningful improvement, and proportions of responders are then compared to determine the efficacy of the tested product. We used a similar definition of responders as that used extensively in IBS drug trials for binary outcome, i.e. improvement during at least 50 % of the time. We show that the responder definition for GI well-being appears to be well correlated with changes in digestive symptoms and other parameters associated with GI health. Subjects classified as responders had a significantly higher decrease in digestive symptoms ( − 38·5 %) when compared with non-responders ( − 9·9 %). This was associated with improved bowel function and with a significantly higher score for a specific digestive comfort dimension of the HRQoL questionnaire. This responder definition has been shown to be responsive to a probiotic intervention in a double-blind, randomised, controlled trial( Reference Guyonnet, Schlumberger and Mhamdi 12 ). An exploratory analysis using a more strict definition of responders (including no worsening at any time point) showed similar differences between responders and non-responders with the same magnitude of symptom decrease ( − 41·8 %) in the responder group.
The present data highlight that changes in GI well-being in this non-IBS population are primarily related to changes in digestive symptoms. The composite score of digestive symptoms showed a higher value of correlation with improvement of GI well-being, and worsening of GI well-being is associated with an increase in digestive symptoms. However, this is not driven by a specific symptom such as abdominal pain/discomfort (as found in IBS), and all the four digestive symptoms measured in the present study contribute in the same way to the improvement of GI well-being. These data confirm the multidimensional nature of this concept.
It has been suggested that the impact of baseline digestive symptom severity is a confounding factor for the effect of treatment outcome in IBS( Reference Passos, Lembo and Conboy 20 , Reference Withehead, Palsson and Levy 22 ). We did not find a significant association between baseline digestive symptom severity and GI well-being improvement in the present study as well as with baseline data of bowel function and HRQoL. Even though responders reported the highest level of abdominal pain/discomfort, composite score of digestive symptoms and stool consistency, no significant correlation was found between all the outcomes and the level of GI well-being improvement.
It is important to point out that some of the findings may reflect specifics of the intervention study from which the data were collected. For example, the fact that digestive symptoms are more related to changes in GI well-being than bowel habit may be a result of greater change in symptoms in the present study v. bowel habit and not inherent in the measure itself. This population has normal bowel habits, and investigations in individuals with altered bowel functions may provide additional information on the factors that could have an impact on this single-item PRO for GI well-being. Data were obtained in a short-term study (4-week intervention) and a longer duration of intervention should allow determining the stability of the measure over a longer period as well as its relationship to changes of other parameters.
This overall assessment may be useful in any clinical trial assessing the effect of a food or nutritional intervention on GI comfort or discomfort, and is complementary to the traditional symptom assessment and bowel function parameters. The choice of the main outcome (overall assessment of GI well-being or digestive symptoms) in a specific clinical trial should be based on the expected effects of the intervention (e.g. what is the main biological/physiological effect of the intervention?). Overall assessment of GI well-being cannot be used alone as the main outcome because such overall improvement should be supported by a concomitant improvement of GI symptoms and/or bowel functions. Taking together, these data could allow us to understand the physiological effect of the intervention and its relevance for a well-defined target population. This overall assessment could be useful as a secondary outcome in trials looking at bowel functions as the main outcome. This may help in determining whether improved bowel functions are associated with GI comfort or discomfort improvement, as the real benefit for a subject for a significant increase of one stool per week could be questionable.
In summary, the overall assessment of GI well-being as used in the present study may be a useful PRO for use in the general non-IBS population which correlates well with changes in digestive symptoms. As such, it appears to be a relevant measure to assess the benefits of food intervention in a healthy population.
Acknowledgements
The present study was funded by Danone Research. The study was performed in one clinical centre (Harrison Clinical Centre, Munich, Germany). D. G., B. N. and O. C. were responsible for the study design and for the interpretation of the study results. P. R. performed the statistical analysis. E. M. was involved in the interpretation of the results. All the authors were involved in the writing and validation of the paper. D. G. and P. R. are employees of Danone Research. B. N., E. M. and O. C. are consultants of Danone Research.












