Licorice root is one of the oldest botanicals in traditional Oriental medicine. Its extract has been utilised worldwide as an additive for flavouring and sweetening of tobacco, candies, chewing gum, toothpaste and beverages. Additionally, it has been demonstrated to perform a variety of pharmaceutical functions, including detoxification, anti-ulcer, anti-inflammatory, anti-viral, anti-atherogenic and anti-carcinogenic activities. Licorice extracts have been shown to evidence anti-carcinogenic activities in both in vivo and in vitro animal models. For example, in a mouse xenograft model, licorice extract has been demonstrated to inhibit the growth of colon tumours(Reference Lee, Park and Lim1). In vitro studies have shown that licorice extract inhibits the proliferation of prostate cancer cells(Reference Adams, Seeram and Hardy2), and induces apoptosis in breast(Reference Jo, Kim and Ra3) and gastric(Reference Ma, Peng and Liang4) cancer cells. These results show that licorice extract has the potential to be developed into a cancer chemopreventive agent.
Licorice contains a considerable quantity of glycyrrhizin, a triterpene compound, which is normally regarded to be its principal biologically active component (reviewed in Wang & Nixon(Reference Wang and Nixon5) and Asl & Hosseinzadeh(Reference Asl and Hosseinzadeh6)). The results of previous in vivo studies have shown that glycyrrhizin inhibits pulmonary metastasis in mice injected with B16 melanoma cells(Reference Kobayashi, Fujita and Katakura7). The results of previously conducted in vitro cell culture studies have demonstrated that glycyrrhizin induces apoptosis in several human cancer cell lines(Reference Hibasami, Iwase and Yoshioka8–Reference Thirugnanam, Xu and Ramaswamy10). However, glycyrrhizin is converted by human intestinal bacteria to glycyrrhetic acid(Reference Hattori, Sakamoto and Kobashi11), which has been reported to induce severe hypertension and hypokalaemia(Reference Stormer, Reistad and Alexander12, Reference van Uum13). Therefore, our research group has attempted to prepare a licorice extract which lacks glycyrrhizin. In order to eliminate glycyrrhizin from the licorice extract, we had previously prepared a hexane–ethanol (9:1, v/v) extract of Glycyrrhiza uralensis (HEGU), which harbours no detectable glycyrrhizin(Reference Choi, Seon and Lim14).
Prostate cancer is one of the most common malignancies in men in the USA(Reference Jemal, Siegel and Ward15), with an increasing incidence worldwide. Morbidity and mortality due to prostate cancer result primarily from metastasis to distant organs. The process of metastasis involves a series of separate steps, which include the separation of tumour cells in the primary tumor, local invasion, intravasation of invading cells into the vasculature or lymphatic systems, extravasations, angiogenesis and proliferation at the new site (reviewed in Steeg(Reference Steeg16)). Matrix-degrading proteases, adhesion molecules and motility factors perform an important function in cancer invasion and metastasis (reviewed in Bogenrieder & Herlyn(Reference Bogenrieder and Herlyn17)). Matrix metalloproteinase (MMP), a zinc-dependent proteinase, is one of the most potent extracellular matrix-degrading enzymes(Reference Yoon, Park and Yun18). MMP-2 (72 kDa gelatinase A) and MMP-9 (92 kDa gelatinase B) appear to be involved in the hydrolysis of basal membrane Type IV collagen, and have also been frequently associated with the invasive metastatic potential of tumour cells, including prostate cancer(Reference Zhang, Shi and Feng19). In addition to MMP, urokinase-type plasminogen activator (uPA) is a serine protease which is involved in tissue remodelling and cell migration(Reference Crippa20). Cellular adhesion molecules are critical participants in cell–cell interactions, and interactions between cells and components of the extracellular matrix(Reference Orian-Rousseau and Ponta21). These molecules have been implicated in a broad variety of cellular functions, including apoptosis and metastasis. Integrins, another important group of cellular adhesion molecules, are involved in the process of local stromal invasion, as well as in invasion at the metastatic site(Reference Okegawa, Pong and Li22).
The principal objective of the present study was to characterise the effects of HEGU lacking glycyrrhizin on the metastatic capacity of androgen-insensitive DU145 prostate cancer cells, and to identify the active component of HEGU. We demonstrated that HEGU inhibits the migration, invasion and adhesion of DU145 cells as well as MMP activities and the expression of adhesion molecules. Additionally, we identified licoricidin as the active component of HEGU.
Experimental methods
Materials
Reagents were purchased from the following suppliers: horseradish peroxidase-conjugated anti-rabbit and anti-mouse IgG from Amersham (Arlington Heights, IL, USA); antibodies against MMP-9, integrin-α2, tissue inhibitor of metalloproteinase-1 (TIMP-1), TIMP-2, intercellular adhesion molecule (ICAM), vascular cell adhesion molecule (VCAM) and vascular endothelial growth factor (VEGF) from Santa Cruz Biotechnology (Santa Cruz, CA, USA); anti-uPA antibody from Calbiochem (Darmstadt, Germany); epidermal growth factor (EGF) from R&D Systems (Minneapolis, MN, USA); Adhesion Assay kit from Chemicon International (Temecula, CA, USA); and Centricon Plus-20 from Millipore Corporation (Billerica, MA, USA). Unless otherwise specified, all other materials were purchased from Sigma (St Louis, MO, USA).
Preparation and fractionation of hexane–ethanol extract of Glycyrrhiza uralensis, and identification of an active component (licoricidin)
Dried G. uralensis Fischer (Leguminosae) was purchased from Dae Kwang Company (Chuncheon, South Korea). A voucher specimen is deposited in the Department of Food Science and Nutrition, Hallym University. HEGU was prepared as described previously(Reference Choi, Seon and Lim14). HEGU (88·0 g) was subjected to flash column chromatography over silica gel and eluted with a gradient system of n-hexane–ethyl acetate (10:0–5:5, v/v) to yield 125 fractions that were pooled on the basis of their TLC patterns. Analytical silica gel (Kieselgel 60 F254, 0·25 mm; Merck, Darmstadt, Germany) plates were utilised for the determination of TLC patterns. The ability of the pooled fractions to inhibit DU145 cell migration was assessed, and the most active G89 (fractions 86–89, 2·0 g) was purified via column chromatography over a Cosmosil C18 eluting with a gradient of methanol–H2O (20:80–10:90) to yield twenty subfractions. The ability of these twenty subfractions to inhibit DU145 cell migration was assessed, and the most efficacious fraction, F7 (5·4 mg), was purified further via recrystallisation. The compound in F7 was structurally identified via 1H NMR and 13C NMR. 1H NMR and 13C NMR spectra were recorded on a Bruker DPX 400 (400 MHz) spectrometer using tetramethylsilane as an internal standard.
Quantitative analysis of licoricidin in hexane–ethanol extract of Glycyrrhiza uralensis
Licoricidin contents in the HEGU were estimated via HPLC using a Thermo HPLC with a P2000 Gradient Pump (Thermo Separation Products, San Jose, CA, USA), an AS3000-021 thermostated autosampler, a SCM 1000-022 degasser and an Agilent Zorbax SB-C18 reversed-phase column (150 mm × 4·6 mm, inner diameter 5 μm) coupled with an Agilent Zorbax Extend C18 guard column (10 mm × 4·6 mm inner diameter 5 μm). The mobile phase consisted of 0·01 % trifluoroacetic acid in water (A) and methanol (B). The gradient program was as follows: 60 % B in 0–10 min, 60–100 % B in 10–30 min at a flow rate of 0·8 ml/min using a Thermo 6000 LP detector at a wavelength of 254 nm. The standard was dissolved in methanol, and the standard curve was plotted using concentrations of 0·01–1 mg/ml, which is almost linear (R 2 0·99). The concentration of licoricidin was calculated via comparison of the peak area of the sample with that of the standard.
Cell culture
DU145 human prostate cancer cells (the American Type Culture Collection, Manassas, VA, USA) were grown in Dulbecco's modified Eagle's medium (DMEM)/F12 containing 100 ml/l of fetal bovine serum (FBS), with 62·5 mg/l of penicillin and 100 mg/l of streptomycin (Gibco BRL, Gaithersburg, MD, USA).
Migration, invasion and adhesion assays
For the transwell migration assay, DU145 cells were serum deprived in DMEM/F12 supplemented for 24 h with 1 % charcoal-stripped FBS. Transwell filters (8 μm pore size) were precoated with 10 μg of Type IV collagen. The lower chambers of the wells of twenty-four-well plates were filled with DMEM/F12 containing 1 % charcoal-stripped FBS and 0·1 % bovine serum albumin in the absence or presence of 10 ng/ml EGF. The cells were then plated onto the filter in the transwell inserts at 2·5 × 104 cells/filter, and treated with the indicated concentrations of HEGU, fractions and licoricidin. The cells were then incubated for 4 h. After incubation, the migrated cells were stained with haematoxylin and eosin. For the invasion assay, procedures same as those described for the migration assay were conducted, except that the cells were plated on a matrigel-coated transwell filter (BD Biosciences, San Jose, CA, USA).
For the adhesion assay, DU145 cells were plated in human collagen Type I-coated CytoMatrix Cell Adhesion Strips. The cells were incubated for 45 min in DMEM/F12 containing 1 % charcoal-stripped FBS with various concentrations of HEGU with or without 10 ng/ml of EGF. The strips were rinsed twice with Ca2+/Mg2+-containing PBS, and stained for 5 min with 0·2 % crystal violet in 10 % ethanol. After staining, the cell-bound stains were quantified by determining the absorbance at 570 nm.
Gelatin zymography
Cells were serum starved in DMEM/F12 and treated with 10 ng/ml EGF for 18 h in the absence or presence of HEGU. Conditioned media were collected, and the proteins were concentrated via centrifugal ultrafiltration using a 10 000 molecular weight (M r) cut-off Centricon Plus-20 filter. Proteins in the conditioned media were separated via SDS-PAGE with 7·5 % acrylamide gel containing 1 % gelatin. The volumes of media loaded onto the gel were adjusted for equivalent protein levels. After electrophoresis, the gels were washed twice in 2·5 % Triton X-100 for 30 min to completely eliminate SDS. The gels were then rinsed twice with zymogen activation buffer (50 mm-Tris–HCl, 0·02 % Brij-35, 5 mm-CaCl2 and 0·2 mm-NaCl), and were incubated for 48 h at 37°C in the same buffer. After incubation, the gels were stained for 2 h with 0·25 % Coomassie Blue solution and destained. Conditioned medium (40 μl) from the human fibrosarcoma cell line HT1080, which secretes MMP-9(Reference Moll, Youngleib and Rosinski23) and MMP-2(Reference Azzam and Thompson24), was used as a control.
Western blot analyses
Total cell lysates were prepared as described previously(Reference Cho, Kim and Kim25), and the protein contents were determined using a bicinchoninic acid protein assay kit (Pierce, Rockford, IL, USA). The total cell lysates (50 μg protein) and concentrated conditioned media (50 or 80 μg protein) were utilised for the Western blot analyses under reducing conditions as described previously(Reference Cho, Kim and Kim25). The relative abundance of each band was quantified using the Bio-profile Bio-1D application (Vilber-Lourmat, Marine La Vallee, France).
Statistical analysis
The data were expressed as the means with their standard errors and analysed via ANOVA. Differences among the treatment groups were evaluated by Duncan's multiple-range test, using the SAS system for Windows version 8.1 (SAS Institute, Cary, NC, USA).
Results
Hexane–ethanol extract of Glycyrrhiza uralensis inhibits the migration and invasion of DU145 cells
We have demonstrated previously that EGF stimulates the migration, invasion and adhesion of DU145 cells(Reference Kwon, Cho and Chung26). In the present study, we cultured the cells in the absence or presence of EGF to determine whether HEGU inhibits the cell migration and invasion of DU145 cells. As anticipated, EGF significantly enhanced the migration of DU145 cells. In the absence of EGF, 7·5 μg/ml of HEGU drastically inhibited the migration of DU145 cells. In the presence of EGF, 2·5–7·5 μg/ml of HEGU inhibited the migration of DU145 cells in a dose-dependent manner (Fig. 1). The transwell invasion assay was also conducted using matrigel-coated filters, which demonstrated that HEGU markedly inhibited the basal and EGF-stimulated invasion of DU145 cells (Fig. 2). We reported previously that HEGU moderately inhibited DU145 cell growth when the cells were treated for 48 h with 5 μg/ml of HEGU(Reference Choi, Seon and Lim14). However, 2·5–7·5 μg/ml of HEGU did not influence cell viability when the cells were treated for 24 h with HEGU (Mira Son & JHY Park, unpublished results), and therefore we utilised 2·5–7·5 μg/ml of HEGU in the experiments conducted in the present study.

Fig. 1 Effect of hexane–ethanol extract of Glycyrrhiza uralensis (HEGU) on the epidermal growth factor (EGF)-induced migration of DU145 cells. DU145 cells were serum deprived for 24 h in Dulbecco's modified Eagle's medium (DMEM)/F12 containing 1 % charcoal-stripped fetal bovine serum (FBS). The lower side of a 6·5 mm transwell filter was precoated with Type IV collagen. Cells were plated into the upper compartment at 25 000 cells/well, and were treated with 0–7·5 μg/ml of HEGU. The lower compartment was then filled with DMEM/F12 with 1 % charcoal-stripped FBS and 0·1 % bovine serum albumin with or without EGF. The cells were incubated for 4 h, and the migrated cells were stained with haematoxylin and eosin (H&E). (A) Photographs of H&E-stained cells (100 × ). (B) Quantitative analysis of the migrated cells. Each bar represents the means with their standard errors (n 3). a,b,c,d,e Mean values with unlike letters were significantly different (P < 0·05).

Fig. 2 Effect of hexane–ethanol extract of Glycyrrhiza uralensis (HEGU) on epidermal growth factor (EGF)-induced invasion of DU145 cells. DU145 cells were serum deprived for 24 h in Dulbecco's modified Eagle's medium (DMEM)/F12 containing 1 % charcoal-stripped fetal bovine serum (FBS). The cells were plated in 6·5 mm matrigel-coated transwells at 25 000 cells/well, and were treated with 0–7·5 μg/ml of HEGU. The lower compartment was filled with DMEM/F12 with 1 % charcoal-stripped FBS and 0·1 % bovine serum albumin with or without EGF. The cells were incubated for 14 h, and the invaded cells were stained with haematoxylin and eosin (H&E). (A) Photographs of the H&E-stained cells (100 × ). (B) Quantitative analysis of the invaded cells. Each bar represents the means with their standard errors (n 3). a,b,c,d,e,f Mean values with unlike letters were significantly different (P < 0·05).
Hexane–ethanol extract of Glycyrrhiza uralensis alters the secretion of matrix metalloproteinase, tissue inhibitor of metalloproteinase and urokinase-type plasminogen activator by DU145 cells
In order to evaluate the possible anti-metastatic mechanisms of HEGU, we evaluated the effects of HEGU on the secretion of MMP using gelatin zymography. EGF stimulated the activity of pro-MMP-9, and HEGU inhibited the activity of both pro-MMP-9 and active MMP-9, whether the cells were treated with EGF or not (Fig. 3(A)). Additionally, the activity of pro-MMP-2 (72 kDa) was increased by EGF treatment and reduced by HEGU, whether the cells were treated with EGF or not. We detected another band with an apparent M r of 43 000, and this band was increased by EGF treatment and reduced by HEGU treatment in a dose-dependent fashion (Fig. 3(A)). Because it has been reported previously that the MMP-2 proenzyme is converted to a 42·5 kDa stable active enzyme(Reference Howard, Bullen and Banda27), we tentatively concluded that the 43 000 M r species is a product of MMP-2 activation. Western blot analysis demonstrated that the secretion of pro-MMP-9 was significantly increased by EGF treatment, and that HEGU treatment effected a reduction in the protein levels of MMP-9 under both basal and EGF-stimulated conditions (Fig. 3(B)).

Fig. 3 Effect of hexane–ethanol extract of Glycyrrhiza uralensis (HEGU) on epidermal growth factor (EGF)-induced secretion of matrix metalloproteinase (MMP), tissue inhibitor of metalloproteinase (TIMP), urokinase-type plasminogen activator (uPA) and vascular endothelial growth factor (VEGF) by DU145 cells. DU145 cells were plated in 100 mm dishes at 2 × 106 cells/dish in Dulbecco's modified Eagle's medium (DMEM)/F12 supplemented with 10 % fetal bovine serum. One day later, the monolayers were serum starved for 24 h with serum-free DMEM/F12. The cells were incubated for 18 h with 0–7·5 μg/ml of HEGU in serum-free media with or without EGF. The media were collected and concentrated for gelatin zymography (A) and Western blotting (B) after 18 h of conditioning. The volumes of media loaded onto the gel were adjusted for equivalent proteins. Photographs of a Coomassie Blue-stained gel (A) and chemiluminescent detection of the blots (B), which are representative of three independent experiments, are shown. In the first lane of (A), serum-free HT1080 cell-conditioned medium was loaded. The relative abundance of each band (B) was estimated via densitometric scanning of the exposed films. The control levels were set to one. The adjusted means with their standard errors (n 3) of each band are shown above each blot. a,b,c,d Mean values with unlike letters were significantly different (P < 0·05).
Because HEGU inhibited the expression and activation of MMP, we subsequently examined the effects of HEGU on the secretion of TIMP, natural inhibitors of MMP(Reference Zhang, Shi and Feng19). The results of Western blot analysis showed that EGF stimulated the secretion of TIMP-1, and that HEGU inhibited the secretion of this protein in the absence or presence of EGF (Fig. 3(B)). However, the secretion of TIMP-2 was not affected by EGF treatment, but was significantly increased as the result of treatment with 7·5 μg/ml of HEGU (Fig. 3(B)).
The uPA system is believed to perform a pivotal function in tissue degradation, cell migration, angiogenesis, and cancer invasion and metastasis(Reference Li and Cozzi28). Thus, we quantified uPA secretion levels via Western blotting. EGF increased the secretion of uPA (53 000 M r pro-form and 33 000 M r active form), and HEGU inhibited the secretion of these proteins. In addition to the pro- and active forms of uPA, we detected another band with a slightly higher M r than that of pro-uPA only in the HEGU-treated cells (Fig. 3(B)). Western blot analysis with a VEGF antibody demonstrated that VEGF secretion was increased as the result of EGF treatment, and also that HEGU inhibited the secretion of VEGF under both basal and EGF-stimulated conditions (Fig. 3(B)).
Hexane–ethanol extract of Glycyrrhiza uralensis inhibits the adhesion of DU145 cells
In order to evaluate the effects of EGF/HEGU on the adhesion of DU145 cells, we used human collagen Type I-coated strips. EGF stimulated DU145 cell adhesion to Type I collagen, and HEGU inhibited basal and EGF-stimulated cell adhesion (Fig. 4(A)). The expression of a variety of adhesion molecules was detected via Western blotting. When the cells were treated with EGF, the levels of integrin-α2 protein were increased by 170 %, an increase which was significantly inhibited by treatment with 5 μg/ml of HEGU. The levels of ICAM and VCAM were not affected significantly by EGF. However, they were lower in cells treated with 7·5 μg/ml of HEGU than in cells treated with EGF only (Fig. 4(B)).

Fig. 4 Effect of hexane–ethanol extract of Glycyrrhiza uralensis (HEGU) on epidermal growth factor (EGF)-induced cell adhesion in DU145 cells. (A) DU145 cells were serum deprived for 24 h in Dulbecco's modified Eagle's medium (DMEM)/F12 containing 1 % charcoal-stripped fetal bovine serum (FBS). Cells were plated in CytoMatrix™ human collagen I cell adhesion strips. Cells were incubated for 45 min in DMEM/F12 containing 1 % charcoal-stripped FBS medium with 0–7·5 μg/ml HEGU with or without EGF. Cells were stained with 0·2 % crystal violet, and the cell-bound stains were colorimetrically quantified. Each bar represents the means with their standard errors (n 4). (B) DU145 cells were plated, serum starved and treated with HEGU as described in Fig. 3. Total cell lysates were subjected to immunoblotting with antibodies raised against integrin-α2, intercellular adhesion molecule (ICAM) or vascular cell adhesion molecule (VCAM). Photographs of chemiluminescent detection of the blots, which are representative of three independent experiments, are shown. The relative abundance of each band was estimated via densitometric scanning of the exposed films, and the expression levels were normalised to β-actin. The adjusted means with their standard errors (n 3) of each band are shown above each blot. a,b,c,d,e,f Mean values with unlike letters were significantly different (P < 0·05).
Licoricidin is an active component of hexane–ethanol extract of Glycyrrhiza uralensis
For the identification of active component(s), HEGU (88·0 g) was fractionated with a gradient system of n-hexane–ethyl acetate on flash column chromatography, and the resulting 125 fractions were pooled into twenty-nine groups on the basis of their TLC patterns. The ability of each group to inhibit DU145 cell migration was determined. Among the twenty-nine groups, G89 most potently inhibited DU145 cell migration (Fig. 5(A)). G89 (2·0 g) was fractionated further by column chromatography over a Cosmosil C18 eluted with a gradient of methanol–H2O to yield twenty subfractions. Among these twenty subfractions, the F7 subfraction (5·4 mg) evidenced the highest inhibitory effect on DU145 cell migration (Fig. 5(B)). After further purification, the structure of the resultant pure compound in F7 was identified as licoricidin (Fig. 5(C)) via comparisons of its 1H, 13C NMR and MS spectra with those reported previously in the literature(Reference Fukai, Toyono and Nomura29). The licoricidin content of the HEGU was 0·35 %.
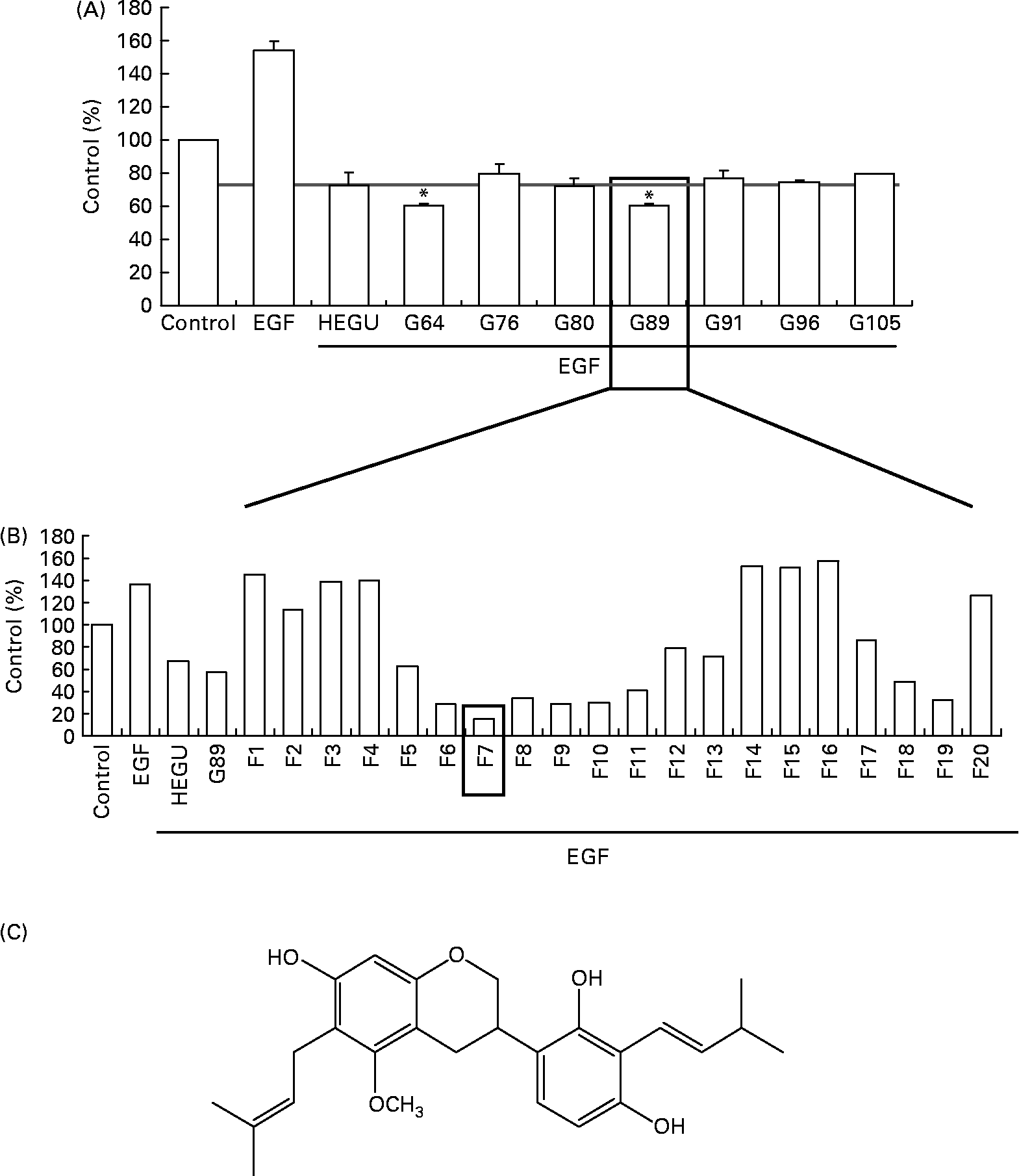
Fig. 5 Purification and characterisation of the active compound licoricidin. DU145 cells were plated, serum starved as described in Fig. 1 and treated with 5 μg/ml of each fraction of hexane–ethanol extract of Glycyrrhiza uralensis (HEGU). The lower compartment of the matrigel-coated transwells was filled with Dulbecco's modified Eagle's medium/F12 with 1 % charcoal-stripped fetal bovine serum and 0·1 % bovine serum albumin with or without epidermal growth factor. The cells were incubated for 4 h, and the migrated cells were stained with haematoxylin and eosin. Quantitative analyses of the migrated cells are shown. (A) n-Hexane–ethyl acetate fractions of HEGU were pooled into twenty-nine groups on the basis of their TLC patterns. Groups (pooled fractions) exhibiting sufficient activity are shown: 64 (fractions 61–65), 76 (fractions 75–78), 80 (fractions 79–82), 89 (fractions 86–89), 91 (fractions 90–93), 96 (fractions 94–96) and 105 (fractions 102–105). Each bar represents the means with their standard errors (n 3). * Mean values were significantly different from HEGU (P < 0·05). (B) Methanol–H2O fractions of G89. (C) Chemical structure of licoricidin. 1H NMR (400 MHz, CD3OD): δ 1·66, 1·67, 1·76, 1·77 (each 3H, s, CH3), 2·72 (1H, dd, 16·0 and 11·0 Hz, H-4ax), 2·90 (1H, ddd, 16·0, 5·1, and 1·8 Hz, H-4eq), 3·23 (2H, t, J = 7·2 Hz, H-9), 3·36 (2H, brd, J = 7·0 Hz, H-7′), 3·32 (1H, m, H-3), 3·67 (3H, s, OCH3), 3·89 (1H, t, J = 10·2 Hz, H-2ax), 4·18 (2H, ddd, J = 2·0, 3·1, and 10·2 Hz, H-2eq), 5·19 (2H, m, H-10 and H-8′), 6·09 (1H, s, H-8), 6·35 (1H, d, J = 8·4 Hz, H-5′), 6·75 (1H, d, J = 8·4 Hz, H-6′). 13C NMR (100 MHz, CD3OD): 158·47 (C-5), 155·90 (C-7), 155·71 (C-4′), 154·78 (C-8a), 154·31 (C-2′), 132·07 (C-9′), 130·84 (C-11), 125·61 (C-8′), 125·27 (C-10), 124·33 (C6′), 121·62 (C-1′), 117·60 (C-3′), 115·12 (C-4a), 108·72 (C-5′), 108·61 (C-6), 100·05 (C-8), 71·26 (C-2), 60·95 (OCH3), 32·79 (C-3), 27·43 (C-4), 26·01 (C-11′), 25·96 (C-13), 23·63 (C-7′, 9), 18·02 (C-10′), 17·97 (C-12).
Licoricidin inhibits the migration of DU145 cells
When DU145 cells were treated with 0–5 μg/ml of licoricidin in the absence or presence of EGF, licoricidin inhibited the migration of DU145 cells in a dose-dependent fashion, whether the cells were treated with EGF or not (Fig. 6). However, 1–5 μg/ml of licoricidin did not affect cell viability when the cells were treated for 24 h with licoricidin (data not shown).

Fig. 6 Effect of licoricidin on epidermal growth factor (EGF)-induced migration of DU145 cells. DU145 cells were plated, serum starved as described in Fig. 1 and treated with 0–5 μg/ml of licoricidin. The lower compartment of the matrigel-coated transwells was filled with Dulbecco's modified Eagle's medium/F12 with 1 % charcoal-stripped fetal bovine serum and 0·1 % bovine serum albumin with or without EGF. The cells were incubated for 4 h, and the migrated cells were stained with haematoxylin and eosin (H&E). (A) Photographs of H&E-stained cells (100 × ). (B) Quantitative analysis of the migrated cells. Each bar represents the means with their standard errors (n 3). a,b,c,d,e Mean values with unlike letters were significantly different (P < 0·05).
Licoricidin alters the expression of proteins involved in the regulation of metastasis in DU145 cells
As HEGU inhibited the secretion of MMP-9, TIMP-1, uPA and VEGF, we evaluated the effects of licoricidin on the secretion of these proteins. Western blot analysis showed that the secretion of MMP-9, TIMP-1, uPA and VEGF was reduced, and that of TIMP-2 was significantly increased in the licoricidin-treated cells (Fig. 7(A)). Similar to the HEGU-treated cells (Fig. 3(B)), a band with a M r slightly higher than that of pro-uPA was detected in the cells treated with 5 μg/ml licoricidin, whether the cells were treated with EGF or not (Fig. 7(A)).

Fig. 7 Effect of licoricidin on proteins involved in the regulation of metastasis in DU145 cells. DU145 cells were plated and serum starved as described in Fig. 3. The cells were incubated with 0–5 μg/ml of licoricidin in serum-free media with or without epidermal growth factor for 18 h. (A) The 18 h conditioned media were collected and concentrated for Western blotting. The volumes of media loaded onto the gel were adjusted for equivalent proteins. The relative abundance of each band was estimated via the densitometric scanning of the exposed films, and the adjusted means with their standard errors (n 3) of each band are shown above each blot. (B) Total cell lysates were subjected to immunoblotting with antibodies raised against integrin-α2, intercellular adhesion molecule (ICAM) or vascular cell adhesion molecule (VCAM). Photographs of chemiluminescent detection of the blots, which are representative of three independent experiments, are shown. The relative abundance of each band was estimated via densitometric scanning of the exposed films, and the expression levels were normalised to β-actin. The adjusted means with their standard errors (n 3) of each band are shown above each blot. a,b,c,d Mean values with unlike letters were significantly different (P < 0·05).
The expression of the adhesion molecules integrin-α2 and ICAM was reduced in a dose-dependent manner in licoricidin-treated cells. The levels of VCAM were significantly reduced in cells treated with 5 μg/ml of licoricidin (Fig. 7(B)).
Discussion
In a previous study, we demonstrated that HEGU, which harbours no glycyrrhizin, suppressed doxorubicin-induced apoptosis in H9c2 rat cardiac myoblasts and induced reductions in the viable cell numbers of a variety of cancer cell types(Reference Choi, Seon and Lim14). We have also observed previously that HEGU exerts potent anti-inflammatory effects in murine macrophages and skin(Reference Cho, Lim and Lee30). As we determined previously that EGF augments the migration, invasion and adhesion of androgen-independent DU145 prostate cancer cells(Reference Kwon, Cho and Chung26), in the present study, an attempt was made to evaluate the effects of HEGU on the basal and EGF-stimulated metastatic potential of DU145 cells. We observed that HEGU at concentrations of 2·5–7·5 μg/ml(Reference Lee, Park and Lim1) markedly inhibits cell migration, invasion and adhesion(Reference Adams, Seeram and Hardy2); reduces the secretion of MMP-2, MMP-9, TIMP-1, uPA and VEGF(Reference Jo, Kim and Ra3); stimulates the secretion of TIMP-2; and(Reference Ma, Peng and Liang4) reduces the expression levels of integrin-α2, ICAM and VCAM proteins. These findings indicate that HEGU may be developed into an agent for the prevention of cancer metastasis.
The constituents of licorice include triterpene saponins, flavonoids, isoflavonoids and chalcones, with glycyrrhizin generally being considered the primary biologically active component (reviewed in Wang & Nixon(Reference Wang and Nixon5) and Asl & Hosseinzadeh(Reference Asl and Hosseinzadeh6)). It has also been demonstrated that glycyrrhetinic acid exerts an inhibitory effect on the proliferation of LNCaP androgen-dependent prostate cancer cells(Reference Hawthorne and Gallagher31). Other components that are present in licorice extracts have also been demonstrated to exert anti-carcinogenic activities. For example, licochalcone A has been shown to inhibit inflammatory ear oedema formation and tumour promotion in mice(Reference Shibata, Inoue and Iwata32). Isoliquiritigenin inhibited pulmonary metastasis(Reference Yamazaki, Morita and Endo33), the migration and invasion of prostate cancer cells(Reference Kwon, Cho and Chung26), the pulmonary metastasis of mouse renal cell carcinoma(Reference Yamazaki, Morita and Endo33), and 7,12-dimethylbenz(a)anthracene/12-O-tetradecanoylphorbol-13-acetate-induced cutaneous oxidative stress and tumour promotion(Reference Agarwal, Iqbal and Athar34). Because glycyrrhizin concentration in dried licorice root ranges between 3·63 and 13·06 g/100 g(Reference Wang and Nixon5), the chronic consumption of licorice in candies and beverages is not recommended. However, licorice contains many bioactive components other than glycyrrhizin, and the identification and characterisation of such compounds may facilitate the development of effective new chemopreventive and/or chemotherapeutic agent(s).
In the present study, we demonstrated that licoricidin is an active component of HEGU, which is responsible, at least in part, for the inhibition of the metastatic potential of prostate cancer cells. To the best of our knowledge, the biological effects of licoricidin have not been abundantly reported, except that licoricidin exerts anti-bacterial effects against Staphylococcus aureus and Helicobacter pylori (Reference Fukai, Marumo and Kaitou35, Reference Hatano, Kusuda and Inada36). This is the first study in which the anti-metastatic effect of licoricidin has been detected and described. However, the absorption and metabolism characteristics of licoricidin from HEGU were not determined in the present study. In order to determine whether the licoricidin concentrations used in the cell culture studies are relevant in an in vivo context, more studies will be required to determine the concentrations of licoricidin in the blood of animals and human subjects after the administration of licoricidin or HEGU.
In the present study, HEGU was demonstrated to markedly inhibit not only the secretion of MMP-2 and MMP-9, but also the activation of these enzymes (Fig. 3). TIMP are natural inhibitors of the MMP found in the majority of tissues and body fluids. By inhibiting the activities of MMP, they participate in the remodelling of ECM tissue(Reference Lambert, Dasse and Haye37). Although the overexpression of TIMP-1 and TIMP-2 has been demonstrated to inhibit tumour growth, invasion and metastasis, conflicting results have also been reported(Reference Jiang, Goldberg and Shi38). In our previous study, we observed that EGF increased TIMP-1 secretion without any detectable changes in TIMP-2(Reference Kwon, Cho and Chung26), a finding which is consistent with the findings of the present study. Herein, we observed that HEGU and licoricidin inhibited both basal and EGF-stimulated TIMP-1 secretion and increased TIMP-2 secretion (Figs. 3 and 7). Consistent with our findings, it has also been reported in previous studies that external stimuli including growth factors (basic fibroblast growth factor, platelet-derived growth factor and EGF), phorbol esters, serum and cytokines (IL-6, IL-1 and IL-1β) induce TIMP-1 expression in a variety of cell types, including FTC-133 thyroid carcinoma cells, whereas TIMP-2 is constitutive(Reference Lambert, Dasse and Haye37, Reference Soula-Rothhut, Coissard and Sartelet39). Furthermore, high serum TIMP-1 levels have recently been associated with adverse prognoses in endometrial carcinoma(Reference Honkavuori, Talvensaari-Mattila and Puistola40). Collectively, these findings are reflective of a more complex role for TIMP-1 during tumourigenesis than in the regulation of MMP activity. The observed increase in the secretion of TIMP-2 may have contributed to reduced MMP activation in HEGU-treated cells.
The uPA/uPA receptor system is profoundly associated with prostate cancer metastasis(Reference Li and Cozzi28). In the present study, EGF increased the protein levels of both 53 000 M r pro-uPA and 33 000 M r active uPA, and HEGU inhibited both the basal and EGF-stimulated secretion of these proteins. Additionally, we detected another band that migrated at a slightly slower rate than the 53 000 M r band, and this band was induced by HEGU/licoricidin treatment (Fig. 3(B)). uPA is secreted as a 411-amino acid inactive proenzyme (pro-uPA), which has an apparent M r of 53 kDa and undergoes several post-translational modifications. The zymogen is cleaved by plasmin or other proteases at Lys158–Ile159 to produce the active two-chain form held together by a single disulphide bond(Reference Crippa20). It has also been reported in previous studies that the primary translation product is the 431-residue pre-pro-uPA which, via the removal of a twenty-residue-long signal peptide, gives rise to the secreted pro-uPA(Reference Blasi and Verde41). Thus, we tentatively concluded that the slightly higher M r band observed in HEGU or licoricidin-treated cells may be attributable to pre-pro-uPA. HEGU may have prevented the removal of the signal peptide, and thus resulted in the accumulation of the higher M r band. These results demonstrate that reduced uPA secretion contributes to reductions in the invasion and migration of HEGU-treated DU145 cells.
The formation of a new tumour vasculature is a critical process supporting tumour growth(Reference Folkman42). VEGF promotes angiogenesis via the activation of a group of signalling pathways that stimulate the growth, migration, and survival of endothelial cells, vasculature permeability and the mobilisation of endothelial precursor cells from the bone marrow into the general circulation(Reference Hicklin and Ellis43). In the present study, HEGU and licoricidin significantly inhibited basal and EGF-induced VEGF production in DU145 cells (Figs. 3(B) and 7(A)). Because VEGF plays a primary role in both tumour progression and angiogenesis(Reference Goh, Sze and Roufogalis44), the HEGU-induced inhibition of VEGF production may constitute a promising strategy for the treatment of prostate cancer.
In addition to the inhibition of migration and invasion, we observed that HEGU inhibited the adhesion of DU145 cells and reduced the levels of the adhesion molecules, integrin-α2, ICAM and VCAM. Among the many changes inherent to the tumour progression process, alterations in cell–cell and cell–matrix adhesions appear to have a crucial role in the promotion of tumour cell invasion, migration and metastatic propagation(Reference Christofori45). Our results show that HEGU inhibits cell adhesion, which is associated with reductions in the expression of integrin-α2, ICAM and VCAM.
In conclusion, in the present study, we have demonstrated that HEGU lacking glycyrrhizin inhibits the migration, invasion and adhesion of DU145 cells, and that this can be associated with reductions in the levels of MMP-9, MMP-2, uPA and VEGF secretion, as well as in the expression levels of adhesion molecules, and increases in the levels of TIMP-2 secretion. We also showed that licoricidin is an active component that is present in HEGU. Because metastasis is the primary cause of cancer deaths and the incidence of hypertension increases as men age, the HEGU-mediated inhibition of prostate cancer cell metastasis may have important preventive and therapeutic benefits. Future in vivo efficacy studies are warranted in light of these findings.
Acknowledgements
This work was supported by a grant (code no. 20070301034039) from the BioGreen 21 Program, Rural Development Administration, and by the grant of the Korean Ministry of Education, Science and Technology (The Regional Core Research Program/Medical and Bio-Materials Research Center). The authors declare that they have no conflict of interest. The authors' contributions are as follows: S. Y. P., I.-J. K., J.-S. K., C. L., J. K. and J. H. Y. P. designed the experiments. S. Y. P. conducted the biochemical analyses and analysed data. S. S. L. and J. K. K. prepared fractions of HEGU and identified the active component. S. Y. P. wrote the first draft, and J. H. Y. P. revised the paper. All the authors read and approved the final manuscript.











