White adipose tissue – a major secretory organ
Our understanding of the physiological role of white adipose tissue (WAT) has changed radically over the past 15 years. What was viewed primarily as a vehicle for the highly efficient storage of fuel in the form of TAG has emerged as a major endocrine and secretory organ(Reference Trayhurn and Beattie1–Reference Rosen and Spiegelman5). Indeed, for many individuals, and certainly the obese, adipose tissue is the largest endocrine organ. This is because white adipocytes, the predominant cell type within the tissue, release a multiplicity of factors in addition to fatty acids – which are quantitatively much the largest secretory product. The ‘secretome’ of the adipocyte also encompasses other lipid moieties, including cholesterol and retinol (neither of which is synthesised de novo by adipocytes), and steroid hormones and prostaglandins.
The paradigm shift in our understanding of the physiological role of WAT came in 1994 with the discovery of the protein hormone leptin, the product of the Lep (or Ob) gene(Reference Zhang, Proenca, Maffei, Barone, Leopold and Friedman6). This resulted in the immediate recognition of WAT as a major endocrine organ. Leptin was not, however, the first protein signal to be identified as being secreted from fat cells. The complement-related factor adipsin had been shown in the mid-1980s to be released from adipocytes(Reference Cook, Min, Johnson, Chaplinsky, Flier, Hunt and Spiegelman7, Reference Flier, Cook, Usher and Spiegelman8), while several years later the synthesis and secretion of the pro-inflammatory cytokine, TNF-α, was demonstrated(Reference Hotamisligil, Shargill and Spiegelman9).
Following the discovery of leptin, which was originally shown to be a satiety factor but is in practice a pleiotropic hormone, a rapidly expanding range of protein signals and factors termed adipokines (originally referred to as adipocytokines) have been found to be released from white adipocytes(Reference Trayhurn and Beattie1–Reference Rosen and Spiegelman5). A number of these adipokines are linked to immunity and the inflammatory response, and their production is generally increased in obesity such that the tissue is in a state of inflammation(Reference Trayhurn and Wood4, Reference Hotamisligil10, Reference Wellen and Hotamisligil11). A key exception is adiponectin, with its anti-inflammatory and insulin-sensitising actions, the production and release of which fall in the obese(Reference Arita, Kihara and Ouchi12). Obesity itself is characterised by a state of chronic mild inflammation, the circulating levels of several inflammatory markers (such as C-reactive protein, haptoglobin, IL-6, monocyte chemoattractant protein-1, plasminogen activator inhibitor-1 (PAI-1), TNF-α) being increased(Reference Hotamisligil10, Reference Wellen and Hotamisligil11). This state of inflammation, particularly in adipose tissue through changes in the release of inflammation-related adipokines and the infiltration of macrophages(Reference Xu, Barnes and Yang13, Reference Weisberg, McCann, Desai, Rosenbaum, Leibel and Ferrante14), is viewed as central to the development of the major obesity-associated diseases – the metabolic syndrome, type 2 diabetes, CVD and certain cancers (such as breast and colon)(Reference Rosen and Spiegelman5, Reference Hotamisligil10, Reference Yudkin15).
Limited attention has been paid, however, as to why adipose tissue should mount an inflammatory response in obesity. The propositions include oxidative stress and endoplasmic reticulum stress(Reference Ozcan, Cao, Yilmaz, Lee, Iwakoshi, Ozdelen, Tuncman, Görgün, Glimcher and Hotamisligil16–Reference Houstis, Rosen and Lander18). An alternative proposal, which we put forward in the British Journal of Nutrition in 2004, is that it is a response to hypoxia(Reference Trayhurn and Wood4). In the present article, we expand on the hypoxia concept and review the developments that have occurred in the past 2–3 years on the relationship between low O2 tension and adipose tissue function. It is emphasised that the different proposals for the basis for the inflammatory response in adipose tissue in obesity are not necessarily mutually exclusive; for example, hypoxia has been reported to induce endoplasmic reticulum stress and the generation of reactive oxygen species(Reference Koumenis, Naczki, Koritzinsky, Rastani, Diehl, Sonenberg, Koromilas and Wouters19, Reference Carriere, Carmona, Fernandez, Rigoulet, Wenger, Penicaud and Casteilla20).
The ‘hypoxia hypothesis’
In the original proposition it was suggested that the dysregulation of the production of inflammation-related adipokines in obesity, linked to the development of the metabolic syndrome and other obesity-associated disorders, is a specific response to relative hypoxia in clusters of adipocytes that become distant from the vasculature as adipose tissue mass expands(Reference Trayhurn and Wood4). The increased production of inflammation-related adipokines, including angiogenic signals, would be expected to lead acutely to increased blood flow and to the long-term stimulation of angiogenesis. The proposal built on the earlier suggestion that hypoxia may stimulate the expression of angiogenic factors and neovascularisation within WAT(Reference Lolmède, Durand de Saint Front, Galitzky, Lafontan and Bouloumié21).
There are several circumstantial arguments in favour of the hypoxia hypothesis. These are, in particular: (i) the proportion of the cardiac output and blood flow that goes to WAT is not increased in the obese, despite the expansion of the tissue mass(Reference Virtanen, Lonnroth and Parkkola22, Reference Kabon, Nagele, Reddy, Eagon, Fleshman, Sessler and Kurz23); (ii) obese subjects do not exhibit the postprandial increase in blood flow to adipose tissue that occurs in the lean(Reference Karpe, Fielding, Ilic, Macdonald, Summers and Frayn24); (iii) hypertrophied adipocytes are larger than the normal diffusion distance of O2 within tissues(Reference Lolmède, Durand de Saint Front, Galitzky, Lafontan and Bouloumié21). With respect to the last point, large adipocytes may be up to 150 (or even 200) μm in diameter(Reference Skurk, Alberti-Huber, Herder and Hauner25) with the normal diffusion distance of O2 being 100–200 μm(Reference Brahimi-Horn and Pouysségur26). Indeed, in some situations and in some tissues, a partial pressure of O2 of close to zero has been reported at only 100 μm from the vasculature(Reference Brahimi-Horn and Pouysségur26–Reference Gatenby and Gillies28).
Hypoxia is well recognised to be characteristic of pathological situations such as wound healing, ischaemic disorders and solid tumours(Reference Brahimi-Horn and Pouysségur26, Reference Semenza29). Tissues in which O2 tension is normally low include the retina, where values of 2–25 mmHg are apparent, while parts of the rat brain may be even more hypoxic at just 0·8–8 mmHg(Reference Brahimi-Horn and Pouysségur26, Reference Erecinska and Silver30). These levels of O2 should be compared with a partial pressure of O2 of approximately 104 mmHg in the blood from the alveolar capillaries of the lungs and the arteries(Reference Brahimi-Horn and Pouysségur26), as summarised in Table 1.
Table 1 Oxygen levels in different tissues, including tissues where hypoxia is evident, as well as recent data on white adipose tissue from lean and obese mice
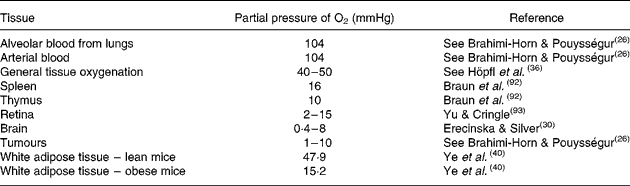
It is widely suggested that WAT is poorly oxygenated in the obese(Reference Virtanen, Lonnroth and Parkkola22, Reference Kabon, Nagele, Reddy, Eagon, Fleshman, Sessler and Kurz23, Reference Fleischmann, Kurz, Niedermayr, Schebesta, Kimberger, Sessler, Kabon and Prager31). Indeed, early data indicate an inverse relationship between fat cell size and the resting blood flow to adipose tissue in dogs(Reference Di Girolamo, Skinner, Hanley and Sachs32). Furthermore, one of us in a study in 1980 on brown adipose tissue thermogenesis found that the proportion of the cardiac output, measured using radioactively labelled microspheres, and the total blood flow to WAT (plus skin) was not increased in obese (ob/ob) mice relative to their lean siblings(Reference Thurlby and Trayhurn33). Similar studies have shown that this is also the case for the Zucker fa/fa rat(Reference West, Prinz, Francendese and Greenwood34).
Molecular signalling of hypoxia
The molecular mechanisms by which cells sense and respond to hypoxia have been extensively investigated and a number of excellent reviews have recently been published on this topic(Reference Brahimi-Horn and Pouysségur26, Reference Semenza35–Reference Rocha39). Here, we outline the key components of the cellular hypoxia signalling pathway.
There are several transcription factors which are implicated in the molecular response to hypoxia, including NFκB and cAMP response element binding protein(Reference Cummins and Taylor38). Hypoxia-induced activation of NFκB, which has recently been demonstrated also in adipocytes(Reference Ye, Gao, Yin and He40), modulates the transcription of target genes such as cyclo-oxygenase 2 and TNF-α(Reference Cummins and Taylor38). However, a pivotal role in the response to hypoxia is played by hypoxia-inducible factor (HIF)-1 which is regarded as the ‘master regulator of O2 homeostasis’. This is a heterodimer composed of α- and β-subunits, one of which, HIF-1β, is constitutively expressed and not regulated directly by O2(Reference Brahimi-Horn and Pouysségur26, Reference Semenza35, Reference Rocha39).
There are at least three α-subunits of HIF-1, namely HIF-1α, HIF-2α and HIF-3α, and the combination of any of these with HIF-1β forms the functional transcription factor. HIF-1α is the most extensively investigated subunit, and appears to be the most important; little is known about HIF-3α(Reference Rocha39). In the presence of O2, HIF-1α (and HIF-2α) is constantly synthesised and degraded via the 26S proteosomal system. This process is initiated by the presence of active prolyl hydroxylation domain proteins which hydroxylate HIF-1α at specific proline residues, thereby presenting binding sites for the von Hippel–Lindau protein(Reference Ivan, Kondo, Yang, Kim, Valiando, Ohh, Salic, Asara, Lane and Kaelin41, Reference Jaakkola, Mole and Tian42). This von Hippel–Lindau protein forms an E3 ligase complex resulting in the polyubiquitylation of HIF-1α, tagging it for destruction. Simultaneously, transcriptional activity is prevented by the action of FIH (factor inhibiting HIF-1) which hydroxylates a specific arginine residue and prevents binding of the cAMP response element binding protein binding protein–p300 complex. Under hypoxic conditions these hydroxylases are rendered inactive. Consequently, HIF-1α is stabilised and HIF-1 moves into the nucleus where it binds to hypoxia response elements within target genes and initiates their transcription (see Fig. 1).

Fig. 1 Diagrammatic view of the molecular signalling response to hypoxia through the hypoxia-inducible factor (HIF) system. PHD, prolyl hydroxylase; FIH, factor inhibiting HIF-1; vHL, von Hippel–Lindau tumour suppressor protein; p300–CBP, p300–cAMP response element binding protein binding protein (transcriptional coactivator); HRE, hypoxia-response element; Ub, ubiquitin.
A substantial number of genes are recognised to be hypoxia sensitive through the hypoxia response elements for HIF-1, and these currently number about seventy. The target genes include those encoding proteins involved in angiogenesis, cell proliferation and survival, apoptosis, vascular tone, and glucose and energy metabolism(Reference Semenza29, Reference Rocha39). Specific hypoxia-sensitive genes include the facilitative glucose transporter, GLUT1, responsible for basal glucose uptake in many cells, leptin, the major angiogenic factor vascular endothelial growth factor (VEGF) and several enzymes involved in glucose metabolism such as phosphofructokinase(Reference Semenza29, Reference Rocha39). GLUT1 in particular, the first of the fourteen-member facilitative GLUT family (see later section ‘Beyond adipokines’) to be identified(Reference Mueckler, Caruso, Baldwin, Panico, Blench, Morris, Allard, Lienhard and Lodish43), is commonly employed as a marker gene of responsiveness to low O2 tension by cells, or a given tissue.
The tools used for investigating the effects of hypoxia and the role of HIF-1 in cell function encompass ‘hypoxia mimetics’ which induce a hypoxic response chemically. These agents include CoCl2, desferrioxamine and dimethyloxallyl glycine, which act by stabilising HIF-1α through inhibition of the prolyl hydroxylases which normally, in the presence of O2, instigate the degradation of the transcription factor subunit. This approach is extensively used in cell-culture studies in addition to, or instead of, incubation under low O2 tension, and helps to define whether signalling through HIF-1α occurs in the case of a particular hypoxia-sensitive gene.
Hypoxia in adipose tissue of obese animals
There are several techniques for measuring whether hypoxia is occurring in specific tissues in vivo, and these include the use of O2 microelectrodes and staining with the chemical marker pimonidazole hydrochloride. The latter is employed primarily in immunohistochemical analyses, forming an adduct with proteins when there is tissue hypoxia. The critical question of whether WAT is hypoxic in obesity has now been directly addressed with pimonidazole in obese mouse models. Using this technique, evidence for hypoxia in WAT has been clearly demonstrated in genetically obese KKAy and ob/ob mice, and in mice made obese through the provision of a high-fat diet(Reference Ye, Gao, Yin and He40, Reference Hosogai, Fukuhara and Oshima44, Reference Rausch, Weisberg, Vardhana and Tortoriello45). Hypoxia in WAT has also been demonstrated in both ob/ob mice and in diet-induced obesity using O2 sensors(Reference Ye, Gao, Yin and He40, Reference Rausch, Weisberg, Vardhana and Tortoriello45). In addition, reduced perfusion of WAT is evident in diet-induced and KKAy obese mice from studies using radiolabelled microspheres(Reference Hosogai, Fukuhara and Oshima44). These reductions in blood perfusion are selective for adipose tissue, there being no decrease in skeletal muscle, the kidney, heart or lungs.
Several molecular indices of hypoxia have also been examined in obese models. Increased HIF-1α protein levels have been found in WAT of both ob/ob and diet-induced obese mice, indicative of hypoxia(Reference Ye, Gao, Yin and He40, Reference Rausch, Weisberg, Vardhana and Tortoriello45). GLUT1 mRNA levels are also increased(Reference Ye, Gao, Yin and He40, Reference Hosogai, Fukuhara and Oshima44, Reference Rausch, Weisberg, Vardhana and Tortoriello45), as is GLUT1 protein in ob/ob mice though not in the diet-induced obese model(Reference Rausch, Weisberg, Vardhana and Tortoriello45). Thus the expression and protein content of a key hypoxia marker are generally increased in WAT in obese mice. Importantly, increased GLUT1 gene expression in ob/ob mice appears selective to WAT, there being no change in GLUT1 mRNA level in skeletal muscle of these obese mutants, consistent with local rather than generalised hypoxia(Reference Ye, Gao, Yin and He40). In parallel with the increased GLUT1 protein in WAT of diet-induced obese and KKAy mice is an increased lactate concentration in the tissue, which again was not evident in skeletal muscle(Reference Hosogai, Fukuhara and Oshima44).
The expression of a number of candidate genes, particularly those related to the inflammatory response, have been screened in WAT of the obese models described above with a view to determining whether hypoxia may underpin any such changes. Increased levels of the mRNA encoding IL-1β, IL-6, leptin, macrophage migration inhibitory factor (MIF), matrix metalloproteinase 2, PAI-1, transforming growth factor-β and TNF-α were each found to be increased, while adiponectin mRNA level is decreased(Reference Ye, Gao, Yin and He40, Reference Hosogai, Fukuhara and Oshima44). Many of these differences have, of course, been widely reported previously, especially the increased expression of leptin, IL-6 and TNF-α, and the reduced adiponectin expression. They have not, however, been linked to hypoxia per se. Interestingly, the expression of the angiogenic factor VEGF, which is well recognised as a hypoxia-sensitive gene, was not elevated in ob/ob mice and only slightly raised in diet-induced obesity(Reference Ye, Gao, Yin and He40).
Changes in the expression of key inflammation-related adipokines in obesity in association with hypoxia do not indicate, of course, a causal link. Indeed, in the case of adipokines such as leptin and adiponectin a number of factors which can modulate expression and which may be altered in obesity are well recognised. These include insulin levels and sympathetic activity, since insulin and β-adrenoceptor agonists can influence the expression of these key adipokines(Reference Leroy, Dessolin, Villageois, Moon, Friedman, Ailhaud and Dani46–Reference Trayhurn, Duncan, Hoggard and Rayner48).
Cell-culture studies: hypoxia-sensitive genes in adipocytes
The effect of hypoxia on the expression and secretion of inflammation-related adipokine genes has been directly examined in adipocytes in culture, both murine and human. The presence of immunoreactive HIF-1α and its induction by hypoxia, or hypoxia mimetics, has been demonstrated in several cell-culture studies employing either the 3T3-F442A or 3T3-L1 adipocyte clonal cell lines(Reference Lolmède, Durand de Saint Front, Galitzky, Lafontan and Bouloumié21, Reference Chen, Lam, Wang, Wu, Lam, Shen, Wong, Hoo, Zhang and Xu49, Reference Segawa, Fukuhara and Hosogai50). Lolmède et al. (Reference Lolmède, Durand de Saint Front, Galitzky, Lafontan and Bouloumié21) in a pioneering report first showed that the production of pro-angiogenic factors such as VEGF, leptin and matrix metalloproteinases is increased in 3T3-F442A adipocytes in response to low O2 tension (5 % O2) or chemical hypoxia. Hypoxia-induced expression of VEGF had also been shown earlier in isolated rat omental adipocytes(Reference Zhang, Magovern, Mack, Budenbender, Ko and Rosengart51).
These observations were followed in 2006 by a report with 3T3-L1 cells that PAI-1 expression and release are augmented by hypoxia and hypoxia mimetics(Reference Chen, Lam, Wang, Wu, Lam, Shen, Wong, Hoo, Zhang and Xu49). Importantly, this study also demonstrated that adiponectin expression and release were inhibited in parallel with the increase in PAI-1, and that the changes were independent of reactive oxygen species. This down-regulation of adiponectin gene expression by hypoxia in 3T3-L1 adipocytes has subsequently been confirmed(Reference Hosogai, Fukuhara and Oshima44), and occurs also in human adipocytes(Reference Wang, Wood and Trayhurn52). Further screening studies on murine adipocytes, both primary and 3T3-L1, have indicated that the expression of a number of genes is stimulated by hypoxia (1 % O2), including GLUT1, MIF, matrix metalloproteinase 9, PAI-1, TNF-α and VEGF(Reference Ye, Gao, Yin and He40, Reference Hosogai, Fukuhara and Oshima44), as illustrated in Fig. 2. However, the changes observed in mRNA levels are rather small, at approximately 1·5–4-fold compared with the controls, in normoxia, and protein secretion (amount in the case of GLUT1) was not measured in these studies.

Fig. 2 Hypoxia-sensitive gene expression in adipocytes. The diagram shows those genes whose expression have been reported to be up-regulated ( ↑ ) or down-regulated ( ↓ ) in adipocytes (murine or human) in cell culture by hypoxia. Genes where changes in the protein, or its activity, have also been documented are shown in red. Where there are conflicting results, as with TNF-α, the gene has not been included. Names and abbreviations are those that are commonly used, rather than the formal gene name. Angptl4, angiopoietin-like protein 4; Hemox, haeme oxygenase 1; MIF, macrophage migration inhibitory factor; MMP, matrix metalloproteinase; MT-3, metallothionein-3; PAI-1, plasminogen activator inhibitor-1; PDK, pyruvate dehydrogenase kinase-1; VEGF, vascular endothelial growth factor.
Visfatin (or pre-B-cell colony-enhancing factor) gene expression, which was originally proposed as an insulin mimetic and to be pro-angiogenic, has additionally been shown to be induced by hypoxia in 3T3-L1 cells; however, the effects recorded are again modest(Reference Segawa, Fukuhara and Hosogai50). Studies on MCF7 breast cancer cells have indicated that visfatin gene expression is directly mediated by HIF-1α(Reference Bae, Kim, Kim, Kim, Koo, Jang, Yun, Yoo and Bae53). Apelin, which has cardioprotective properties, has also been shown to be hypoxia sensitive in 3T3-L1 adipocytes, as in cardiomyocytes(Reference Ronkainen, Ronkainen, Hanninen, Leskinen, Ruas, Pereira, Poellinger, Vuolteenaho and Tavi54), through the mediation of HIF-1α, both cellular mRNA levels and protein in the medium being increased by low O2 tension and hypoxia mimetics(Reference Glassford, Yue, Sheikh, Chun, Zarafshar, Chan, Reaven, Quertermous and Tsao55).
In the case of humans, expression of the HIF-1α gene has been reported in WAT, the level of the mRNA being increased in obese subjects(Reference Cancello, Henegar and Viguerie56). HIF-1α mRNA is evident in both isolated adipocytes and macrophages, the relative level being much greater in the latter(Reference Cancello, Henegar and Viguerie56). Increased HIF-1α mRNA levels in WAT in obese humans is not, however, necessarily evidence that there is hypoxia in the tissue in human obesity; in contrast to the majority of situations where mRNA and protein levels change in parallel, in our experience while the amount of HIF-1α protein increases in adipocytes in cell culture in response to hypoxia, mRNA levels may actually fall.
Our own studies on the effects of hypoxia on adipokine production have focused directly on human adipocytes differentiated from preadipocytes in primary culture. Immunoreactive HIF-1α protein is present in cultured human adipocytes, the level increasing rapidly and substantially (up to 8-fold in 4 h) with exposure to either 1 % O2 or to CoCl2(Reference Wang, Wood and Trayhurn52). The level of 1 % O2, which is a level of oxygenation widely used in in vitro studies of hypoxia, is equivalent to a partial pressure of O2 of 7·6 mmHg, and this is close to that observed in WAT in ob/ob mice(Reference Ye, Gao, Yin and He40). GLUT1 gene expression also increases, indicating that the cells respond to the hypoxia-induced rise in HIF-1α.
A candidate gene approach has been used to examine the effects of hypoxia on the expression of key inflammation-related adipokines in human adipocytes(Reference Wang, Wood and Trayhurn52). Hypoxia induces the expression of several inflammation-related adipokines; these include angiopoietin-like protein 4 (also known as fasting-induced adipose factor), IL-6, leptin, MIF, PAI-1 and VEGF(Reference Wang, Wood and Trayhurn52). The secretion of IL-6, leptin, MIF and VEGF into the medium is also increased by hypoxia (the other adipokines were not measured). In contrast, the expression and secretion of adiponectin, on the other hand, were inhibited by low O2 tension in human adipocytes, as in 3T3-L1 cells, but the effect was modest(Reference Wang, Wood and Trayhurn52). The expression of the acute-phase protein haptoglobin was also decreased under hypoxic conditions.
In array studies on human adipocytes, using a hypoxia signalling pathway PCR array (SuperArray), the expression of one particular gene – metallothionein (MT)-3 – was found to be very highly induced by hypoxia(Reference Wang, Wood and Trayhurn57). MT-3 mRNA levels increased > 600-fold in 24 h, with a > 100-fold increase by just 60 min after exposure of the adipocytes to 1 % O2. This response is not general to the MTs, since expression of the MT-2A gene, which we have previously shown occurs in human adipocytes(Reference Do, Nam, Hong, Kim, Duncan, Beattie and Trayhurn58), is essentially unaltered by hypoxia(Reference Wang, Wood and Trayhurn57). The functional significance of MT-3 expression by hypoxia in adipocytes is unclear, but previous work has shown that MT-3 is highly hypoxia inducible in human astrocytes where it is suggested to be protective against hypoxic damage(Reference Tanji, Irie, Uchida, Mori, Satoh, Mizushima and Wakabayashi59).
Overall, the cell-culture observations are generally consistent with hypoxia leading to an increase in the production of inflammation-related adipokines, both in murine and human adipocytes, this being mediated through HIF-1α as indicated by the studies with the hypoxia mimetics. The hypoxia-induced increase in the expression of the major angiogenic factor VEGF is consistent with a stimulation of angiogenesis. Other adipokines have also been implicated in angiogenesis, including angiopoietin-like protein 4 and leptin(Reference Sierra-Honigmann, Nath and Murakami60–Reference Le Jan, Amy and Cazes63). The inhibition of adiponectin production and release by hypoxia demonstrated by the cell-culture studies is of particular significance since this adipokine has important anti-inflammatory and insulin-sensitising actions(Reference Ouchi, Kihara and Arita64–Reference Yamauchi, Kamon and Waki67). It has been suggested that hypoxia may lead to a decrease in adiponectin production in adipocytes via the induction of endoplasmic reticulum stress(Reference Hosogai, Fukuhara and Oshima44).
Beyond adipokines: hypoxia and other adipocyte cellular processes
Hypoxia has been considered so far in terms of adipokine synthesis, reflecting the importance of these factors in WAT physiology. However, low O2 tension would be expected to impact on adipocyte function in multiple ways and two particular areas have been explored – cell differentiation and glucose utilisation. Several studies have examined the effects of hypoxia on adipocyte differentiation using various cell systems, including 3T3-L1 preadipocytes and bone marrow stromal cells. In all but one case, hypoxia or the CoCl2 mimetic inhibited differentiation(Reference Carriere, Carmona, Fernandez, Rigoulet, Wenger, Penicaud and Casteilla20, Reference Yun, Maecker, Johnson and Giaccia68–Reference Lin, Lee and Yun71). However, a study employing mouse bone marrow stromal cells suggested that hypoxia accelerates differentiation into adipocytes(Reference Ren, Cao and Zhao72). Whether this different response is a reflection of the specific cell system, or the precise culture conditions, is unclear. Our own exploratory studies on human preadipocytes suggest that adipocyte differentiation in these cells is indeed inhibited by hypoxia (B Wang, IS Wood and P Trayhurn, unpublished results).
As described above, hypoxia leads to an increase in GLUT1 gene expression in white adipocytes, and this appears to be HIF-1 dependent in that incubation with CoCl2 under normoxic conditions leads to an increased GLUT1 mRNA level(Reference Wang, Wood and Trayhurn52). White adipocytes express several of the fourteen members of the facilitative GLUT gene family (GLUT1–GLUT12, HMIT, GLUT14; gene name SLC2A), including the insulin-sensitive transporter, GLUT4(Reference Wood and Trayhurn73, Reference Wood, Hunter and Trayhurn74). Exposure of human adipocytes to hypoxia for up to 24 h has been shown to lead to increased expression of the GLUT3 and GLUT5 genes, as well as of GLUT1, while GLUT4, GLUT10 and GLUT12 expression remains unchanged(Reference Wood, Wang, Lorente-Cebrián and Trayhurn75). The increase in GLUT1 mRNA level in hypoxia is paralleled by a substantial (10-fold) increase in the cellular level of GLUT1 protein(Reference Wood, Wang, Lorente-Cebrián and Trayhurn75). However, despite the increase in GLUT5 mRNA, GLUT5 protein level is unchanged, while GLUT3 protein could not be detected.
Importantly, functional studies with 2-deoxy-d-glucose indicate that glucose uptake by human adipocytes is strongly stimulated by hypoxia, presumably as a consequence of the increased amount of GLUT1(Reference Wood, Wang, Lorente-Cebrián and Trayhurn75). Increased glucose utilisation by adipocytes in response to hypoxia is, of course, a reflection of the Pasteur effect. It seems probable from these results that the metabolic activity of adipocytes is widely influenced by hypoxia. Certainly, glycolysis would be expected to be greatly stimulated and the utilisation of other nutrients may well also change.
Of particular significance is the possibility that insulin sensitivity may alter, either acutely or chronically, in response to hypoxia. This has been demonstrated in in vivo studies under conditions such as high altitude (particularly with acute exposure) and with obstructive sleep apnoea syndrome(Reference Larsen, Hansen, Olsen, Galbo and Dela76, Reference Oltmanns, Gehring, Rudolf, Schultes, Rook, Schweiger, Born, Fehm and Peters77). Intermittent hypoxia is commonly used to simulate obstructive sleep apnoea syndrome in experimental models and has been shown to induce insulin resistance(Reference Polotsky, Li, Punjabi, Rubin, Smith, Schwartz and O'Donnell78, Reference Iiyori, Alonso, Li, Sanders, Garcia-Ocana, O'Doherty, Polotsky and O'Donnell79). Although there is some evidence for insulin resistance following hypoxia in certain tissues, such as skeletal muscle, the overall picture is unclear(Reference Iiyori, Alonso, Li, Sanders, Garcia-Ocana, O'Doherty, Polotsky and O'Donnell79, Reference Gulve, Ren, Marshall, Gao, Hansen, Holloszy and Mueckler80). Specific studies are needed on whether local hypoxia leads to a reduction in insulin sensitivity in adipocytes, perhaps through the translocation of GLUT4 between the intracellular storage sites and the plasma membrane or by alterations in the insulin signalling pathway.
Hypoxia and the other cells in adipose tissue
The effects of hypoxia on WAT function have been discussed in terms of adipocytes, reflecting the fact that these are the cells that are characteristic of adipose tissue and which greatly enlarge in obesity. Adipocytes generally account, however, for no more than 50 % of the total cell content of WAT, the exact proportion varying between depots. The other cells include preadipocytes, endothelial cells, mast cells and macrophages, and it is argued from in vitro studies that adipocytes are only minor contributors compared with the non-adipocyte fraction in the production and release of many inflammatory cytokines and chemokines from WAT(Reference Fain, Madan, Hiler, Cheema and Bahouth81, Reference Fain82). However, the precise contribution of adipocytes, macrophages and other cells to the production of specific adipokines (other than leptin and adiponectin which essentially are produced in WAT only by mature adipocytes) is difficult to assess quantitatively. For example, is not certain whether the process of preparing and subsequent incubation of the different cell fractions alters their relative rate of production and release of various adipokines. In addition, large adipocytes show the highest rates of production of inflammatory adipokines(Reference Skurk, Alberti-Huber, Herder and Hauner25), but because of their greater fragility they are likely to be under-represented, or absent, from most cell fractionations.
Macrophages have attracted considerable recent attention in adipose tissue biology following the discovery that they infiltrate the tissue in obesity and appear to play a substantial role in the inflammatory process(Reference Xu, Barnes and Yang13, Reference Weisberg, McCann, Desai, Rosenbaum, Leibel and Ferrante14). These immune cells are recognised to respond to hypoxia with changes in the production of key inflammatory mediators(Reference Lewis, Lee, Underwood, Harris and Lewis83–Reference Oda, Hirota and Nishi85). This has been directly examined recently in the context of macrophage involvement in adipose tissue function, with the induction of IL-1β, IL-6, MIF, TNF-α and VEGF gene expression under hypoxia being evident in peritoneal macrophages(Reference Ye, Gao, Yin and He40). Given the importance attributed to macrophages in the inflammatory response in WAT in obesity, it is of particular interest that immunohistochemical evidence suggests that F4/80+ macrophages are located primarily in the areas of the tissue exhibiting hypoxia(Reference Rausch, Weisberg, Vardhana and Tortoriello45). This suggests that there is a link between pockets of inflammation within WAT and the recruitment of macrophages into these same areas of the tissue through the hypoxia-induced production of inflammation-related adipokines.
The preadipocytes within WAT also exhibit major inflammatory responses, expressing and secreting a range of inflammation-related adipokines, including IL-1β, IL-6, IL-8, monocyte chemoattractant protein-1, MIF and TNF-α(Reference Cousin, Munoz, Andre, Fontanilles, Dani, Cousin, Laharrague, Casteilla and Penicaud86–Reference Chung, LaPoint, Martinez, Kennedy, Boysen Sandberg and McIntosh91). Indeed, preadipocytes (human) are highly responsive to lipopolysaccharide challenge and it is suggested that they may play a pivotal role in the inflammatory response within WAT(Reference Chung, LaPoint, Martinez, Kennedy, Boysen Sandberg and McIntosh91). Studies on the effects of hypoxia on preadipocytes have focused on adipogenesis, as described above, rather than on the inflammatory response or general metabolic function. We have, however, recently found some similar responses to hypoxia in human preadipocytes as in adipocytes – together with some differences (B Wang, IS Wood and P Trayhurn, unpublished results). The similarities include increases in GLUT1 gene expression, and in VEGF expression and secretion. In contrast to mature adipocytes, however, hypoxia did not lead to major changes in preadipocytes in the expression of either IL-6 or angiopoietin-like protein 4.
Conclusions and implications
It is evident that hypoxia can influence the function of both adipocytes and adipose tissue. In particular, the expression and secretion of several key adipokines linked to inflammation are altered by hypoxia; indeed, the increase in leptin and reduction in adiponectin in obesity may be partly a direct result of hypoxia within WAT. While the changes in the production of inflammation-related adipokines are generally consistent with the original hypoxia hypothesis(Reference Trayhurn and Wood4), some major inflammatory adipokines, particularly TNF-α, may not be up-regulated under hypoxic conditions, at least in human adipocytes(Reference Wang, Wood and Trayhurn52). However, even if low O2 tension proves not to be a critical factor in initiating the inflammatory response in WAT, it is nonetheless likely to impact broadly on adipocytes and on adipose tissue.
We suggest that the hypoxic environment in which current evidence suggests enlarged adipocytes exist needs to be taken into account in studies on WAT. Thus the possibility that most metabolic systems, or the response to particular agents or factors, might be modified (directly or indirectly) by hypoxia should be considered. Hypoxia as a factor in adipose tissue biology has been viewed exclusively in terms of obesity, but in principle it could underlie other situations in which there is a marked increase in adipose tissue mass. For example, hypoxia may well occur in WAT during the physiological fattening that can take place in late pregnancy, and during the pre-hibernatory and pre-migratory period in those species which hibernate or migrate, respectively. Hypoxia, at least initially, could also occur in WAT on acute cold exposure as blood is channelled to brown adipose tissue and skeletal muscle for the generation of thermoregulatory heat by non-shivering and shivering mechanisms.
Finally, targeting the hypoxia signalling pathway, whether through HIF-1α itself or another key intermediate, may provide a novel route for the treatment of obesity-associated diseases, as noted previously(Reference Hosogai, Fukuhara and Oshima44, Reference Chen, Lam, Wang, Wu, Lam, Shen, Wong, Hoo, Zhang and Xu49). Directing any potential such treatment specifically to adipose tissue, and more particularly to the adipocyte, may alleviate many of these disorders.
Acknowledgements
We are grateful to the Biotechnology and Biological Sciences Research Council (BBSRC UK) for funding our studies on hypoxia in adipose tissue.
The authors declare that they have no conflicts of interest.
P. T. wrote the draft of the article, with subsequent additions and revisions from B. W. and I. S. W.; P. T. prepared the final version.







