The global obesity epidemic and the growing burden of associated co-morbidities have galvanised the scientific community in its efforts to identify the aetiological triggers that determine individual susceptibility to weight gain and attendant metabolic dysregulation( Reference Grattan and Connolly-Schoonen 1 , Reference Maclean, Bergouignan and Cornier 2 ). Genetic, behavioural and environmental factors have been implicated as contributing to the obese-susceptible phenotype( Reference Davis, Curtis and Levitan 3 , Reference Bell, Walley and Froguel 4 ). What is not clear is the extent to which dietary intake, and in particular, dietary macronutrient intake and substrate oxidation, may be causally associated with obesity, per se and metabolic dysregulation in the obese( Reference Blaak, Hul and Verdich 5 , Reference Heilbronn, Gregersen and Shirkhedkar 6 ). For example, in some studies, the development of obesity has been shown to be associated with a high-fat diet( Reference Ravussin and Swinburn 7 , Reference Macdiarmid, Cade and Blundell 8 ) as it leads to increased energy intake and has been found to be less satiating than did the other macronutrients( Reference Rolls, Kim-Harris and Fischman 9 , Reference Speechly and Buffenstein 10 ). However, a positive fat balance and subsequent weight gain may not only be linked to intake, but also associated with a reduced rate of fat oxidation( Reference Zurlo, Lillioja and Esposito-Del Puente 11 – Reference Simoneau, Veerkamp and Turcotte 14 ).
Blundell et al. ( Reference Cooling and Blundell 15 ) have previously characterised a specific dietary phenotype that consumes a high-energy, high-fat diet (44 % energy from fat) but remains weight stable and non-obese. In comparison with lean habitual low-fat consumers (32 % energy from fat), these individuals are characterised by having higher RMR, significantly lower RER( Reference Cooling and Blundell 15 ) and higher fasting plasma leptin concentrations( Reference Cooling, Barth and Blundell 16 ). These characteristics may serve to protect such individuals from weight gain. Furthermore, they found that these two groups displayed different appetite control characteristics( Reference Cooling and Blundell 17 ). When challenged with high-fat meals, the lean high-fat (LHF) consumers ate a constant weight of food, whereas the lean low-fat (LLF) consumers ate a constant amount of energy. Taken together, these findings suggest that these groups make up distinct, specific, dietary metabolic phenotypes.
Blundell et al. ( Reference Blundell, Stubbs and Golding 18 ) have also compared LHF consumers with obese high-fat (OHF) consumers (>43 % of energy) to identify the characteristics that may make some individuals more susceptible to weight gain. They found that, in comparison with LHF dietary phenotypes, susceptible individuals showed a preference for high-fat foods, a weak satiety, but strong hedonic response to high-fat foods, and also had high scores for disinhibition and hunger as measured by the Three Factor Eating Questionnaire (TFEQ). Thus, the capacity of individuals to remain normal-weight despite ingesting a high-fat diet appears to be related, at least in part, to the capacity to oxidise dietary fat and to exhibit the appropriate satiety and hunger signals in response to certain macronutrients in the diet. A mismatch in these characteristics may lead to an increased susceptibility for obesity. For example, Marrades et al. ( Reference Marrades, Martínez and Moreno-Aliaga 19 ) found that obese-susceptible individuals were more likely to be in positive fat balance, following a lipid-containing meal, than did their lean, diet- and age-matched counterparts.
An imbalance between energy intake and energy expenditure may also be partly a consequence of inadequate appetite regulation. Numerous gastrointestinal hormones have been implicated in appetite regulation, including long-acting adiposity hormones such as leptin and insulin, and short-term hunger and satiety signals such as ghrelin and peptide YY (PYY)( Reference Cummings and Overduin 20 ). Leptin is largely produced by adipose tissue and released into the circulation in proportion to the size of the adipose tissue stores( Reference Weigle, Duell and Connor 21 ). Leptin signals to key regulatory centres in the brain to inhibit food intake and regulate body weight and energy homoeostasis( Reference Halaas, Gajiwala and Maffei 22 ). In contrast, PYY, a short-term satiation hormone, produced mainly by distal-intestinal L cells in response to a meal, is lower in obese individuals( Reference Batterham, Cohen and Ellis 23 ). Although obese individuals do not display PYY resistance, lower endogenous production of PYY in response to a meal may translate into reduced satiety( Reference Le Roux, Batterham and Aylwin 24 ). Ghrelin, produced by the stomach and proximal small intestine, functions and is regulated oppositely to satiation hormones. Ghrelin acts as an orexigenic signal promoting meal initiation, as circulating ghrelin levels increase before a meal( Reference Cummings, Purnell and Frayo 25 ) and decline postprandially( Reference Tschöp, Wawarta and Riepl 26 ).
Ghrelin levels correlate negatively with BMI, and obese individuals display an attenuated ghrelin response to a meal( Reference English, Ghatei and Malik 27 ). Meal-induced suppression of ghrelin is dependent on the composition of the meal( Reference Cummings, Foster-Schubert and Overduin 28 ). Although carbohydrate (CHO)-rich meals induce a pronounced short-term suppression of ghrelin, levels are shown to subsequently rebound above those recorded at baseline. In contrast, fat- and protein-rich meals suppress ghrelin levels for up to 6 h after ingestion( Reference Foster-Schubert, Overduin and Prudom 29 ). PYY secretion is also shown to be greater in response to lipid-rich compared with CHO-rich meals( Reference Degen, Oesch and Casanova 30 ), whereas leptin secretion is not altered by macronutrient composition( Reference Weigle, Duell and Connor 21 ). It is, however, not clear how the habitual diet or dietary phenotype alters the responsiveness of the satiety hormones to an acute meal, and how this is altered in the obese state.
Further exploration of these dietary and metabolic phenotypes may help to elucidate the underlying aetiology of obesity as well as the propensity for weight-loss relapse and diet-resistant obesity. Therefore, the aims of the present study were the following: (i) to characterise and compare LLF and LHF dietary phenotypes as well as lean and OHF dietary phenotypes in terms of body composition, appetite, fasting metabolic rate and substrate oxidation; (ii) to compare changes in metabolic rate, substrate oxidation and appetite in response to a high-fat meal, in these groups; and (iii) to examine the relationships between hormonal modulators of appetite in response to a high-fat meal.
Methods
Ethics approval
The study was conducted according to the guidelines laid down in the Declaration of Helsinki, and all procedures involving human participants were approved by the Human Research Ethics Committee of the Faculty of Health Sciences of the University of Cape Town (REC REF 064/2003). Written consent was obtained from all participants before participation in the study.
Subject screening and selection
Sample size determination was based on previous studies( Reference Cooling and Blundell 31 , Reference Zwirska-Korczala, Konturek and Sodowski 32 ), in which mean differences between LHF and LLF phenotypes in RMR of 10·0 (sd 7·1) kJ/kg (2·4 (sd 1·7) kcal/kg) fat-free mass (FFM) and RER 0·05 (sd 0·04)( Reference Cooling and Blundell 31 ), as well as mean differences in fasting serum ghrelin concentrations of 295 (sd 241) pg/ml between lean and obese women( Reference Zwirska-Korczala, Konturek and Sodowski 32 ) were observed. Using a power of 80 % and α-level of 0·05, the estimated sample size was eight to eleven men per group. A total of thirty healthy male subjects participated in the trial, ten per group. Subjects were recruited via advertisements in local newspapers and were selected according to their BMI and dietary phenotype, as previously described by Cooling & Blundell( Reference Cooling and Blundell 17 , Reference Cooling and Blundell 31 ). These phenotypes were determined on the basis of self-reported habitual dietary intake as either high-fat (>40 % total energy (TE)) or low-fat (<30 % TE). Inclusion criteria were the following: (i) BMI<25 kg/m2 for the lean groups and >30 kg/m2 for the obese group; (ii) total average energy intake from dietary fat >40 % for high-fat consumers and <30 % for low-fat consumers; (iii) no known medical conditions or medications that may adversely affect metabolism; (iv) weight stable (<3 kg weight fluctuation in past 6 months); and (v) exercise not more than three times per week. These criteria allowed the subjects to be placed into one of three groups: LHF consumers, LLF consumers and OHF consumers. By design, there was a significant difference in the Short Fat Questionnaire (SFQ) score and self-reported dietary fat intake between the LLF and LHF groups (Table 1).
Table 1 Subject characteristics (Mean values and standard deviations; n 10)

CHO, carbohydrate; HOMA-IR, homoeostatic model assessment for insulin resistance( Reference Matthews, Hosker and Rudenski 33 ); LHF, lean high-fat group; LLF, lean low-fat group; OHF, obese high-fat group; PYY, peptide YY; SFQ, Short Fat Questionnaire; TE, total energy; TFEQ, Three Factor Eating Questionnaire.
Weight and height were measured, and body composition was estimated using near infrared reactance (Futrex – 6100/XL; Futrex Inc.). Subjects were initially screened for habitual dietary fat intake using the validated SFQ( Reference Dobson, Blijlevens and Alexander 34 ). The SFQ estimates habitual dietary fat intake from a series of seventeen questions. A score of <17 correlates with a fat intake of <30 % TE, and a score of >28 correlates with a fat intake of >37·5 % TE( Reference Dobson, Blijlevens and Alexander 34 ). For the purpose of this trial, low-fat consumers were categorised on the SFQ as having a score of <18 and high-fat consumers as having a score of >30. Dietary intake was confirmed using a 3 d dietary record, which was analysed for TE and macronutrient intake using Food Finder II Dietary Analysis Programme (MRC). Dietary fat intakes of <30 or >40 % TE were used as thresholds for identifying the low-fat and high-fat consumers, respectively. Subjects also had completed a 24 h dietary record on the day before the experimental trial to confirm that the subjects’ habitual dietary intakes did not change. Dietary restraint, hunger and disinhibition were also measured using the Three Factor Eating Inventory (TFEQ)( Reference Stunkard and Messick 35 ).
Experimental trial
Subjects reported to the laboratory after an overnight fast (10–12 h). Fasting RMR, RER and appetite sensations were measured, and a fasting blood sample was drawn for the determination of serum glucose, insulin, leptin, ghrelin and PYY concentrations. The subjects then ingested a standardised high-fat test meal, following which RMR, RER and appetite sensations were measured hourly for 6 h, and blood samples for the determination of plasma ghrelin and PYY levels were drawn hourly for 3 h. At the end of the trial, subjects consumed a quantified but self-selected portion of a standardised lunch, after which appetite sensations were measured again. Subjects remained in a resting state for the duration of the trial and were permitted to drink 250 ml of water.
Test meal
The test meal consisted of a 4723 kJ milkshake (200 ml cream, 200 g full-fat ice-cream and 100 ml full-cream milk), comprising 77 % fat (98 g), 20 % CHO (56 g) and 3 % protein (11 g). The high-fat (77 %) content of the test meal was chosen in order to optimise the chances of detecting metabolic and appetite differences between the groups in response to dietary fat. All subjects were fed a fixed volume of the test meal to replicate the protocol of Cooling & Blundell( Reference Cooling and Blundell 31 ). It was also intended to reflect an energy-dense food choice available to free living individuals. This allows the opportunity to assess differences in metabolic and appetite hormone response between dietary phenotypes, as well as between lean and obese individuals of the same dietary phenotype, to the identical meal.
Metabolic measurements and calculations
RMR was measured on all subjects in the morning after a 12 h overnight fast. Subjects rested in supine position, in a quiet, isolated room, with a temperature ranging from 21 to 24°C, after which the basal respiratory exchange was measured for 20 min and again, every hour for 6 h after the consumption of the test meal using the ventilated hood technique (VMax Metabolic Cart 229; SensorMedics). Before each experimental trial, the metabolic cart was calibrated with a Hans Rudolph 3L syringe, and the analysers were calibrated using standard gas mixtures of oxygen (26 % O2 with the balance nitrogen) and carbon dioxide (4 % CO2, 16 % O2 and the balance nitrogen) (BOC Special Gas).
RMR and total rates of fat and CHO oxidation were calculated using the equations of Weir( Reference De Weir 36 ) and Frayn( Reference Frayn 37 ), respectively. The thermic effect of feeding (TEF) was calculated as the increased energy expenditure after the test meal, expressed as a percentage of fasting RMR (TEF=((AUCRMR/RMR0 h)−1)×100). Energy balance was calculated as the difference between the energy ingested in the test meal and the energy expended during the postprandial period (EI−AUCRMR). Fat balance was calculated as the difference between fat ingested and fat oxidised (Fat intake−AUCfat oxidation)( Reference Schutz 38 ).
Blood sampling and analysis
Following the fasting respiratory exchange measurements, a cannula attached to a three-way stopcock was inserted into the antecubital vein for blood sampling. A fasting blood sample (approximately 18 ml) was drawn for the determination of serum glucose, insulin, leptin, ghrelin and PYY concentrations. Thereafter, samples for the determination of serum ghrelin and PYY concentrations were drawn 1 and 3 h following the test meal. Samples were kept on ice until centrifuged at 3000 rpm at 4°C for 10 min and were then stored at −80°C for later analysis. Plasma glucose concentrations were determined using the glucose oxidase method (Glucose Analyzer 2; Beckman Instruments). Commercial radio-immunoassays were used to measure plasma insulin (Axsym Insulin Assay; Abbott Laboratories), leptin (Human Leptin RIA Kit, LINCO Research Inc.), ghrelin (Human Ghrelin RIA Kit; Phoenix Pharmaceuticals Inc.) and PYY (Human PYY RIA Kit, LINCO Research Inc.) concentrations. Insulin resistance was estimated using the formula of the homoeostatic model assessment for insulin resistance (HOMA-IR=(fasting glucose (mmol/l)×fasting insulin (mU/l))/22·5)( Reference Matthews, Hosker and Rudenski 33 ).
Appetite questionnaires
Ratings of behavioural appetite (hunger, fullness, prospective consumption and satiety) were investigated before, every 1 h following the test meal, and immediately after the post-test lunch, using a validated 100 mm line scale( Reference Flint, Raben and Blundell 39 , Reference Raben, Tagliabue and Astrup 40 ). Each sensation was assessed with a separate line scale anchored with the opposing extremes of the sensation (e.g. ‘Not at all full’ (0 mm) to ‘Extremely full’ (100 mm)).
Post-test meal (lunch)
After 6 h of the test meal, subjects were invited to eat a self-selected quantity of a standardised lunch of ready-made macaroni cheese (40 % fat, 45 % CHO and 15 % protein; Today’s Frozen Foods), which was of similar composition to a typical Western South African diet( Reference Steyn, Langenhoven and Joubert 41 ). The meal was served in a large casserole dish from which the subjects served themselves ad libitum. Food quantity was covertly measured by weighing the plate before and after completion of the meal and by measuring plate wastage.
Statistical analysis
For the analyses, the LHF group was compared with both the LLF group and the OHF group independently, allowing for the following comparisons: (i) between groups with different dietary phenotypes but similar body composition (LHF v. LLF); and (ii) between groups of similar dietary phenotype but different body composition (LHF v. OHF). We included ten men per group. A Bonferroni correction was used to correct for pair-wise comparisons (two groups) throughout, and, as a result, only a P value <0·025 (0·05/2) was considered statistically significant. An independent t test was used to analyse differences between groups at the outset. Two-way ANOVA for repeated-measures was used to analyse the differences between groups over time. A Tukey honest significant difference post hoc test was performed to locate specific differences between groups over time. Peason’s correlation was used to assess whether energy intake and macronutrient composition – as analysed from the 3 d dietary records and the 24 h record before the trial – were similar and reflected usual dietary intake. Results are expressed as means and standard deviations. Data were normalised by log transformation where required. STATISTICA (version 10; Statsoft) was used to perform the statistical analyses.
Results
Body composition and dietary intake
The characteristics of the LLF, LHF and OHF groups are presented in Table 1. There were no differences in body composition between the LLF and LHF groups, whereas participants of the OHF group were heavier, and had greater body fat and FFM than that of the LHF group. The LHF group had a significantly higher fat intake (g and % TE) and consequently a higher TE intake than that of the LLF group. Absolute CHO and protein intakes were not different between the LLF and LHF groups, but, when expressed in terms of TE, the LHF group ingested less CHO than did the LLF group. In contrast, there were no differences in SFQ score or TE or macronutrient intakes between the LHF and OHF groups (Table 1). Dietary intake recorded using the 3 d dietary record and the 24 h record on the day before the experimental trial correlated significantly for energy (r 0·87, P<0·001) and macronutrient intake (r 0·90, 0·60 and 0·66 for fat, CHO and protein intakes, respectively, P<0·001) for all three groups (group data not shown). On the basis of the results of the TFEQ, the OHF group had significantly higher levels of disinhibition and hunger than that of the LHF. In contrast, there were no differences in dietary restraint, disinhibition or hunger between the LHF and LLF groups (Table 1).
Circulating metabolite and hormone concentrations
Fasting plasma glucose and hormone levels, as well as insulin resistance estimated using HOMA, are presented in Table 1. There were no differences in fasting plasma glucose or hormone levels between the LLF and LHF groups. In contrast, the OHF group had higher fasting plasma glucose and insulin levels, and consequently were more insulin resistant than that of the LHF group. In addition, fasting plasma leptin levels were significantly higher, and fasting plasma ghrelin levels tended to be lower in the OHF than the LHF group. Fasting plasma PYY levels were not different between groups.
Energy and substrate balance
Energy and substrate balance in the fasted state and in response to the high-fat meal are presented in Table 2. Fasting RMR was not different between the lean groups. However, the LHF group had a significantly lower fasting RER than that of the LLF group. In contrast, there were no differences in RER between the LHF and OHF groups, but the OHF group had a significantly higher fasting RMR, expressed both in absolute terms and relative to FFM, than that of the LHF group.
Table 2 Energy and substrate balance in the fasted state and in response to the high-fat meal (Mean values and standard deviations)
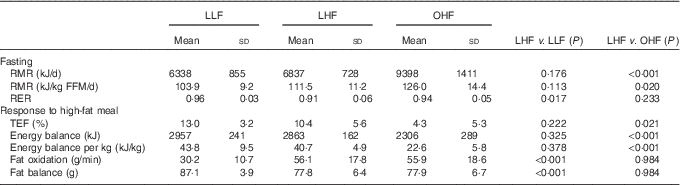
FFM, fat-free mass; LHF, lean high-fat group; LLF, lean low-fat group; OHF, obese high-fat group; TEF, thermic effect of feeding.
There were no significant differences in energy balance in response to the high-fat meal between the LLF and LHF groups. The change in substrate oxidation in response to the test meal was similar in the LLF and LHF groups (Fig. 1). However, the lower baseline fasting RER, in the LHF compared with the LLF group, was maintained following the meal so that the LHF effectively oxidised significantly more fat in the postprandial period than that the LLF group consumed (Table 2). By virtue of their greater size, the OHF group compared with the LHF group showed a significantly lower thermic response and was in less positive energy balance following the high-fat test meal. There were no differences in RER (Fig. 1) or fat balance between the LHF and OHF groups.

Fig. 1 Changes in RER in response to the high-fat meal in the (a) lean high-fat (![]() , LHF) v. lean low-fat (
, LHF) v. lean low-fat (![]() , LLF) groups, and (b) LHF v. obese high-fat (
, LLF) groups, and (b) LHF v. obese high-fat (![]() , OHF) groups. Values are means and standard deviations. (a) LLF v. LHF: † P<0·001 for group effect; * P<0·001 for time effect from baseline for both groups (no group×time effect indicating similar response to the test meal). (b) LHF v. OHF: * P<0·001 for time effect from baseline for both groups.
, OHF) groups. Values are means and standard deviations. (a) LLF v. LHF: † P<0·001 for group effect; * P<0·001 for time effect from baseline for both groups (no group×time effect indicating similar response to the test meal). (b) LHF v. OHF: * P<0·001 for time effect from baseline for both groups.
Appetite ratings and satiety hormones
Appetite ratings (AR) in response to the high-fat meal are presented in Fig. 2. There were no differences in fasting AR or AR in response to the high-fat meal or the post-test meal between the LHF and LLF groups. Similarly, there were no significant differences in AR between the LHF and OHF groups when measured over time or as the AUC (data not shown). There were, however, significant and appropriate changes in AR in response to the high-fat meal and the post-test meal. For example, hunger ratings decreased after the high-fat meal and then increased significantly 4–5 h after the high-fat meal, and then decreased dramatically and significantly after the post-test meal in all groups. Post-test meal consumption was also not different between the LLF and LHF groups (533·7 (sd 156·2) v. 509·1 (sd 186·5) g, P=0·752) or between the LHF and OHF groups (509·1 (sd 186·5) v. 482·7 (sd 175) g, P=0·748). However, when expressed relative to body size, the OHF group ate significantly less than that of the LHF group (4·58 (sd 1·48) v. 7·23 (sd 2·67), P=0·013).

Fig. 2 Changes in ratings of hunger, fullness, prospective consumption and satiety in response to the high-fat meal in the (a) lean high-fat (![]() , LHF) v. lean low-fat (
, LHF) v. lean low-fat (![]() , LLF) groups; and (b) LHF v. obese high-fat (
, LLF) groups; and (b) LHF v. obese high-fat (![]() , OHF) subjects. Values are means and standard deviations. * P<0·001 for time effect from baseline for all four ratings of appetite. AL, after lunch.
, OHF) subjects. Values are means and standard deviations. * P<0·001 for time effect from baseline for all four ratings of appetite. AL, after lunch.
Plasma ghrelin and PYY responses to the high-fat meal are presented in Fig. 3. Plasma ghrelin levels decreased similarly in the LLF and LHF groups in response to the high-fat meal. In contrast, in the OHF group, plasma ghrelin levels did not change in response to the high-fat meal, with a significant group×time interaction between the LHF and OHF groups (P=0·01). In contrast to ghrelin, plasma PYY levels increased significantly in response to the high-fat meal in the LLF and LHF groups, with a tendency for a group×time interaction (P=0·04). Compared with the LHF group, the OHF group had a blunted PYY response to the high-fat meal (P=0·002 for group×time interaction).

Fig. 3 Changes in plasma ghrelin and peptide YY (PYY) levels in response to the high-fat meal in the (a) lean high-fat (![]() , LHF) v. lean low-fat (
, LHF) v. lean low-fat (![]() , LLF) groups; and (b) LHF v. obese high-fat (
, LLF) groups; and (b) LHF v. obese high-fat (![]() , OHF) groups. Values are means and standard deviations. (a) LLF v. LHF: * P<0·01 for time effect from baseline for plasma ghrelin and PYY levels in both groups; P=0·04 for group x time effect for PYY. (b) LHF v. OHF: * P<0·01 for time effect from baseline for plasma ghrelin and PYY levels in the LLF group only; † P=0·01 for group×time effect for plasma ghrelin; ‡ P=0·002 for group×time effect for plasma PYY levels.
, OHF) groups. Values are means and standard deviations. (a) LLF v. LHF: * P<0·01 for time effect from baseline for plasma ghrelin and PYY levels in both groups; P=0·04 for group x time effect for PYY. (b) LHF v. OHF: * P<0·01 for time effect from baseline for plasma ghrelin and PYY levels in the LLF group only; † P=0·01 for group×time effect for plasma ghrelin; ‡ P=0·002 for group×time effect for plasma PYY levels.
Discussion
The main findings of this study were that we were able to characterise LLF as well as LHF and OHF dietary phenotypes, based on habitual low-fat (<30 % of TE intake) or high-fat (>40 % of TE) dietary intake. These comparisons reveal underlying differences that may help to explain why some individuals on a high-fat diet are protected from weight gain, whereas others are not. Compared with the LLF group, the LHF group showed higher baseline fasting fat oxidation, which was maintained in the postprandial period, and this meant that they oxidised a greater amount of fat ingested in the high-fat test meal, suggestive of some adaptive response to the high-fat diet, whereas there were no differences in rates of fat oxidation between the LHF and OHF groups. In contrast, although the lean dietary phenotypes did not differ in energy expenditure or appetite responses to the high-fat meal, the OHF group showed significantly higher rates of energy expenditure, along with dampened ghrelin and PYY responses to the high-fat meal. The blunted appetite hormone signals in the OHF group were not reflected in subjective AR, which were similar to those of the lean phenotypes. However, significantly higher scores for hunger and disinhibition on the TFEQ in the OHF compared with the LHF group indicate that the OHF group experiences persistent difficulties with appetite control.
Similar to earlier findings of Cooling & Blundell( Reference Cooling and Blundell 15 – Reference Cooling and Blundell 17 , Reference Cooling and Blundell 31 ), we identified lean dietary phenotypes, and despite the differences in dietary macronutrient intake, these individuals were all lean. Although cross-sectional, comparisons between the LLF and LHF groups allow us to explore differences that may help to explain why the LHF appears to be resistant to weight gain, despite a higher energy and dietary fat intake. Our results showed that the LHF was able to oxidise a greater amount of fat, both in the fasted state and postprandially, than that of the LLF group. It is understood that LHF phenotypes develop an adaptive response to habitual high fat intake( Reference Schrauwen, van Marken Lichtenbelt and Saris 42 , Reference Phinney 43 ), and potentially a higher metabolic rate( Reference Cooling and Blundell 15 , Reference Cooling and Blundell 31 ), which may be protective against weight gain. It has been suggested that a higher metabolic rate in high-fat compared with low-fat consumers may be explained by higher leptin levels( Reference Cooling, Barth and Blundell 16 ) and/or linked to their higher energy and protein intake (g/d)( Reference Cooling and Blundell 31 ). Our results do not, however, confirm these findings, and despite a higher energy intake, LHF consumers show similar energy expenditure to that of LLF, both in the fasted state and in response to the test meal. There were also no differences in fasting plasma glucose or leptin levels, and, although the energy intake was higher in the LHF v. LLF group, the protein intake between the lean groups was not significantly different (98 (sd 34) v. 88 (sd 32) g protein/d, respectively, P=0·076). However, in the study of Cooling & Blundell( Reference Cooling and Blundell 31 ), although the high-fat group also consumed more TE, their protein intake was almost double that of the low-fat group (high fat: 158·8 (sd 17·9) v. low fat: 80·8 (sd 7·2) g protein/d, P<0·05), which may account for the higher energy expenditure observed in the high-fat group. This highlights the potential role of a higher absolute amount of dietary protein intake in the context of a high-fat diet, conferring a protective effect against weight gain by increasing energy expenditure. Higher protein intake has been shown to be effective in countering declines in energy expenditure both during dynamic weight loss( Reference Soenen, Martens and Hochstenbach-Waelen 44 , Reference Pesta and Samuel 45 ) and in weight loss maintenance( Reference Ebbeling, Swain and Feldman 46 ).
Cross-sectional comparisons between LHF and OHF groups suggest possible explanations as to why one group is lean, whereas the other is obese, despite a similar dietary regimen. All participants were matched for habitual levels of physical activity to control for the effect this may have had on rates of fat oxidation. Overall the LHF and OHF groups showed no significant differences in the absolute rate of fat oxidation or fat balance, demonstrating similar adaptation to a high-fat diet and the standardised meal challenge. Nonetheless, there was large variability in rates of fat oxidation in response to the high-fat meal within the OHF group. In particular, two individuals showed a reduced capacity for fat oxidation, which was more in line with that demonstrated by the LLF group, despite the habitual high fat intake. Future studies should therefore investigate potential genetic or environmental factors that may explain this individual variability in dietary adaptation and response to high fat intake. This may help to further our understanding of the development of obesity and improve optimisation of individualised dietary macronutrient recommendations.
In terms of energy expenditure, as shown previously( Reference Kunz, Schorr and Römmling 47 , Reference Thomas, Peters and Reed 48 ), the OHF group demonstrated a significantly greater fasting RMR than did the LHF group, even after adjusting for FFM, and several factors may contribute to this. Both leptin and insulin are involved in signalling body energy stores and influencing energy expenditure to bring about long-term energy balance( Reference Lecoultre, Ravussin and Redman 49 – Reference Simonds, Cowley and Enriori 52 ). Both of these hormones were elevated in our OHF group, and this would be expected to moderate energy intake while increasing energy expenditure. Furthermore, although contributing only 15–20 % of the energy expenditure of each gram of FFM, the greater size of adipose tissue of obese individuals suggests that this contribution should not be overlooked( Reference Kaiyala, Morton and Leroux 53 , Reference Kaiyala and Schwartz 54 ). Finally, it has been shown that with increasing adiposity, the mass of highly metabolically active components of FFM (heart, liver and kidney) is increased, and, as a result, there is a far greater increase to RMR/kg FFM with increasing percentage FM( Reference Bosy-Westphal, Braun and Schautz 55 , Reference Pourhassan, Bosy-Westphal and Schautz 56 ). The TEF in response to the test meal was not different between the lean groups but was significantly lower for the OHF compared with the LHF group. This is believed to be the result of the study protocol that used a standardised test meal for all participants, which resulted in the OHF group consuming a lower energy intake per unit body mass. Similarly, energy balance during the postprandial period was significantly lower in the OHF than the LHF group, confirming higher rates of energy utilisation by the obese group by virtue of their greater body size and FFM. Indeed, earlier studies showed that increased meal size, along with FFM, are shown to increase the TEF( Reference Reed and Hill 57 ). It is therefore likely that differences between these two groups would have been diminished, had the study protocol assigned test meals that were normalised for body size.
There were a number of key differences in hormones involved in appetite regulation that distinguished lean and obese groups. Compared with the LHF group, the OHF group had higher plasma leptin and insulin levels, together with increased resting energy expenditure, and yet had higher hunger and disinhibition scores on the TFEQ, suggesting ‘selective leptin and insulin resistance’( Reference Heymsfield, Greenberg and Fujioka 58 , Reference Könner and Brüning 59 ). Possible mechanisms include down-regulation of hypothalamic leptin receptor expression causing resistance to leptin signalling( Reference Zhang and Scarpace 60 ) and impaired leptin transport across the blood brain barrier in obese individuals( Reference Caro, Kolaczynski and Nyce 61 ), potentially increasing the risk of diet-induced obesity. Tschop et al.( Reference Tschop, Weyer and Tataranni 62 ) also showed a negative correlation between insulin and leptin levels and the hunger hormone ghrelin. The reduced ghrelin hunger signal in the OHF group was, however, not reflected in their subjective hunger ratings, which were similar to the lean group. Postprandial hyperglycaemia in healthy overweight and obese humans has been shown to reduce PYY3–36 and ghrelin responses to a meal challenge and ‘abolish meal-induced satiety’, measured using a visual analogue scale( Reference Knudsen, Karstoft and Solomon 63 ). This suggests that blunted appetite hormone responses may be secondary to the development of obesity-related insulin resistance and may subsequently contribute to the maintenance or worsening of obesity, rather than its development. As ghrelin is also involved in nutrient partitioning, this constant signal may also promote fat storage independent of increased food intake, through the up-regulation of lipogenic enzymes in white adipose tissue( Reference Perez-Tilve, Heppner and Kirchner 64 ). Although obese subjects ate less of the post-test meal than did their lean counterparts, this may be because of the feelings of self-consciousness about body size( Reference Rogers 65 ). Therefore, it would appear that there is an impaired ability of these hormones to control short- and long-term energy intake and to restore long-term energy balance within the obese group, and, although they report similar subjective AR to the LHF group, under free-living conditions, hedonic factors may override natural satiety signals in obese individuals, causing them to overeat( Reference Blundell and Finlayson 66 ).
There were a number of limitations with this study. Subjects in this study are exclusively men so results cannot be more generally extrapolated. The study design also required that all participants ingest the same volume of the standardised test meal, unadjusted for body size or energy expenditure. Although this presents a potential limitation, our rationale is that this test meal reflects an energy dense consumer option available to free-living individuals (e.g. a standardised size, energy dense, high-fat milkshake). It then allows us to observe differences in metabolic and physiological responses to the consumption of this choice between lean and obese dietary phenotypes. For example, the muted response of appetite hormones to the meal challenge in the OHF group highlights that a standardised, energy dense, high-fat meal choice will not produce the same alteration in postprandial appetite signalling as that is shown in lean individuals. It may then be likely that obese individuals require ‘super-sized’ versions of the meal, possibly as a function of body size and composition, in order to achieve the same appetite response. Future studies should employ alternative protocols to allow the investigation of the energy and macronutrient composition required to achieve this. It is interesting to note, however, that subjective AR were similar despite test meal standardisation across groups, although this may not allude to subsequent food choices by the different groups under free-living conditions. As our design is cross-sectional, we are also unable to determine cause and effect and cannot be certain that the LHF group is protected from weight gain in the face of higher energy intake over the long term.
In conclusion, LHF consumers are adapted to their habitual dietary intake, metabolising greater amounts of fat in the fasted state and following ingestion of a high-fat meal compared with LLF consumers, which potentially enables them to remain lean. OHF consumers, however, were more insulin resistant and, despite higher leptin levels, showed selective leptin resistance with increased levels of energy expenditure, whereas their TFEQ scores suggest that they experienced greater difficulty in controlling appetite, and this may drive excess energy intake. It is notable that appetite hormone responses in obese individuals, both in the fasted state and in response to the high-fat meal challenge, were blunted in comparison with lean individuals, even after controlling for habitual dietary macronutrient intake. It therefore appears that hormonal signals responsible for reducing the drive for energy intake, are altered in obesity and insulin resistant states. Under free-living conditions this may result in increased energy intake despite adequate energy stores, thus contributing towards steady weight gain over time.
Acknowledgements
The authors would like to thank the subjects who gave of their time to participate in the trial. They are grateful to Today’s Frozen Foods, Cape Town, for their generous donation of food. They are also grateful to Lara Keytel and Judy Belonje for their expert technical assistance.
This study received no specific grant from any funding agency, commercial or not-for-profit sectors.
J. G. and E. V. L. designed the study. J. G., A. H. and C. B. carried out the study. L. C., A. H. and J. G. analysed the data. L. C., A. H., J. G., C. B. and E. V. L. contributed to the writing of the manuscript and approved the final submission.
There are no conflicts of interest to declare.










