Lutein is a non-provitamin A carotenoid that is present in many commonly consumed fruits and vegetables( Reference Calvo 1 ). Due to ingestion of these foods by animals, lutein is also present in animal products such as eggs and dairy products. As an antioxidant, lutein has the potential to protect against oxidative stress by quenching lipid peroxide radicals, and could therefore protect an individual from CVD and metabolic diseases( Reference Calvo 1 ).
Previous literature has shown associations between higher lutein intake or blood levels and a decreased risk of metabolic syndrome( Reference Beydoun, Shroff and Chen 2 – Reference Suzuki, Ito and Inoue 6 ). In particular, lutein has been shown to be inversely related to waist circumference( Reference Suzuki, Ito and Inoue 6 , Reference Waters, Clark and Greene 7 ) and BMI( Reference Sugiura, Nakamura and Ogawa 5 , Reference Waters, Clark and Greene 7 ). Nevertheless, reverse causation could apply as lutein is fat-soluble, and thus adiposity might lead to lower levels of lutein due to lutein absorption by fat tissue. However, a causal relation might also be possible, because lutein has been linked to adipocyte differentiation, and through this mechanism lutein might reduce abdominal adiposity( Reference Gunanti, Marks and Al-Mamun 8 ).
Bioavailability of lutein depends on many other dietary factors, such as the presence of dietary fibre (which inhibits lutein uptake)( Reference Yeum and Russell 9 ) and the presence of dietary fat (which increases lutein uptake)( Reference Roodenburg, Leenen and van het Hof 10 ). Dietary factors might, however, not only cause interactions but also might confound the relationship between lutein intake and cardiometabolic health. In addition, because there is a high amount of lutein in vegetables, high lutein intake might reflect the overall healthy diet, or even healthy lifestyle in general, and it is, thus, important to take other dietary and lifestyle factors into account.
We recently reviewed the literature about the relationship between lutein and cardiometabolic health and observed that, despite these suggested positive effects of lutein in adults, studies in children are scarce (ETM Leermakers, SKL Darweesh, CP Baena, EM Moreira, D Melo van Lent, MJ Tielemans, T Muka, A Vitezova, R Chowdhury, WM Bramer, JC Kiefte-de Jong, JF Felix and OH Franco, unpublished results). To our knowledge, only one article has been published so far that related lutein to cardiometabolic health in children( Reference Beydoun, Canas and Beydoun 11 ). In this cross-sectional study among 1339 US adolescents, lutein levels were not significantly associated with metabolic syndrome diagnosis, but higher level of lutein was significantly inversely associated with a continuous score of a number of metabolic syndrome components.
It is important to study the effects of nutrition early in life as dietary behaviours track throughout the life course( Reference Mikkila, Rasanen and Raitakari 12 ), and therefore early interventions can have benefits to improve health later in life. In addition, there is no recommended intake for lutein at present, and studying the effects of lutein is important from a public health perspective to determine whether recommendations are required. We aimed to assess the relationship between lutein intake at 13 months of age and cardiometabolic outcomes at the age of 6 years in a prospective population-based cohort study. The second objective was to assess whether there is an interaction of lutein intake with dietary fat and fibre intake.
Methods
Study population
The present study is a part of The Generation R Study, a population-based prospective cohort study in Rotterdam, the Netherlands, which has previously been described in detail elsewhere( Reference Jaddoe, van Duijn and Franco 13 ). This study was approved by the Medical Ethics Committee at Erasmus MC, University Medical Center, Rotterdam (MEC 198.782/2001/31). Written informed consent was obtained from all participating mothers. We restricted our analyses to Dutch children, because the FFQ was designed and validated for dietary assessment of a Dutch population. The FFQ was implemented in a later stage of the study, and was therefore available to 71 % of the total population. Children without information on dietary intake at 13 months of age (n 1775) were excluded. As blood samples were not collected from all children, the population for analysis ranged from 1305 to 2044 children, depending on the outcome of interest (Fig. 1).
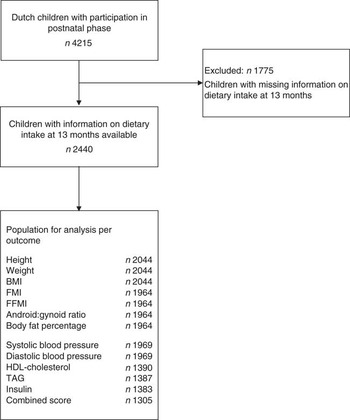
Fig. 1 Flow chart of the participants included for analysis. FMI, fat mass index; FFMI, fat-free mass index.
Dietary assessment
At the age of 13 months (median 12·9, 95 % range 12·2, 19·2), the primary caregiver of the child (mother 86·2 %, father 3·8 %, both 9·8 % or other 0·2 %) completed a 211 item semi-quantitative FFQ( Reference Kiefte-de Jong, de Vries and Bleeker 14 ). The FFQ asked for habitual diet of the last month, thereby covering diet from the age of 12 months onwards. At the dietary assessment, only 6·8 % of the children received any breast-feeding and all the children received complementary feeding. The FFQ was developed in co-operation with the division of Human Nutrition of Wageningen University, The Netherlands. The final FFQ included questions on the frequency of consumption of the food items over the last month, the serving sizes, the type of the food item and the preparation methods – for example, for the types of vegetables, it was assessed whether they were cooked or stir-fried or whether they were raw. For each type of fruit and vegetables, it was asked whether it was consumed ‘Never’, ‘Sometimes’, ‘Often’ or ‘Always’, and consumption was weighted accordingly. Based on frequencies and servings, the food items were converted into g per d using standardised portion sizes( Reference Donders-Engelen, Heijden van der and Hulshof 15 ). For all individual items of fruits and vegetables, as well as for eggs and dairy products, lutein values per gram were extracted from The Dutch Food Composition Table (NEVO)( 16 ). This FFQ was validated against three 24-h recalls in a representative sample of Dutch children (n 32), which showed the following intra-class correlation coefficients for macronutrients: total energy, 0·4; total protein, 0·7; total fat, 0·4; carbohydrates, 0·4; and dietary fibre, 0·7; and for micronutrients: calcium, 0·53; iron, 0·48; vitamin C, 0·63; vitamin D, 0·74; and vitamin E, 0·42( Reference Kiefte-de Jong, de Vries and Bleeker 14 , Reference Voortman, Kiefte-de Jong and Geelen 17 ). In a later phase of the study, dietary lutein intake was estimated from this FFQ data, based on the Dutch Food Composition Table (NEVO)( 16 ), which included lutein intake from fruits and vegetables, as well as eggs and dairy products. As our objective was to assess the effects of lutein intake, we assessed lutein independent of β-carotene. The correlation between intake of lutein and intake of β-carotene was 0·94; thus, adjusting for β-carotene in the regression model was not possible due to multicollinearity. Therefore, we standardised lutein intake for β-carotene using the residual method( Reference Willett, Howe and Kushi 18 ), together with total energy to account for measurement error( Reference Kipnis, Subar and Midthune 19 ). sd scores (SDS) for (β-carotene-standardised and energy-standardised) lutein intake were created and SDS of lutein intake were categorised into quartiles. The median absolute intake of lutein within each quartile was 264, 766, 1470 and 2274 mcg/d, respectively.
The Supplementary material shows the results for lutein unstandardised for β-carotene (online Supplementary Tables S1B and S2B) and the results for β-carotene individually (online Supplementary Tables S1C and S2C).
Cardiometabolic outcomes
At a median age of 5·9 (95 % range 5·7, 6·5) years, children visited our dedicated research facility at the Erasmus Medical Center, Sophia’s Children Hospital. Weight and height were measured (without shoes and heavy clothing) using an electronic scale (SECA) and stadiometer (Holtain Limited). Total and regional fat mass was measured by Dual-energy X-ray absorptiometry scans (iDXA; General Electric, 2008)( Reference Kaul, Rothney and Peters 20 ). Percentage body fat was calculated as 100 %×(total body fat mass (g))/(total body mass (fat mass+lean mass+bone mass) (g)), android:gynoid fat mass ratio was calculated as (abdominal fat mass (g)/fat mass around hips, thighs and buttocks (g)). The fat-free mass index was calculated as (lean mass (kg)+bone mass (kg))/(height2 (m)). The fat mass index was calculated as (fat mass (kg))/(height2 (m)). Age- and sex-specific SDS were calculated for all outcomes based on the total population.
Non-fasting blood samples were drawn by antecubital venepuncture. Insulin, C-peptide, cholesterol (total, HDL and LDL) and TAG concentrations were measured using enzymatic methods (using a Cobas 8000 analyser; Roche). Quality-control samples demonstrated intra-assay and inter-assay coefficients of variation ranging from 0·69 to 1·57 %.
Systolic and diastolic blood pressures (SBP and DBP) were measured at the right brachial artery with the child in the supine position, using the validated automatic sphygmomanometer Datascope Accutor Plus TM( Reference Wong, Sung and Leung 21 ). Blood pressure was measured four times with 1-min intervals, and the first measurement was excluded for the calculation of mean SBP and DBP. Mean arterial pressure (MAP) was calculated as MAP=(mean SBP+2×mean DBP)/3. In addition to the individual cardiometabolic outcomes, we calculated a continuous score following examples of previously defined metabolic syndrome scores for children( Reference Eisenmann 22 ), including body fat percentage, blood pressure (DBP and SBP) and serum levels of HDL-C, TAG and insulin. The cardiometabolic risk factor score was calculated as the sum of age- and sex-specific SDS of these five components, as proposed previously for paediatric populations( Reference Eisenmann 22 ). The SDS for HDL-C were multiplied by –1 as a higher HDL-C represents a better cardiometabolic profile. The SDS for SBP and DBP were multiplied by 0·5 so that each contributed half to the blood pressure component. In summary, the cardiometabolic risk factor score was calculated as: SDS BF %+0·5×SDS SBP+0·5×DBP+SDS TAG+(–1×SDS HDL-C)+SDS insulin, and was standardised to facilitate interpretation of effect estimates.
Covariates
Information on maternal and paternal age, household income, educational level, maternal parity, smoking, alcohol use and folic acid use during pregnancy was obtained from questionnaires during pregnancy. Maternal and paternal weight and height were measured at enrolment and BMI was calculated (kg/m2). Information on sex, gestational age at birth and birth weight of the children was obtained from midwife and hospital registries. Information about breast-feeding, introduction of complementary feeding, history of allergy to cows’ milk and any hospitalisation in 1st year of life was derived from questionnaires at 6 and 12 months of age. The number of hours spent watching television was used as a proxy for sedentary lifestyle of the child and was derived from the questionnaire at 2 years of age. Participation in sports was used as a proxy for physical activity of the child and was derived from the questionnaire at 6 years of age. Total energy intake, food intake and nutrient intake were derived from the FFQ at 13 months. We used a modified version of the diet quality score as previously constructed by Voortman et al. ( Reference Voortman, Kiefte-de Jong and Geelen 17 ), including components for cereals, potatoes, fish, fats, sugar-containing beverages and snacks and candy.
Statistical analysis
Age- and sex-specific SDS were created for all outcomes. Non-normally distributed variables were transformed before standardising. Lutein was not normally disturbed, and was therefore square root transformed. We used linear regression to estimate the sd change in cardiometabolic outcomes for each quartile of lutein intake, using the lowest intake as reference category. We tested for a linear relationship using lutein intake (SDS) as a continuous variable.
Crude models were adjusted for the child’s age at the dietary assessment. Multivariable models were additionally adjusted for potential confounders that were selected on the basis of the literature. The following confounders were included: maternal and paternal age and BMI, household income, maternal education, parity, smoking, alcohol and folic acid use during pregnancy, child’s sex, birth weight (SDS), gestational age at birth, breast-feeding, timing of introduction to solid foods, hospitalisation in the 1st year of life, allergy to cows’ milk, age at dietary assessment, total energy intake, diet quality score, watching television at age of 2 years and playing sports at the age of 6 years.
Analyses with android:gynoid fat ratio, body fat percentage or blood pressure as outcome were adjusted for child height (SDS) at the age of 6 years. We tested for interactions between lutein intake, dietary fat intake and dietary fibre intake as these factors may interact with lutein in relation to cardiometabolic health. In addition, as there may be sex differences in factors associated with childhood obesity( Reference Govindan, Gurm and Mohan 23 ), we assessed possible heterogeneity effect by sex. These interactions were tested in the multivariable model with lutein intake (SDS) as the continuous variable.
To reduce potential bias associated with attrition, a multiple imputation procedure was performed to impute missing values on covariates( Reference Sterne, White and Carlin 24 ). Missing values on exposures and outcomes were not imputed. We used the Fully Conditional Specification method (predictive mean matching), assuming no monotone missing pattern( Reference Sterne, White and Carlin 24 ). Auxiliary variables were used to improve the model (online Supplementary material). We created ten multiply imputed data sets instead of five (default) to improve the variance. Analyses were performed in each of the ten imputed data sets separately, and the final results were pooled. Regression coefficients were pooled by taking the mean coefficient of the ten imputed data sets. The pooled standard errors were calculated according to Rubin’s rules( Reference Rubin and Schenker 25 ). Characteristics of the children before and after multiple imputation are shown in the online Supplementary material. A P-value<0·05 was considered as statistically significant. Statistical analyses were performed by E. T. M. L. using SPSS, version 21.0 (SPSS Inc.).
Results
Subject characteristics
Median energy intake of the children at 13 months of age was 5301.1 kJ/d (1267 kcal/d), with a median lutein intake of 1317 mcg/d (95 % range 87, 6069 mcg/d) (Table 1). The main contributors to lutein intake were spinach, broccoli, Brussels sprouts, green beans, curly kale, legumes and fruits, which explained in total 67 % of the variation in lutein intake. All these products, except for curly kale, also significantly contributed to β-carotene intake, in addition to carrots and dairy products, and explained in total 79 % of the variation in β-carotene intake.
Table 1 Basic characteristics of the children (Mean values and standard deviations; medians and 95 % ranges; numbers and percentages)

FMI, fat mass index; FFMI, fat-free mass index.
Body composition
There were no significant associations between lutein intake and height, weight, fat mass index, fat-free mass index, android:gynoid ratio and body fat percentage. There was no significant linear association between lutein intake and BMI, and there were no consistent associations across quartiles of lutein intake (Table 2). The highest quartile of lutein intake was associated with higher BMI, and the crude associations (online Supplementary material) suggest that this increase in BMI was driven by an increase in the fat-free mass index.
Table 2 Association of lutein intake at 13 months with body composition at the age of 6 yearsFootnote † (Regression coefficients and 95 % confidence intervals)

AG, android:gynoid; FMI, fat mass index; FFMI, fat-free mass index; Ref., reference; SDS, sd score.
* P<0·05.
† Linear regression coefficients reflect the difference in outcome (age- and sex-specific SDS) for mid-low, mid-high and high intake, as compared with low intake of energy and β-carotene-adjusted lutein intake.
Continuous model reflects the difference in outcome (age- and sex-specific SDS) per sd increase of energy and β-carotene-adjusted lutein intake. Models are adjusted for maternal and paternal age and BMI, household income, maternal education, parity, smoking, alcohol and folic acid use during pregnancy, child sex, birth weight (SDS), gestational age at birth, breast-feeding, timing of introduction of solids, hospitalisation in the 1st year of life, allergy to cows’ milk, age at dietary assessment, total energy intake, diet quality score, television watching at age 2 years and playing sports at age 6 years. Models for android:gynoid ratio and body fat percentage are additionally adjusted for child height.
Cardiometabolic risk factors
There was no association between lutein intake at 13 months of age and the cardiometabolic risk factor score at the age of 6 years (Table 3). In addition, there were no significant linear associations between lutein intake and any of the individual components of the cardiometabolic risk factor score, nor were there consistent associations across quartiles of lutein intake. In addition to the components of the metabolic syndrome score, we examined total cholesterol, LDL-cholesterol, C-peptide, MAP and pulse wave velocity, and found no significant associations (data not shown). Crude associations are shown in the online Supplementary material.
Table 3 Association of lutein intake at 13 months of age with cardiometabolic outcomes at the age of 6 yearsFootnote †(Regression coefficients and 95 % confidence intervals)

Ref., reference; SDS, sd score.
** P<0·01.
† Linear regression coefficients reflect the difference in outcome (age- and sex-specific SDS) for mid-low, mid-high and high intake, as compared with low intake of energy and β-carotene-adjusted lutein intake.
Continuous model reflects the difference in outcome (age- and sex-specific SDS) per SD increase of energy and β-carotene-adjusted lutein intake. Models are adjusted for maternal and paternal age and BMI, household income, maternal education, parity, smoking, alcohol and folic acid use during pregnancy, child sex, birth weight (SDS), gestational age at birth, breast-feeding, timing of introduction of solids, hospitalisation in the 1st year of life, allergy to cows’ milk, age at dietary assessment, total energy intake, diet quality score, television watching at age 2 years and playing sports at age 6 years. Models for systolic and diastolic blood pressure are additionally adjusted for child height.
Interactions
We found a significant interaction between lutein intake and fat intake on HDL-cholesterol levels, but stratification for below and above the median of total fat intake showed no significant associations.
We found a significant interaction between lutein intake and fibre intake on weight. After stratification for above and below the median of fibre intake, we observed that there was a significant positive association between lutein intake and weight in the group with fibre intake below the median (increase in weight SDS 0·06 (95 % CI 0·02, 0·11)).
We also found significant sex interactions for weight, for body fat percentage and diastolic blood pressure. Stratification for sex showed that there was a significant positive association between lutein intake and weight in girls (0·06 sd (95 % CI 0·02, 0·11)), but not in boys. Stratification for sex showed no significant associations between lutein intake and body fat percentage or diastolic blood pressure in either boys or girls. There were no significant interactions between any of the other outcomes.
Discussion
In a population-based prospective cohort study, we assessed whether lutein intake at 13 months of age was associated with cardiometabolic outcomes at the age of 6 years and whether there could be an interaction of lutein intake with sex, dietary fat intake and dietary fibre intake. We observed that there were no consistent associations between lutein intake in toddlers and a cardiometabolic risk factor score at the age of 6 years, nor with any of the individual cardiometabolic risk factors. In addition, there were no consistent interactions with factors that may influence cardiometabolic risk or bioavailability of lutein such as sex, dietary fat intake and dietary fibre intake.
Our findings that lutein was not associated with cardiometabolic health is different from those from previous studies, although all the studies were conducted in older populations. Previous studies in adults have shown that higher lutein intake was associated with lower risk of metabolic syndrome( Reference Beydoun, Shroff and Chen 2 – Reference Suzuki, Ito and Inoue 6 ). Only one of these studies assessed dietary intake( Reference Sluijs, Beulens and Grobbee 4 ), and all the other studies used blood levels of lutein. In addition, the only study in adolescents that assessed lutein intake in relation to cardiometabolic health used blood levels of lutein, and observed that higher lutein levels were associated with a lower metabolic syndrome score( Reference Beydoun, Canas and Beydoun 11 ).
As compared with the use of dietary intake levels, the use of blood levels of lutein might provide a more direct measurement of how much lutein is available. Absorption of lutein in the body depends on multiple factors, including other dietary factors such as fibre( Reference Yeum and Russell 9 ) and fat( Reference Roodenburg, Leenen and van het Hof 10 ). In our study, we observed significant interactions of lutein intake with fibre and fat intakes with some outcomes, which could indeed be due to the fact that the bioavailability of lutein depends on dietary fibre and fat, and these factors are thus important to be taken into account in studies assessing dietary lutein. However, studies that assess blood levels of lutein already have a direct measure of the available lutein. Furthermore, when lutein is measured in the blood, other factors that affect bioavailability are taken into account as well. This is of particular benefit because there are large inter-individual differences in the bioavailability of lutein( Reference Gruber, Chappell and Millen 26 ), which are only partly explained by known factors that we can adjust for (e.g. age and sex), but unmeasured factors (e.g. genetic variance) might play an equally important role( Reference Gruber, Chappell and Millen 26 , Reference Borel, Desmarchelier and Nowicki 27 ). All these factors contribute to the fact that the correlation between lutein intake and lutein levels is low( Reference Gruber, Chappell and Millen 26 ). Dietary lutein is, therefore, not a direct marker of lutein status, which might explain why we did not observe beneficial effects of dietary intake of lutein.
However, the use of blood levels can also have challenges. As blood levels of antioxidants are being used to counteract oxidative stress, disease processes with high oxidative stress may result in depletion of lutein levels, and lower levels of lutein in metabolic disorders might thus be a result of these processes( Reference Ford, Mokdad and Giles 28 ). This reverse causation is of particular concern in cross-sectional studies, when there could already be an active disease process. However, in pre-clinical stages of diseases, there might already be increased oxidative stress, and thus lower levels of antioxidants. For example, it has been shown that subjects with obesity or metabolic syndrome have higher levels of oxidised LDL, a marker of oxidative stress, that might deplete antioxidant levels( Reference Van Guilder, Hoetzer and Greiner 29 ). Moreover, it is important to address this topic in studies with sufficiently long follow-up periods. Studies assessing lutein in relation to metabolic syndrome were all cross-sectional, and thus the inverse relationships that were observed could potentially be explained by reverse causation. Prospective studies in adults that investigated lutein (levels or intake) in relation to risk of type 2 diabetes found inconsistent results( Reference Hozawa, Jacobs and Steffes 30 – Reference Wang, Liu and Pradhan 33 ), and these studies all had a follow-up period of >10 years, and are thus less prone to risk of reverse causation.
The large sample size and the prospective longitudinal design are important strengths of our study. In addition, we were able to adjust for a wide range of potential confounders such as socio-economic and other dietary and lifestyle factors. It is important to note that all studies on lutein in relation metabolic syndrome and diabetes in adults were observational. Besides issues with reverse causation, observational studies are subject to confounding. As nutritional and lifestyle factors are clustered( Reference Leech, McNaughton and Timperio 34 ), and intakes of nutrients are highly correlated, it is difficult to conclude from observational studies that any beneficial effect that is found is attributable to one specific nutrient. In the case of lutein, it could also be that not only lutein was responsible for the observed effect but also another carotenoid, the group of carotenoids as a whole, or other nutrients, foods or nutritional factors that are correlated with lutein intake could have been responsible. Indeed, we found in our study that β-carotene and lutein were highly correlated. In addition to dietary factors, socio-economic and lifestyle variables such as sedentary behaviour and physical activity are highly related to diet, and could often not be fully adjusted for in these studies.
Another possible explanation for our null findings is that we were unable to detect a relevant difference in our study. It may be argued that associations between lutein and health are easier to detect in studies where extremely low lutein intake is present among participants, and that our study had negative findings due to a relatively high lutein intake in our population. Unfortunately, there are no dietary recommendations regarding lutein intake to establish whether children have sufficient intake. Moreover, there are no other studies on lutein intake in young children in relation to cardiometabolic health, but lutein intake in our study was comparable with a study on 6-year-old Canadian children, which studied lutein in relation to cognitive function( Reference Mulder, Innis and Rasmussen 35 ). In addition, it can be argued that diet in early life may be subject to large changes. However, we used ranking instead of absolute values, and it has been shown that diet during early life tracks into later life( Reference Craigie, Lake and Kelly 36 – Reference Schwartz, Scholtens and Lalanne 38 ).
We assessed lutein intake with the use of an FFQ, and therefore measurement error in dietary intake may be present( Reference Kipnis, Subar and Midthune 19 ). Although the FFQ that we used showed good validation against 24-h recalls for some micronutrients, we have no validation measure for lutein specifically, and we did not have blood levels of lutein to assess the correlation with estimated intake of lutein. We standardised lutein intake for total energy using the residual method( Reference Willett, Howe and Kushi 18 ), which reduces the magnitude of systematic measurement error( Reference Kipnis, Subar and Midthune 19 ). However, random error may still be present. For example, we had information on preparation method (cooking, stir-frying) for vegetables, but no information on cooking time. Moreover, lutein values might vary within products. For example, nutritional values of eggs are influenced by the diet of the hens( Reference Leeson and Caston 39 ). As this random error generally leads to bias towards the null, this could, therefore, have led to an underestimation of the true effect(40).
We found significant interactions of lutein with fat intake, fibre intake and sex on several outcomes, but not on all. We might have been underpowered to detect possible interactions on other outcomes; therefore, the results should be interpreted with caution. In addition, we looked at the interaction with total fat or fibre intake, although a possible interaction might depend on which point in time they are consumed.
Finally, for this analysis, we included only Dutch children with complete nutritional data and a visit to the research centre at the age of 6 years. Although this approach reduces heterogeneity of the study population and related measurement of diet, it may decrease the generalisability of our results.
In conclusion, although earlier studies in adults did show a beneficial effect of lutein on cardiometabolic health, we found no association between lutein intake at the age of 1 year and cardiometabolic outcomes in children at 6 years of age. However, this was the first study in this age-group, and further studies are needed to elucidate the role of lutein in young children.
Acknowledgements
The Generation R Study is conducted by the Erasmus Medical Center in close collaboration with the School of Law and Faculty of Social Sciences of the Erasmus University Rotterdam, the Municipal Health Service Rotterdam area, the Rotterdam Homecare Foundation and the Stichting Trombosedienst and Artsenlaboratorium Rijnmond (STAR). The authors gratefully acknowledge the contribution of participating mothers and children, general practitioners, hospitals, midwives and pharmacies in Rotterdam.
The Generation R Study was made possible by the financial support from the Erasmus Medical Center, Rotterdam, the Erasmus University Rotterdam and the Netherlands Organization for Health Research and Development. V. W. V. J. received an additional grant from the Netherlands Organization for Health Research and Development (ZonMw – VIDI 016.136.361). E. T. M. L., O. H. F. and J. C. K.-d J. work in ErasmusAGE, a centre for ageing research across the life course funded by Nestlé Nutrition (Nestec Ltd), Metagenics Inc. and AXA. Nestlé Nutrition (Nestec Ltd), Metagenics Inc. and AXA had no role in design and conduct of the study, collection, management, analysis and interpretation of the data as well as preparation, review or approval of the manuscript.
E. T. M. L., J. C. K.-d. J., A. H., V. W. V. J. and O. H. F. designed the research. E. T. M. L., J. C. K.-d. J., A. H., V. W. V. J. and O. H. F. conducted the research. A. H., V. W. V. J. and O. H. F. provided essential materials. E. T. M. L. analysed the data. E. T. M. L. and J. C. K.-d. J. wrote the paper. J. C. K.-d. J. had primary responsibility for the final content. All the authors read and approved the final version of the manuscript.
There are no conflicts of interest.
Supplementary Material
For supplementary material/s referred to in this article, please visit http://dx.doi.org/doi:10.1017/S0007114515002779









