Introduction
Endovascular therapy (EVT) is highly efficacious for acute ischemic stroke due to large-vessel occlusion, Reference Goyal, Menon and van Zwam1 up to 24-hours post-onset in select cases. Reference Nogueira, Jadhav and Haussen2,Reference Albers, Marks and Kemp3 Thrombolysis with alteplase is efficacious in ischemic stroke within 4.5 hours of onset Reference Emberson, Lees and Lyden4 and in selected patients in later time windows. Reference Ma, Campbell and Parsons5,Reference Thomalla, Simonsen and Boutitie6 However, randomized evidence is lacking in patients with multiple comorbidities or pre-stroke disability, who have generally been excluded from stroke trials. Reference Ganesh and Goyal7 For instance, most EVT/alteplase trials excluded patients with premorbid modified Rankin Scale (mRS) score ≥2, although they may constitute up to one-third of all patients with ischemic stroke. Reference Quinn, Taylor-Rowan and Coyte8 This approach is not based on any mechanistic hypothesis about reduced benefit, but simply reflects the fact that such patients cannot contribute to typical dichotomy-based definitions of favorable outcome (e.g. mRS 0–1 or 0–2). Reference Ganesh, Luengo-Fernandez, Wharton, Rothwell and Oxford Vascular9 Nevertheless, comorbidities and premorbid disability will commonly cluster together (as comorbid illness commonly causes disability) and are often cited as a reason for excluding patients from acute therapies. Reference Cappellari, Bosco and Forlivesi10
The reticence to treat patients with high prestroke disability/comorbidity is partly due to their poorer prognosis, with registry data reporting high mortality and complication rates. Reference Quinn, Taylor-Rowan and Coyte8,Reference Kwok, Clark and Ford11 Although post-stroke mortality has improved over the past two decades, comorbidity burden remains a strong prognostic factor. Reference Schmidt, Jacobsen, Johnsen, Botker and Sorensen12 Comorbidities like hypertension and diabetes are associated with poorer outcomes after thrombolysis or EVT, particularly among older patients. Reference Borggrefe, Gluck and Maus13–Reference Lu, Ren, Zhang, Zu and Shi16 However, stroke commonly occurs in patients with multiple comorbidities, and in acute stroke settings, the prognosis of these conditions may be elusive. Reference Gallacher, Jani, Hanlon, Nicholl and Mair17 Decisions to treat with EVT/alteplase will partly depend on how physicians weigh potential benefits and risks in light of the patients’ baseline status, comorbidities, and/or life expectancy. Therefore, we explored decision-making by physicians regarding EVT and/or thrombolysis in the setting of pre-stroke comorbidities/disability.
Methods
We conducted an international cross-sectional web-based survey (UNMASK-EVT) Reference Saposnik, Menon and Kashani18 of stroke physicians to understand their current treatment practice and EVT/thrombolysis decision-making in acute stroke. Participants were assigned to 10 case scenarios from a pool of 22 and were asked how they would treat the patient in the given scenario, under their current practice conditions and under ideal conditions (i.e. assuming the absence of any local resource limitations or other practice constraints). Case scenarios were developed by consensus of the steering committee and were designed to represent common clinical situations. Each scenario was written to represent either a clear standard of care indication (guideline based) for treatment or a deviation from that standard of care, so that we could specifically assess how respondents interpreted the indications for treatment. The survey was distributed in waves by country/region leads in each region of the world to each region’s available distribution list. Responses were obtained from November 26, 2017 to March 27, 2018. Approval was obtained from the local research ethics board, and participants provided electronic informed consent.
In total, 1330 stroke physicians (neurologists, interventional neuroradiologists, neurosurgeons, internists, geriatricians, and other physicians [including emergency] directly involved in acute stroke care) from 38 countries were invited to participate. Whereas geriatricians and internists may not play a role in acute stroke decision-making in North American centers, they often serve as acute stroke physicians in countries like the United Kingdom; consequently, some stroke physicians in our study from such practice settings were geriatricians and internists. Reference Oliver and Burns19 No restrictions with respect to case volume or experience levels were applied, and participants had both academic and non-academic backgrounds.
Participants were simply asked to provide their favored treatment approach to 10 randomly assigned case scenarios, five involving major comorbidities (A: Stage-IV metastatic prostate cancer, B: heart failure, Chronic Obstructive Pulmonary Disease (COPD), and renal insufficiency requiring dialysis, C: non-metastatic prostate cancer with anti-hormonal treatment, D: non-disabling (mild) cognitive impairment, and E: rheumatoid arthritis (RA) requiring care in a nursing home but without cognitive impairment). All five patients had proximal Internal Carotid Artery (ICA)/Middle Cerebral Artery (MCA) occlusions and were otherwise eligible for treatment with both alteplase and EVT. Of the other 17 scenarios, six were designed to represent “level-1A” evidence for which treatment with EVT and alteplase was clearly indicated per current AHA/ASA guidelines (see supplementary appendix). Reference Powers, Rabinstein and Ackerson20
Statistical Analysis
The proportions of respondents who decided to proceed with EVT were determined and compared across scenarios to determine if presence of comorbidities would deter the physicians from selecting EVT. We first compared the proportion of respondents favoring EVT for the five comorbidity-related scenarios (examining the scenarios together and individually) to that for the six 1A scenarios and then to that for all the remaining 17 scenarios.
We used logistic regression to determine the influence of each comorbidity-related factor (cancer [none, managed with hormonal therapy, vs metastatic], multiple comorbidities, non-disabling cognitive impairment, and dependent functional status with preserved cognition) on EVT decision rates under current resources and under assumed ideal conditions, unadjusted and adjusted for other scenario characteristics: baseline Alberta Stroke Program Early CT Score (ASPECTS), Reference Barber, Demchuk, Zhang and Buchan21 onset-to-presentation time, patient age, sex, National Institutes of Health Stroke Scale score, and occlusion site (ICA, M1, proximal or distal M2). We first did this analysis including the responses for only one comorbidity-related scenario at a time (examining the influence of each individual premorbid illness/disability factor) and then all the five scenarios (adjusting for all comorbidity-related factors), together with the “ideal” 1 A scenarios alone. We then repeated this process including the comorbidity-related and all 17 non-comorbidity-related scenarios, to see if the influence of comorbidity-related factors was different on accounting for all the other potentially “non-ideal” factors that may be encountered in practice. We used logistic regression to determine the influence of respondent characteristics (age, sex, speciality, region, hospital type [teaching/non-teaching], EVT cases/year) in univariable and multi-variable models, on EVT decision for the five comorbidity-related scenarios examined together and individually. Since respondents may have answered more or less of the same types of questions owing to the random scenario assignment, we adjusted the covariance matrix of the logistic regressions to account for lack of independence of observation within-respondent (or within-respondent correlations).
Similar analyses were performed to examine the decision to provide alteplase, with/without EVT.
Statistical significance was defined as p < 0.05. Analyses were performed in STATA 13.1. Figures were created using Microsoft Power BI and the Mapbox Visual Plugin.
Results
We received responses from 607 physicians from 38 countries in different specialties. They provided 6070 scenario-based responses, with 1379 for five comorbidity-related scenarios (Table 1). Patients in comorbidity-related scenarios were generally older. Respondents had a median age of 44 years (interquartile range 39–50, Table 1), 15.9% were women, and the main specialities were neurology (53.7%), neurosurgery (13.3%), interventional neuroradiology (28.5%), and geriatrics or internal medicine (1.2%).
Table 1: Baseline scenario and respondent characteristics for the comorbidity-related case scenarios
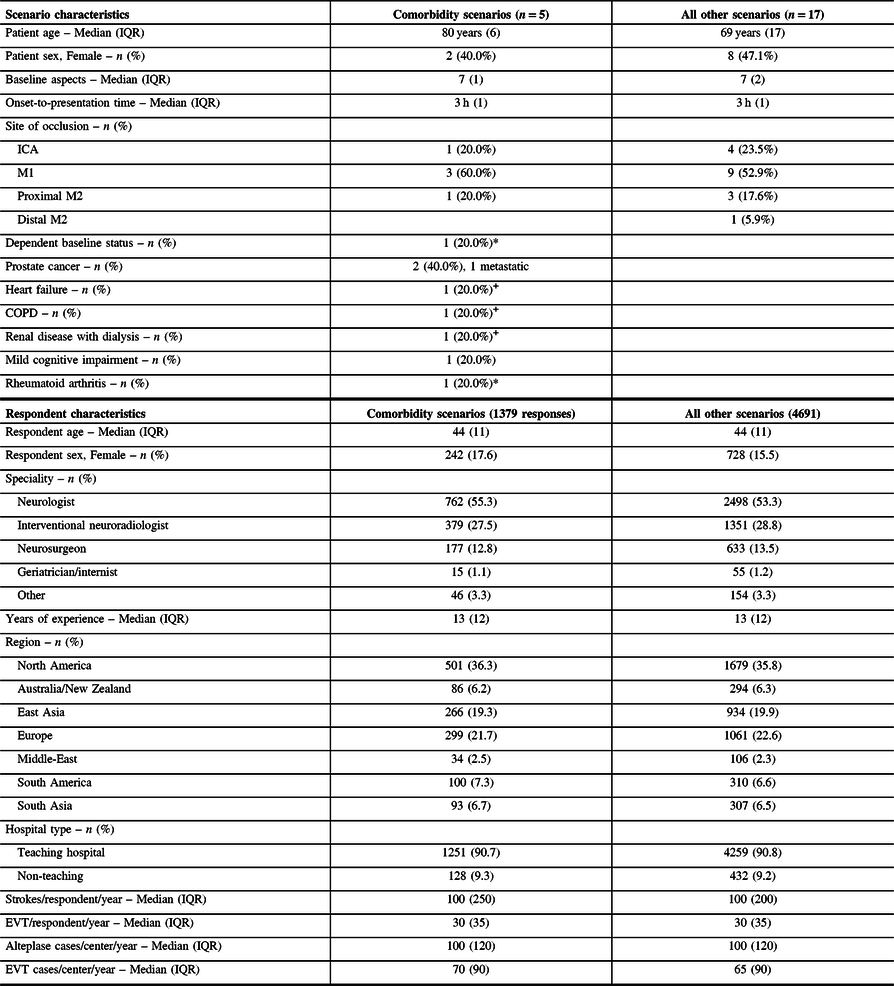
Items tagged with a * or + were represented in the same scenario (each symbol being one scenario).
Respondents favored EVT in 1097/1379 (79.6%) responses for the comorbidity-related scenarios under current resources and in 1140 (82.7%) under assumed ideal conditions; these rates were lower than those for six scenarios with level-1A evidence (for which, EVT and alteplase were clearly indicated per current guidelines): 1510/1657 (91.1%, odds ratio [OR]: 0.38, 95% confidence interval [CI] 0.31–0.47) and 1575 (95.1%, OR: 0.25, 0.19–0.33), respectively. Proportions favoring intravenous alteplase did not differ between comorbidity-related and level-1A scenarios (under current resources: 75.0% vs 72.2%, OR: 1.15, 0.99–1.33; assuming ideal conditions: 72.2% vs 69.5%, OR: 1.14, 0.99–1.31). Comparing each individual comorbidity-related scenario to level-1A scenarios, rates favoring EVT were lower for scenarios A (metastatic prostate cancer), C (non-metastatic prostate cancer), and D (mild cognitive impairment/MCI) under current resources and when assuming ideal conditions (Supplementary Table 1). Corresponding rates favoring alteplase (with/without EVT) were lower for scenario A (metastatic prostate cancer, Supplementary Table 2) but higher for C (non-metastatic cancer) and E (dependent in nursing home), the latter difference explained by more respondents choosing EVT without alteplase for some 1-A scenarios. When all comorbidity-related factors were considered in adjusted logistic regressions including comorbidity-related and level-1A scenarios (Supplementary Table 3), D (MCI) was the only scenario with lower EVT odds. On similar analyses for alteplase decisions, all scenarios except D (MCI) were associated with lower alteplase odds (Supplementary Table 4); respondents more often chose alteplase alone with MCI. That being said, this alteplase model had a very wide range of ORs, warranting a cautious interpretation.
In contrast, response rates favoring EVT with comorbidity-related scenarios were higher than those seen when considering all the remaining scenarios (Figure 1): 3489/4691 (74.4%, OR: 1.34, 1.17–1.54) under current resources and 3653 (77.9%, OR: 1.36, 1.17–1.57) assuming ideal conditions, corresponding numbers for alteplase were 2832 (60.4%, OR: 1.97, 1.73–2.24) and 2722 (58.0%, OR: 1.87, 1.66–2.12). Respondents favored treatment as or more frequently in each of the five comorbidity-related scenarios versus all others, except D (MCI) for EVT (Supplementary Table 5) and A (metastatic cancer) for alteplase (Supplementary Table 6). When all comorbidity-related factors were considered in adjusted logistic regressions, MCI and dependent status were associated with higher odds of favoring EVT (Table 2) and alteplase (Supplementary Table 7), while the rest of the comorbidities (metastatic/non-metastatic cancer and multi-system dysfunction [B]) were associated with lower alteplase odds. Older age and female sex were associated with lower EVT odds, but while female sex was also associated with lower alteplase odds, older age was associated with higher odds.
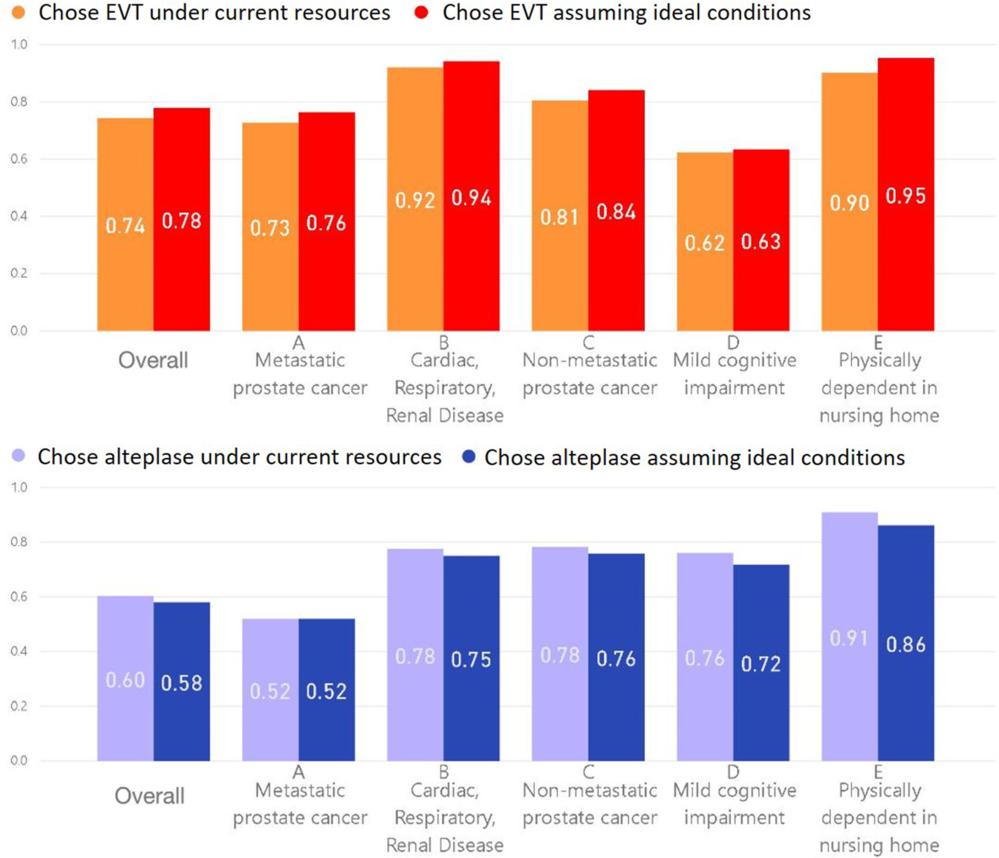
Figure 1: The proportion of respondents who chose EVT and alteplase under current resources (i.e. assuming their current practice conditions) and when assuming ideal conditions (i.e. assuming the absence of any local resource limitations or other practice constraints) for all scenarios (overall) and for each of the five comorbidity-related scenarios.
Table 2: Multivariable logistic regression for the association of comorbidity-related factors (bolded) with the decision to pursue EVT under current resources and under assumed ideal conditions, adjusted for key scenario characteristics, containing all comorbidity-related and non-comorbidity-related scenarios (5792 responses, P > χ 2 < 0.0001)

On multivariable logistic regression examining EVT decision-making for the comorbidity-related scenarios, adjusted for respondent characteristics (Table 3), EVT odds were higher with more EVT cases/year reported per respondent and center, with practicing in East Asia versus North America, and lower with more alteplase cases/center/year. With assuming ideal conditions, practising in East Asia remained significant. Compared to respondents in East Asia, those in South Asia were less likely to choose EVT under current resources (adjusted odds ratio [aOR]: 0.33, 0.16–0.67, p = 0.002, regional heterogeneity illustrated in Figure 2), but this difference was attenuated on assuming ideal conditions (aOR: 0.81, 0.35–1.85, p = 0.61). On similar multivariable regression for alteplase decisions (Supplementary Table 8), alteplase odds were lower with interventional neuroradiologists vs neurologists (both under current resources and assuming ideal conditions), more EVT cases/center/year, and female versus male respondents (under current resources). On examining each individual scenario, EVT odds were higher under current resources for neuroradiologists and neurosurgeons versus neurologists in scenario A (metastatic cancer, e.g., aOR [neuroradiologist]: 3.31, 1.15–9.53), with more EVT cases/respondent/year in scenarios C (non-metastatic cancer, aOR: 1.03, 1.003–1.05) and D (MCI), and with practising in East Asia in scenario D (MCI, aOR vs North America: 3.51, 1.41–8.75). Although respondents in Australia/New Zealand also seemed to favor EVT more than those in other parts of the world (78.6% vs 60% in North America, Figure 2), higher EVT odds were not seen for this region on multivariable analysis. EVT odds were lower under current resources with more alteplase cases/center/year in scenarios A (metastatic prostate cancer) and B (heart failure/COPD/dialysis, aOR: 0.995, 0.992–0.999), and with geriatricians/internists versus neurologists (aOR: 0.04, 0.005–0.35) or practicing in Australia/New Zealand in scenario E (nursing home with RA; aOR vs North America: 0.08, 0.02–0.40). Alteplase odds were lower with more EVT cases/center/year in scenario A (metastatic cancer, aOR [under current resources]: 0.993, 0.986–0.999), with interventional neuroradiologists in scenario B (heart failure/COPD/dialysis, aOR vs neurologists: 0.30, 0.11–0.82), with geriatricians/internists in scenario E (nursing home with RA; aOR [assuming ideal conditions]: 0.05, 0.004–0.61), and with practicing in Europe versus North America in scenarios A (metastatic cancer, aOR [assuming ideal conditions]: 0.34, 0.15–0.79) and B (heart failure/COPD/dialysis).
Table 3: Multivariable logistic regression for the association of respondent characteristics with the decision to pursue EVT for the five comorbidity-related scenarios under current resources and under assumed ideal conditions (1379 responses, P > χ 2 < 0.0001)

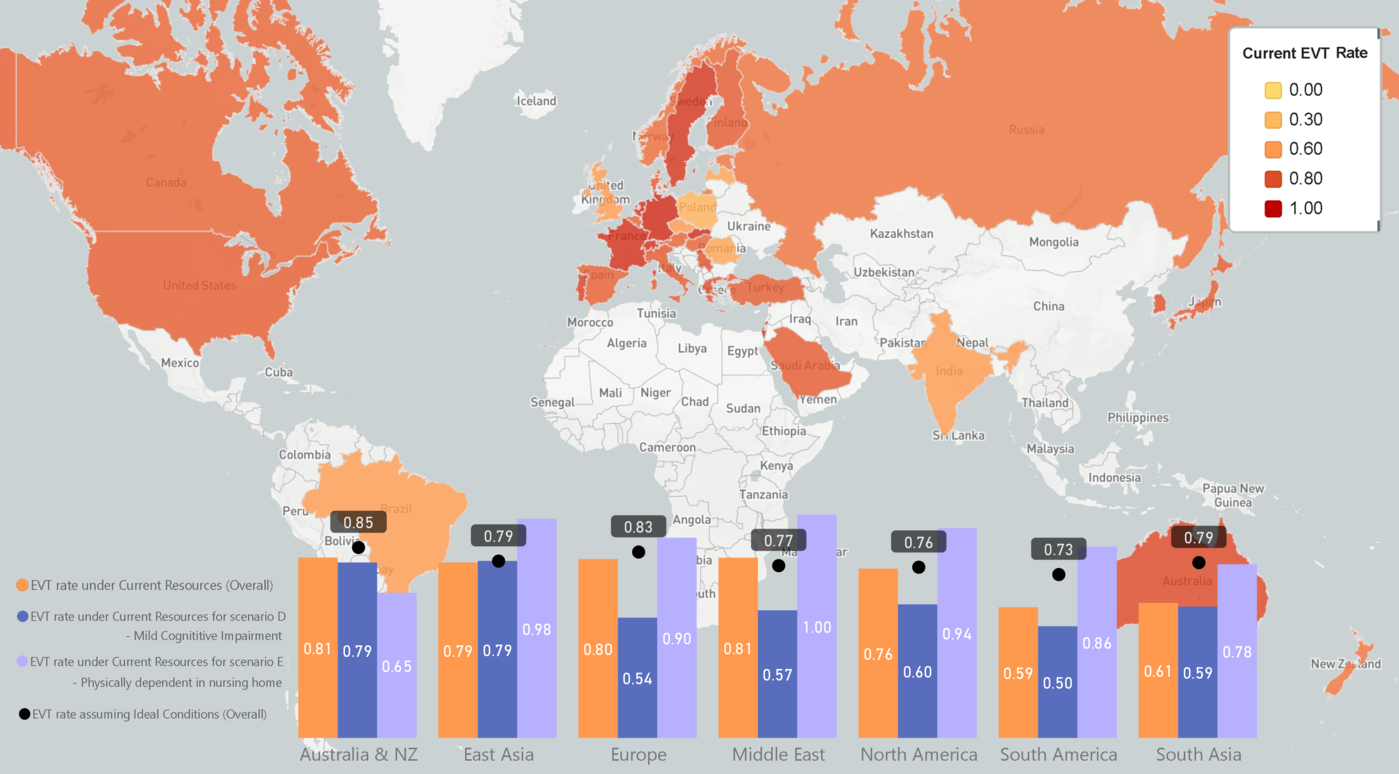
Figure 2: The proportion of respondents from key world regions who chose EVT under current resources for all scenarios (overall) and for comorbidity-related scenarios D (mild cognitive impairment) and E (physically dependent in nursing home). The proportion of respondents who chose EVT assuming ideal conditions (overall) in each region is also shown. The world map shows the overall proportion of responses in each country that favored EVT under current resources, when considering all 22 scenarios. The color scheme is based on a spectrum for the proportion ranging from 0.00 to 1.00, with key colors and corresponding proportions indicated in the legend on the upper right. Hues of yellow evolving to orange represent the range of proportions from 0.00 to 0.60, with the hues then evolving from orange to red as the proportion rises from 0.60 to 1.00. Countries that were either not captured in the study or for which we had fewer than three respondents appear white.
Discussion
In this international multidisciplinary scenario-based survey, respondents were less likely to favor EVT in the presence of patient comorbidities – particularly cognitive impairment – compared to ideal scenarios meeting level-1A evidence, but by a small absolute difference. However, when considering all scenarios presented, the presence of comorbidities and disability did not deter respondents from pursuing EVT, especially on accounting for additional factors like baseline stroke severity, extent of ischemic changes (ASPECTS), and occlusion site. On the other hand, respondents were less likely to include alteplase in the setting of metastatic/non-metastatic cancer or multi-system dysfunction but were comfortable giving alteplase with pre-stroke dependence or MCI. These findings have implications for our understanding of physician decision-making in acute stroke and for informing the care of patients with comorbidities.
First, the finding that respondents less often favored EVT/alteplase in the setting of comorbidity when compared to ideal level-1A scenarios unsurprisingly confirms the relative uncertainty experienced by physicians when faced with premorbid illness/disability. However, on adjusting for other scenario characteristics, cognitive impairment was the only comorbidity-related factor associated with lower EVT odds versus level-1A scenarios. This suggests that cognitive impairment is overvalued by stroke physicians in EVT decision-making, especially since we specified the patient had MCI or non-disabling cognitive impairment (versus dementia). While there are few data on thrombectomy in these patients, observational studies of thrombolysis in patients with dementia suggest that mortality and hemorrhage risks are comparable to those without dementia, though functional outcomes may be worse. Reference Saposnik, Kapral and Cote22–Reference Zupanic, von Euler and Kareholt24 In this regard, it is noteworthy that most respondents chose alteplase in the scenario with MCI.
Second, the absence of any consistent association between the comorbidities presented and decreased odds of favoring EVT (on considering all scenarios) demonstrate a lack of consensus among stroke experts about excluding patients based on pre-stroke comorbidity/disability. There is no mechanistic basis for why patients with comorbidities cannot benefit from EVT/alteplase, although the degree of benefit may not be as robust as in healthier patients. We know that these patients accumulate disability and experience worse clinical and health economic outcomes if acute treatments are routinely withheld, Reference Ganesh, Luengo-Fernandez, Pendlebury and Rothwell25 and there is preliminary evidence that some of these patients can retain their premorbid state with EVT/alteplase without increased risk of harm. Reference Goldhoorn, Verhagen and Dippel26 Adding in the absence of any clear comorbidity-based rationale for decision-making among our surveyed experts, the routine exclusion of patients based on some combination of pre-stroke comorbidities/disability may not be tenable. A crucial modifier in practice will be the values of patients as voiced by themselves or alternative decision-makers. The perspectives of patients with pre-stroke multi-morbidity/disability regarding EVT/alteplase remain an avenue for further study.
Third, our results demonstrate uncertainty among respondents regarding the added value of intravenous alteplase when pursuing EVT in setting of comorbidities. We found alteplase was often favored in scenarios where respondents were reluctant to pursue EVT (older age, MCI) but was often left out in other comorbidity-based scenarios where respondents chose EVT, despite no obvious alteplase contraindication. Respondents reporting more EVT cases/year and interventional neuroradiologists were more likely to forego alteplase in scenarios where they favored EVT, suggesting a perceived lack of benefit or risk of harm. This equipoise may indicate the value of trials comparing alteplase plus EVT to EVT alone, 27,Reference Bellwald, Weber and Dobrocky28 but unless such studies enroll patients with pre-stroke comorbidity/disability, they are unlikely to definitively settle this issue.
Fourth, our findings that patients’ older age and female sex were associated with lower odds of respondents favoring EVT (and alteplase for female sex), whereas comorbidities themselves were not, raise questions about implicit biases in EVT decision-making. Age and sex may well have contributed to the apparent instability of the association of certain comorbidities with lower enthusiasm for treatment in our analysis. For example, while the MCI scenario had an overall lower proportion of respondents choosing EVT, the MCI characteristic itself was associated with higher rather than lower odds once adjusting for other scenario characteristics including age and sex (the patient in this scenario was an 85-year-old woman). In individual patient meta-analyses, age and sex did not modify EVT treatment effect. Reference Goyal, Menon and van Zwam1 Agism has been noted in interventional stroke studies, Reference Hadbavna and O’Neill29 and older patients and women appear less likely to receive appropriate acute stroke care. Reference Asdaghi, Romano and Wang30,Reference Kimball, Neal, Waters and Hoh31 Our finding that age and sex further modify EVT decisions merits further validation.
Fifth, our results imply that EVT/alteplase decision-making in the setting of comorbidities is strongly associated with physician characteristics. Interventional neuroradiologists and neurosurgeons more often favored EVT than neurologists, as did those reporting more EVT cases/year, whereas geriatricians/internists were less likely to choose EVT/alteplase, particularly for an already dependent patient. Regional differences included respondents in East Asia more often favoring EVT than North-American counterparts, whereas those in South Asia less often chose EVT under current resources but became more likely to do so on assuming ideal conditions. This further emphasizes the importance of investing in stroke-care resources in Asia, where there is a known disparity between availability of acute stroke infrastructure in East Asia (high) versus the rest of the region. Reference Toyoda, Koga, Hayakawa and Yamagami32
A few limitations merit discussion. While the overall completion rate was high (45.6%) compared to other surveys, we only had a few participants from some countries. As there is no comprehensive international register of stroke physicians, participant enrollment relied on institutional networks and collaborations, potentially limiting the study’s representativeness. In addition, survey-based data may not accurately reflect decision-making in routine practice, although care was taken to frame the scenarios to realistically reflect routine practice. However, the physicians’ real-life decisions may still differ from the hypothetical ones made in these case scenarios. Our ability to assess the relative importance of each comorbidity was limited by respondents only being presented with one comorbidity factor (e.g. cancer or MCI) at a time. Future studies of the influence of comorbidities on EVT/alteplase decisions may consider presenting respondents with multiple different combinations of these comorbidity factors. Since this study ultimately relied on mock scenarios rather than real-life cases, there were expected limitations in the ranges, permutations, and combinations of demographic and clinical variables that could be captured within those cases, which may have contributed to instability or wide ranges of ORs in some of the multi-variable logistic models. Although the scenarios were chosen by consensus among a steering committee of stroke experts, the range or severity of the comorbidities presented may not reflect the experiences of stroke physicians around the world. In addition, we were unable to calculate a response rate for our survey; the denominator for distribution is not known because each list was known only to each country/region lead and distribution occurred in waves, with the surveys likely forwarded to known colleagues and thereby resulting in challenges to the representativeness of our sample. Therefore, our results must be interpreted knowing the limitations of this type of survey-based approach to assessing attitudes about standards of care. Despite these limitations, our findings can help further understand the influence of comorbidities and premorbid disability on treatment attitudes of stroke physicians across a broad, international, multidisciplinary spectrum.
Conclusion
In this international survey, we sought to explore EVT/alteplase decision-making by stroke experts in the setting of comorbidity/disability. Moderate and even severe disabling comorbidities did not consistently deter experts from choosing EVT in this international survey, arguing against the routine exclusion of such patients. However, intravenous alteplase was often foregone in these scenarios when respondents chose EVT. Differences in approach based on patient age and sex merit further investigation. Inter-speciality and regional differences in EVT/alteplase decision-making could potentially be mitigated by improving access to stroke care resources.
Disclosures
Aravind Ganesh reports membership in the editorial boards of Neurology, Neurology: Clinical Practice, and Stroke; speaker honoraria from NHS Health Education England; consulting fees from MD Analytics, MyMedicalPanel, Adkins Research Group, and Genome BC; research support from The Rhodes Trust, Wellcome Trust, the University of Calgary, Alberta Innovates, the Canadian Cardiovascular Society, and the Canadian Institutes of Health Information; stock/stock options from SnapDx, TheRounds.ca, and Advanced Health Analytics (AHA Health Ltd); and a provisional patent application (US 63/024,239) for a system to deliver remote ischemic conditioning or other cuff-based therapies. Gustavo Saposnik reports a Heart and Stroke Foundation of Canada Career Award. Mayank Goyal reports personal fees from Medtronic, personal fees from Stryker, personal fees from Microvention, personal fees from Mentice, outside the submitted work; in addition, Dr. Goyal has a patent Systems of Stroke Diagnosis licensed to GE Healthcare. Michael Hill reports grants from Covidien (Medtronic), personal fees from Merck, non-financial support from Hoffman La Roche Canada, outside the submitted work. In addition, Dr. Hill has a patent 62/086,077 for triaging systems in ischemic stroke issued and Research support from Alberta Innovates Health Solutions, Heart & Stroke Foundation, Hotchkiss Brain Institute, Canadian Stroke Prevention Intervention Networks (Institute of Circulatory and Respiratory Health, CIHR), Calgary Stroke Program, Department of Clinical Neurosciences, University of Calgary; nonfinancial support from Alberta Health Services; stock in Calgary Scientific Incorporated, a company focusing on medical imaging software. Bijoy Menon reports personal fees from Penumbra Incorporated, outside the submitted work. In addition, Dr. Menon has a patent 62/086,077 for triaging systems in ischemic stroke issued and Membership of the Steering/Executive Committee, ESCAPE trial with support from Covidien; Site Principal Investigator, SOCRATES Trial, sponsored by Astra Zeneca; research funding from Canadian Institutes of Health Research (CIHR), Heart and Stroke Foundation of Canada, Alberta Innovates Health Solutions, Hotchkiss Brain Institute and Faculty of Medicine, University of Calgary and salary support from the CIHR New Investigator Award and Heart and Stroke Foundation/University of Calgary Professorship in Stroke Imaging. Johanna Ospel reports funding support from the Julia Bangerter Rhyner Foundation, University of Basel Research Foundation, and Freiwillige Akademische Gesellschaft Basel. The remaining authors have nothing to disclose.
Acknowledgements
The authors are most grateful to all the physicians who participated in the study.
Statement of authorship
AG performed statistical analysis and interpretation, wrote and revised the manuscript. NK and JMO acquired data, performed statistical analysis, and revised the manuscript. ATW and MMF were involved in study concept and design, data acquisition, and manuscript revision. MG, MAA, MDH, BKM, and GS conceived and designed the study, provided supervision and funding, analyzed and interpreted data, and revised the manuscript.
Supplementary material
To view supplementary material for this article, please visit https://doi.org/10.1017/cjn.2020.158.
Funding
The study was funded by Stryker Inc. through an unrestricted research grant to the University of Calgary. The company was not involved in the design, execution, analysis, interpretation, or reporting of the results.









