INTRODUCTION
Nosocomial hepatitis B virus (HBV) outbreaks have been reported in association with failure to apply universal precautions or sterile surgical techniques in the USA and Europe. Patient-to-patient transmission of HBV in these settings has been linked to blood contamination of subdermal EEG electrodes, intravenous cannulae and multiple-dose vials [Reference Stryker, Gunn and Frances1–Reference Hlady6], but one of the most common exposures implicated is the use of ‘finger-stick’ lancet devices for blood glucose testing [Reference De Schrijver7–Reference Perz and Fiore20]. This report is the first account of investigations into a series of hepatitis B outbreaks linked to the use of lancing devices in community healthcare settings in the UK. The occurrence of these outbreaks has highlighted the confusion that exists and the need for clear recommendations regarding the use of such devices in the UK.
OUTBREAKS
Northwest London
In February 2004, an elderly diabetic resident of a private residential home in northwest London was reported to have acute hepatitis B. Investigations identified that individual patient, self-use lancing devices for blood glucose monitoring were being shared between patients. Recommendations were made to the home regarding the appropriate use of these devices and a national alert was issued by the Medicines and Health Care Product Regulatory Agency (MHRA) [21]. However, in November 2004 a second resident of the home, who was also diabetic, was reported to have acute hepatitis B.
Hertfordshire
In May 2005, acute hepatitis B was reported in a long-term resident of a nursing and residential home in Hertfordshire. The individual had learning difficulties, epilepsy and diabetes and had random blood glucose monitoring performed by staff at the home.
Manchester
In June 2005, the Health Protection Agency (HPA) was informed of two individuals with acute hepatitis B. Both these individuals were elderly residents from the Manchester area. Initial enquiries revealed that one of them was a current resident at a local private nursing and residential home and that the other had also been a resident in the same home for 2 months during the previous 6 months. Both individuals were known to be diabetics who required frequent blood glucose monitoring. A further diabetic resident at the same home was identified with acute hepatitis B 2 weeks later.
Wiltshire
In August 2006, the HPA in Wiltshire were informed of an individual with acute hepatitis B who was a resident in a local care home. This resident had learning difficulties and was a non-insulin-dependent diabetic requiring regular blood glucose monitoring by staff at the home.
Southwest London
In September 2006, an individual with acute hepatitis B was reported to the HPA in southwest London. Initial enquiries revealed that the individual was an elderly diabetic resident of a local care home. Six days later, the HPA were notified of acute hepatitis B in another diabetic resident from the same home. Both individuals were non-insulin-dependent diabetics undergoing regular blood glucose monitoring.
METHODS
Multi-agency outbreak control teams were convened in response to these outbreaks to coordinate their investigation and management and to decide upon control measures. Most teams were led by local HPA staff and included individuals from a wide range of organizations including infection control and public health teams from the local primary-care trusts, local virology staff and the Commission for Social Care Inspection (CSCI).
The epidemiological investigations included the collection of demographic and risk-factor information from each of the individuals with HBV infection in order to help determine the source and mode of spread of infection. This was conducted by reviewing hospital, nursing and GP notes and by interviewing patients and key staff to identify any potential exposures. In all of the homes, residents and staff were offered HBV screening. In two of the homes all residents were offered screening and in the other homes screening was limited to selected groups. These groups included all diabetics, residents who had been finger-pricked and residents from the same area of the home as the index cases. In Manchester and southwest London, samples were also requested from residents discharged within the past 6 months. Blood samples were tested for HBsAg and anti-HBc with additional hepatitis B markers (anti-HBc IgM, anti-HBe, HBeAg) for any positive on initial tests. In southwest London, a second round of testing was conducted around 3 months later. Positive samples from all investigations other than in Wiltshire were referred to the Centre for Infections (CfI) at Colindale for detection of HBV DNA followed by sequencing of the surface and pre-core/core regions.
Additional investigations that took place in one or more of the outbreaks included reviewing death certificates from residents who had died in the preceding 6 months, screening for other bloodborne viruses, and DNA testing to detect infections early in the incubation period.
Environmental investigation consisted of infection control assessment of all clinical practices, including blood glucose testing and the administration of insulin through interviewing patients and key staff, and observing practice. In one incident environmental samples were obtained from several key sites around the home including clinical areas, patients' rooms and blood glucose testing equipment. These samples were sent to the virology laboratory for HBV DNA identification.
RESULTS
Epidemiological investigations
The testing of residents in all homes found a high proportion (20/221, 9·0%) to be HBsAg positive, including one resident in southwest London (SWL6) who was HBsAg negative and DNA negative on the first round of testing but HBsAg positive when tested again 3 months later. (Table 1) The proportion of residents seropositive for HBsAg was higher in diabetics (19/66, 28·8%) than non-diabetics (1/155, 0·6%) (P<0·0001). In all five outbreaks, no other common risk factors were identified from individuals that could explain their infections. In addition to those infections detected in current residents, one ex-resident tested in the southwest London incident was found to be HBsAg positive (SWL7).
Table 1. Summary of serological testing for hepatitis B infection in residents and staff at each of the homes
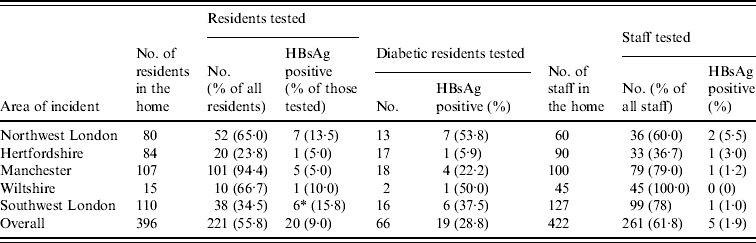
* In addition to the total one ex-resident tested positive.
Seventeen of the 21 patients with HBsAg had clear serological evidence of acute hepatitis B infection, being anti-HBc IgM positive. Two additional patients (NWL5 and SWL6), had altered serological markers consistent with resolving acute infection and early acute infection, respectively (Table 2). Only 11 individuals had symptoms consistent with acute hepatitis, seven of whom died, with hepatitis B infection recorded as a contributory or underlying factor in five of these patients. HBV DNA sequencing performed on all seven patients from northwest London were found to be genotype A and identical to each other (Fig. 1). The strain found in the Hertfordshire patient was identical to those identified in northwest London. In the Manchester outbreak, HBV DNA could only be recovered from three of the patients with acute hepatitis and were found to be genotype D and identical to each other. All seven patients from southwest London were shown to be genotype A and identical to each other, but distinct from those in both northwest London and Hertfordshire (Fig. 1).
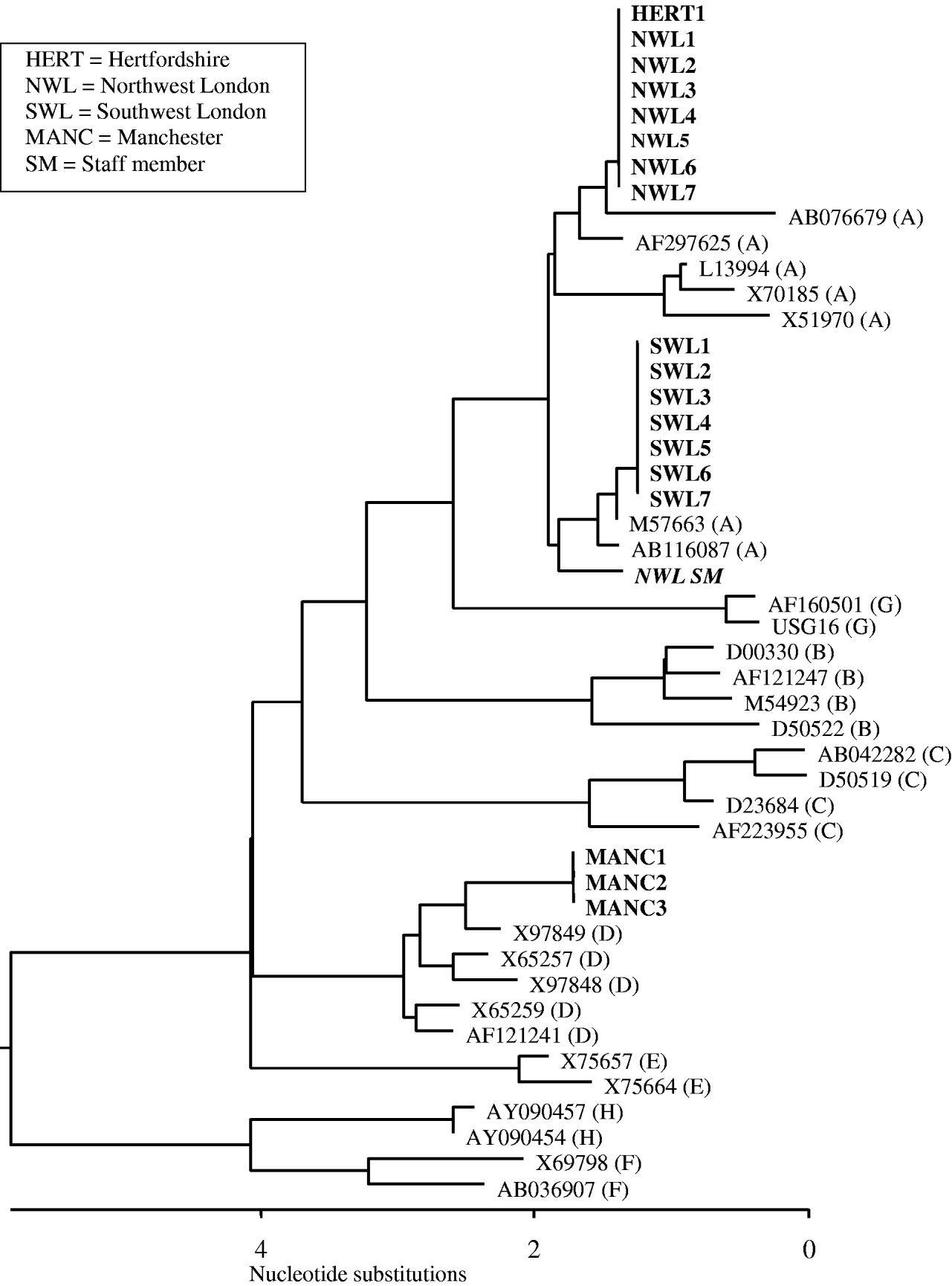
Fig. 1. Dendogram of the HBsAg region showing the phylogenetic relationship of the sequences involved in the outbreaks. The residents with HBV infection are shown in bold; sequence from the staff member is shown in bold italics. The accession numbers of the HBV reference sequences are shown with the associated genotype in parentheses.
Table 2. Summary of residents with evidence of acute or chronic hepatitis B virus infection in each of the outbreaks
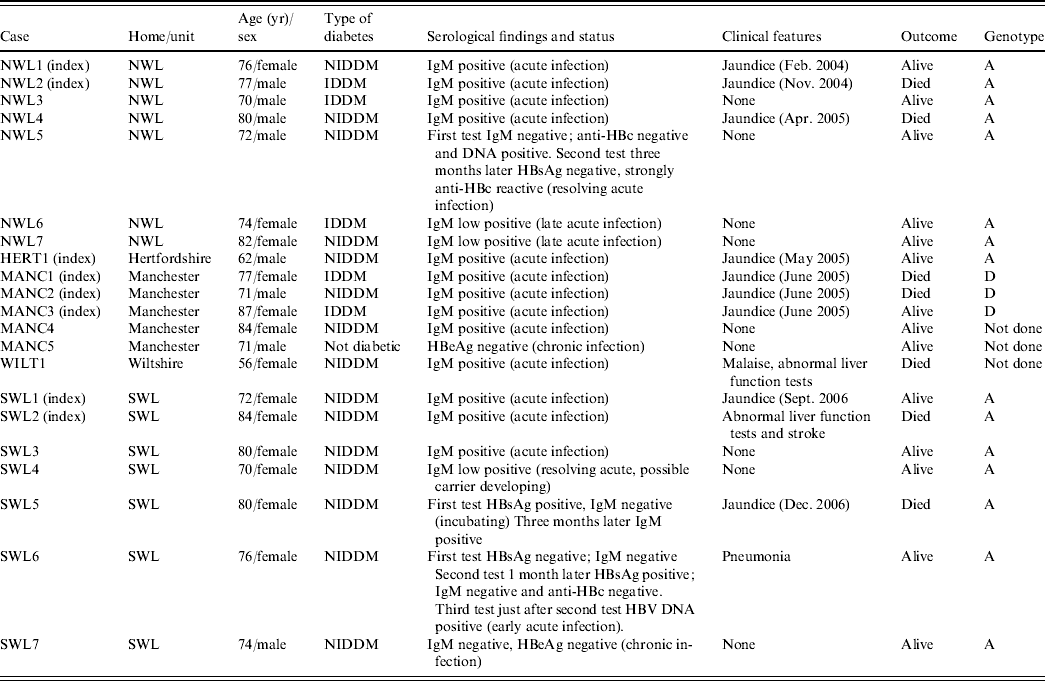
NWL, Northwest London; HERT, Hertfordshire; MANC, Manchester; WILT, Wiltshire; SWL, southwest London; NIDDM, non-insulin-dependent diabetes mellitus.
In southwest London one ex-resident infected with the incident strain had markers suggestive of chronic infection (SWL7), and could therefore have been the source of infection for the outbreak. In northwest London, one elderly asymptomatic patient (NWL7) had a low stable level of anti-HBc IgM which was also consistent with chronic infection. This individual was still chronically infected 12 months later, and therefore could not be excluded as a possible source of the outbreak. In Manchester, the only resident with markers of chronic infection (MANC5) was not diabetic, resided in a different section of the home to the three residents with acute infection, had insufficient DNA for typing and was not considered a likely source. In both Hertfordshire and Wiltshire no residents with evidence of current infection were found although one resident with resolved infection was identified in each setting. In both individuals, serum anti-HBc IgM was negative and it was considered unlikely that these were the source of infection for both index cases.
A total of 261 staff were tested and five (1·9%) were HBsAg positive. All of the positive staff members were asymptomatic and none were positive for IgM. One staff member in northwest London was found to be infected with genotype A but the phylogenetic sequence was different from the residents in this incident (Fig. 1). Four staff members from homes in northwest London, Hertfordshire, southwest London and Manchester were found to be infected with genotype E.
Environmental investigations
Infection control assessments highlighted several areas of concern in relation to basic infection control standards. In one of the affected homes inadequate standards of hygiene were identified in both the clinical and residential areas of the house where the residents with acute hepatitis B resided. Clinical areas at the home were untidy, waste disposal was inappropriate, and hand washing facilities were poor. Staff had limited access to personal protective equipment and the wearing of gloves was discouraged to avoid the home appearing to be a clinical environment. Environmental swabs taken at this home failed to yield any HBV DNA upon testing.
Similar problems regarding infection control were found in the other homes where it was also noted that infection control policies were incomplete or out of date and few staff had adequate, up-to-date infection control training. The vaccination status of staff was poorly documented in all instances.
Specific problems were identified with regard to blood glucose testing in all homes. All the diabetics had regular blood glucose monitoring performed by nursing staff using lancing devices. There are various models on the market and different models were used by the homes affected. Most of the devices designed for individual patient use have a cap at the end through which a lancet punctures the skin when the device is activated in position on the patient's fingertip. The lancets, which are disposable and for single use only, require careful manipulation by nursing staff to be inserted. With the alternative devices designed for multi-patient use, the lancet retracts and becomes disabled after use and the cap and lancet are then replaced, thus avoiding the potential for contaminated blood from another patient remaining on the cap.
All the devices used in the homes were those designed for self-testing personal use and were not designed for multi-patient use. Investigations at the homes revealed that these devices were either being shared incorrectly or individual residents were assigned their own lancing devices with the intention of the equipment not being shared between residents; however, the potential for sharing was noted. In the northwest London outbreak the home had been advised to change to multi-use devices after the first case; however, this was not done as staff at the home had misunderstood the advice. The home involved in the southwest London outbreak was run by the same company as the home in northwest London but, after taking advice from a local diabetic nurse, the recommendations on blood testing following the earlier incident had not been followed. Staff in the homes reported that the single-patient self-testing lancing devices were preferred as they were often supplied free with insulin prescriptions and doctors could prescribe lancets under the NHS. In contrast the multi-patient devices were expensive and necessitated the home purchasing, disposable lancets, at extra expense, for use with this professional device.
In one of the homes affected, all diabetics shared a common glucometer, which was not routinely cleaned between patient use, and equipment for all patients was carried on single tray. This tray was stored in the clinical room and also not routinely cleaned between use. Another infection control concern regarding blood glucose testing was poor hand hygiene in staff members.
Control measures
In all homes, immediate control measures included a review of infection control procedures, and replacement of single-use devices with those approved for multi-patient use or single-use disposable lancing devices. In response to the outbreaks, hepatitis B vaccine was offered as post-exposure vaccination, either to all residents or to the diabetic residents. In addition to this, in the Hertfordshire outbreak hepatitis B immunoglobulin was given to residents who had undergone blood glucose monitoring at the home and lived on the same floor as the index case.
Various actions taken in relation to staff at the homes included testing for hepatitis B markers, hepatitis B immunization and implementation of robust occupational health arrangements to deliver these actions on an ongoing basis. Staff in all of the homes were offered testing for anti-HBs and vaccination against hepatitis B where appropriate.
The MHRA issued a total of three medical device alerts. The first was issued in September 2004 after the first case in the northwest London outbreak. This alert was designed to go to a wide range of settings being cascaded through NHS trusts, Primary Care Trusts, the Healthcare Commission, Social Services and the CSCI. The alert advised staff to check that devices designed for self-use were not being used for more than one patient. [21]. When the second infection occurred the alert was circulated via CSCI to all registered nursing homes.
After the outbreaks in Manchester and Hertfordshire were reported, another alert was issued by the MHRA in November 2005, advising that self-use devices were not to be used for sampling from multiple patients [22]. Following the outbreak reported in southwest London and Wiltshire a third alert was issued in December 2006, which advised that staff in nursing and care homes should only use disposable, single-use lancing devices or non-disposable lancing devices intended for multi-patient use [23].
DISCUSSION
Between 1990 and 2003 a total of 9336 individuals with acute hepatitis B were reported by laboratories in England and Wales to the HPA CfI [24]. Exposure information was available for 5463 and only four individuals were associated with diabetic blood sampling; two sporadic cases and two linked cases on a hospital ward. Between February 2004 and December 2006, nine individuals with acute hepatitis B were reported to five local units of the HPA.
The epidemiological and environmental evidence in relation to most of these individuals suggests that HBV transmission occurred as a result of a breakdown in infection control measures in blood glucose testing. This is supported by the high attack rate in diabetics in four out of five of the homes, the identification of identical strains in linked cases from the three large outbreaks and the absence of any other common risk factors for infection in most of these individuals. The exact modes of transmission cannot be determined but sharing of lancing devices designed for single patient use was reported in two of the outbreaks and considered a likely source following investigation of two of the three other outbreaks, and was identified as a possible cause of transmission for the remaining outbreak.
The identification of hepatitis B infection associated with diabetic blood sampling after 2004 corresponds with a major shift in the supply of blood lancets towards those suitable for use in safety lancing devices [25]. In 2004/2005, 10·5 million lancing devices and 18·8 million safety lancets were supplied to the NHS in England [26]. The increased market for diabetic sampling in particular, was facilitated by the free supply of lancing devices with insulin and the ability of doctors to prescribe safety lancets suitable for self-use under the NHS drug tariff.
Because HBV is stable at ambient temperatures and it is possible for asymptomatic individuals with hepatitis B infection to have a high amount of the virus in their blood [Reference De Schrijver9], transmission can occur by unapparent blood contamination of a surface that has contact with broken skin. In the process of glucose testing, blood could also have been transferred between the patient's finger, the healthcare worker's hands, glucometers, insulin vials and other surfaces. The high attack rate in diabetics in the three homes having more than one resident with hepatitis B infection, the clustering of likely onset dates, and the finding of identical strains, imply that a single source led to widespread exposure in the diabetic populations of the home. It is likely that the vehicle for transmission in all these outbreaks was the cap at the end of the lancing device.
Despite thorough epidemiological investigation, no source patient was identified for the individuals infected in Hertfordshire and Wiltshire or for the Manchester outbreak. The finding of an identical strain in Hertfordshire to that found in northwest London is not surprising, given that genotype A is common in the UK and well conserved. This emphasizes the importance of adequate epidemiological and virological investigation in these outbreaks.
Despite MHRA alerts on the use of blood glucose monitoring equipment in response to the northwest London outbreak, a further outbreak, associated with breaches in recommended practice, occurred in southwest London. This highlights the potential for confusion regarding the correct use of blood glucose monitoring equipment, as the models for self-use and professional use appear very similar. Following the outbreaks, informal surveys conducted in London indicated that incorrect use of the devices was widespread in a range of healthcare settings. The outbreak teams concluded that the manufacturer's information that accompanies these devices is not always clear. These concerns were reported to the MHRA for their attention. The MHRA are the competent authority charged with the responsibility of enforcing the Medical Devices Directive 93/42/EEC in the UK and for ensuring that medical devices meet the essential requirements of the Directive in terms of quality, safety and performance. These include requirements for labelling, packaging, and the instructions necessary for safe use of the medical devices. The MHRA is conducting an investigation into the concerns reported by the HPA, and at present, its enquiries are ongoing. A subsequent report of misuse of devices intended for self-testing in community pharmacies led to a further MHRA alert in 2008 [27].
In December 2006 the HPA held a national outbreak team meeting with representatives from a variety of agencies and observers from MHRA and NHS Logistics. The committee recommended that, unless nursing homes could ensure that professional devices would not be confused with self-testing devices brought in by residents, single-use, disposable safety lancing devices are preferred in these settings. Such devices can now be prescribed on the NHS drug tariff, so the cost will be free to most residents. The HPA has also developed guidelines for diabetic blood sampling in nursing homes [28]. In addition to advising on appropriate infection control, the document refers to guidance from the National Institute for Clinical Excellence on the potential role of regular blood glucose monitoring for those with type 2 diabetes [29]. The latter document advises that regular blood glucose monitoring for non-insulin-dependent diabetics should only be performed as a part of self-management. Given that the majority of patients infected in these outbreaks were frail and elderly residents with non-insulin-dependent diabetes it appears likely that some of the monitoring performed in the home was unnecessary.
Lack of occupational healthcare for staff working at the homes was also a common problem, particularly in the privately run care homes. Many of the staff were recruited from countries abroad where hepatitis B vaccination is not a prerequisite for working in healthcare. This resulted in poor levels of hepatitis B vaccination coverage in healthcare staff performing risky procedures.
A key additional factor underlying the system of failures in all of these outbreaks is that of inadequate training for staff in the homes with regard to infection control. These outbreaks highlight the need to specifically address training in blood glucose testing for staff working in care homes in the community and also identify a broader need to strengthen basic infection control practice in care homes in the community [Reference Patel30]. Providing training to staff working in residential and nursing-home settings in many urban areas is a challenge, as staff turnover is often high.
Incidences of hepatitis B transmission linked to blood glucose monitoring in care homes have been reported in the USA and various European countries since the early 1990s [Reference De Schrijver7–Reference Perz and Fiore20, Reference Patel30, Reference Thompson31]. The five outbreaks described in this paper occurred within a 2-year period and outbreaks due to incorrect sharing of equipment have been reported for the same period in Belgium and The Netherlands [Reference De Schrijver7, Reference De Schrijver9, Reference Götz32]. The association with the major increase in the use of safety lancing devices in the UK, primarily introduced to reduce sharps injuries, highlights the need for training and guidance in infection control practice to keep up with developments in technology. It also highlights the need to ensure that unnecessary blood sampling is avoided wherever possible. The urgent attention of public health authorities at local, national and international level is required.
APPENDIX. Local incident teams
L. Benson, J. Chaloner, K. Mutton, S. Webster (Manchester); S. Hart, J. Kearney, M. Reacher (Hertfordshire); R. Ruggles, L. Harvey, S. Mandal, P. Rice, A. Stewart, H. Maguire, K. Lewis, V. Gilfilian, L. Venables, J. Pitt, (South West London); M. A. Balogun, S. L. Ngui, H. Kikuchi, C. G. Teo (Centre for Infections); M. Evans, A. Shelley, M. Donati, A. Jones (Wiltshire); G. Fraser, M. Barnard, E. James, G. Corre, R. Sooriah (North West London).
ACKNOWLEDGEMENTS
The authors thank all the members of local incident teams and laboratory staff involved in the processing of specimens.
DECLARATION OF INTEREST
None.







