Introduction
Hepatitis A infection causes a spectrum of illness from asymptomatic disease to fulminant hepatitis. Severe disease occurs more commonly in adults and fatalities predominantly occur in those over 50 years of age or with underlying chronic liver disease [Reference Heymann and Heymann1, Reference Hawker2]. Up to 70% of infections are asymptomatic in children under 6 years of age, yet this group is a source of transmission due to suboptimal personal hygiene [3].
The mean incubation period is 28–30 days and the maximum infectivity occurs during the second half of the incubation period, while asymptomatic [Reference Heymann and Heymann1]. Most cases are considered non-infectious after the first week of symptoms.
Faecal–oral, person-to-person spread is the principal mode of transmission in developed countries [Reference Bell4]. Common source outbreaks associated with contaminated water or food have also been reported, as well as transmission via contaminated blood products [Reference Xu5–Reference Hettmann7].
Hepatitis A vaccine is widely available and confers 95% immunity within 4 weeks of one dose. This vaccine is not part of the primary immunisation schedule in Ireland [3].
In Ireland, the incidence of hepatitis A has declined since 2000, with fewer than 50 cases now notified annually at the national level [3, 8]. Under Irish law, clinicians and clinical directors of laboratories must notify cases of hepatitis A to the Medical Officer of Health (MOH) at the regional Department of Public Health (Dept. PH).
We describe an outbreak of hepatitis A associated with an urban childcare facility (CCF) in Ireland in which 12 confirmed, symptomatic cases were identified over a period of 23 weeks and 554 contacts were followed-up.
Detection and chronology of the outbreak
On 17 April 2015, a case (case B) of hepatitis A in an adult male was notified to the regional Dept. PH. Case B reported that his child (case A) had been unwell 3 weeks previously with fever, fatigue, abdominal pain, diarrhoea, pale stools and possible jaundice. He had attended a Paediatric Emergency Department (ED) but hepatitis serology was not performed at the time. Subsequently, serology confirmed that case A was IgM and IgG positive. Serological tests on the other household members indicated that all (apart from cases A and B) were IgM negative and IgG positive and therefore immune. None of the household members recalled previous hepatitis A infection or vaccination. Case A attended a local CCF.
On 17 April 2015 case C, a frequent visitor to case B's house was admitted to hospital with suspected hepatitis A infection which was subsequently confirmed. Close contacts of case C were offered hepatitis A vaccination. Cases A, B and C did not have a history of recent foreign travel. Two restaurants where cases A, B and C had eaten within the incubation period were referred to the local Environmental Health Officers (EHOs) for investigation.
On 27 April, a cousin of case A (case D) – was notified as a case of hepatitis A to the Dept. PH. Case D also attended the CCF. While it was initially considered that this was an extended family outbreak, no obvious source of infection was identified. Therefore it was decided to further investigate the CCF to rule this out as a source of infection. A CCF outbreak of hepatitis A was declared on 28 April 2015.
The CCF accommodated 100 children full-time, aged from 6 months to 5 years, in six rooms. The CCF also provided after school care to seven children aged between 5 and 8 years. At the time of the outbreak, 93 children were attending the CCF which had a staff of 23.
Between 28 April and 6 July 2015, seven additional cases of hepatitis A, associated with the outbreak were notified to the Dept. PH. In addition, a review of the hepatitis A surveillance system identified case X as a case with an epidemiological link to the CCF. All confirmed cases identified were investigated to elicit potential sources of infection.
Methods
Epidemiological methods
Case definition
The following case definitions were used. A probable outbreak case was a person(s) with symptoms suggestive of acute hepatitis±fever±jaundice±elevated aminotransferase levels with onset since January 2015 with an epidemiological link to the CCF. A confirmed outbreak case met the criteria for the probable case in addition to meeting the following laboratory criteria: Evidence of hepatitis A virus (HAV) nucleic acid in serum or stool or evidence of HAV -specific IgM antibody response.
All confirmed and suspected cases of hepatitis A, notified to the Dept. PH between January and September 2015 were reviewed and investigated for possible epidemiological links to the outbreak.
Microbiological methods
Twelve confirmed cases were confirmed by hepatitis serology – at local laboratories or the National Virus Reference Laboratory (NVRL) where Abott Architect and BioMerieux platforms were used to detect both hepatitis A IgM and IgG. The NVRL conducted phylogenetic analysis on four cases (cases X, A, B and D). HAV sequences, comprising a region spanning the VP1/2A junction of the viral genome, were generated at the National Virus Reference Laboratory using a protocol adapted from that published by Stene-Johansen et al. [Reference Stene-Johansen, Skaug, Blystad and Grinde9]. A dataset of HAV VP1/2A reference sequences, corresponding with HAV genotypes IA, IB, IIA, IIB, IIIA, IIIB and V, was prepared using sequences downloaded from the GenBank (http://www.ncbi.nlm.nih.gov/genbank/) and HAVNet (http://www.secure.rivm.nl/mpf/database/hav/) databases. These sequences were aligned with the study sequences and contemporaneous HAV sequences from Irish patients using Bioedit version 7.05 [Reference Hall10]. A phylogenetic tree was constructed using the neighbour-joining distance method under a Kimura-2-parameter model of evolution in PAUP* version 4.0 beta10 [Reference Swofford11].
Environmental methods
Potential environmental exposures were investigated. Previous inspection reports for the CCF were reviewed. Hygiene and infection prevention and control practices at the CCF were reviewed by the Dept. PH. All relevant food premises identified from case interviews were inspected by EHOs.
Outbreak control methods
Following identification of the outbreak of hepatitis A, the Dept. PH staff contacted cases to identify contacts and institute control measures. As the outbreak was associated with a CCF, all CCF staff, registered CCF attendees and their households were also identified in order to implement control measures. Additional remediation measures included a review of: child care practices, food sources and preparation and hygiene practices in order to identify possible sources of infection and transmission routes.
Outbreak cost methods
The cost of the outbreak was calculated from a health service perspective. Inpatient hospital costs and costs due to Emergency Department attendances were calculated using available Health Service Executive (HSE) data [12, 13]. GP consultation costs to cases were calculated using an average cost of a GP visit [14]. The NVRL provided serology and genotyping costs associated with the outbreak. However, it was not possible to get cost details from local hospital laboratories. As vaccine costs are commercially sensitive the National Immunisation Office provided us with an estimate for hepatitis A vaccine cost. An outbreak vaccination fee was paid to GPs per outbreak-related dose of hepatitis A administered.
Results
Epidemiology results
Between January and July 2015, 12 hepatitis A cases met the confirmed case definition. Table 1 shows cases X to K in chronological order of notification to the Dept. PH. Seven adults and five children were identified as symptomatic cases. All seven adult cases were household contacts of CCF attendees. No CCF staff members developed symptomatic hepatitis A. Six of the cases required hospitalisation. Four cases notified had received post-exposure hepatitis A vaccination prior to developing symptoms.
Table 1. Characteristics of Hepatitis A cases identified during the outbreak investigation, Ireland 2015

a Child <18 years of age.
b GP general practitioner.
c A&E Accident and Emergency.
Case X (school child) was identified during the review of hepatitis A cases reported to the Dept. PH. Case X's sibling attended the CCF, therefore providing case X with an epidemiological link to the outbreak. At the time of case X's diagnosis this sibling was asymptomatic and therefore was not tested for hepatitis A but received hepatitis A vaccine as per national recommendations [3]. The public health investigation of Case X's illness did not reveal any history of foreign travel or local source of infection, though several food premises were investigated by local EHOs.
Table 1 highlights the time interval between symptom onset and notification of hepatitis A to Dept. PH, laboratory results, level of medical care and vaccination uptake.
The complex relationships between symptomatic and possible asymptomatic CCF cases from the same households are outlined in Figure 1. We assumed that symptomatic adults contracted hepatitis A from asymptomatic infected CCF attendees in their household. In households where more than one child in the household attended the CCF, we also assumed that all CCF attendees were infected with hepatitis A. None of the asymptomatic CCF attendees in this diagram were tested for hepatitis A. This diagram reflects the potential transmission routes both within the CCF and individual households.
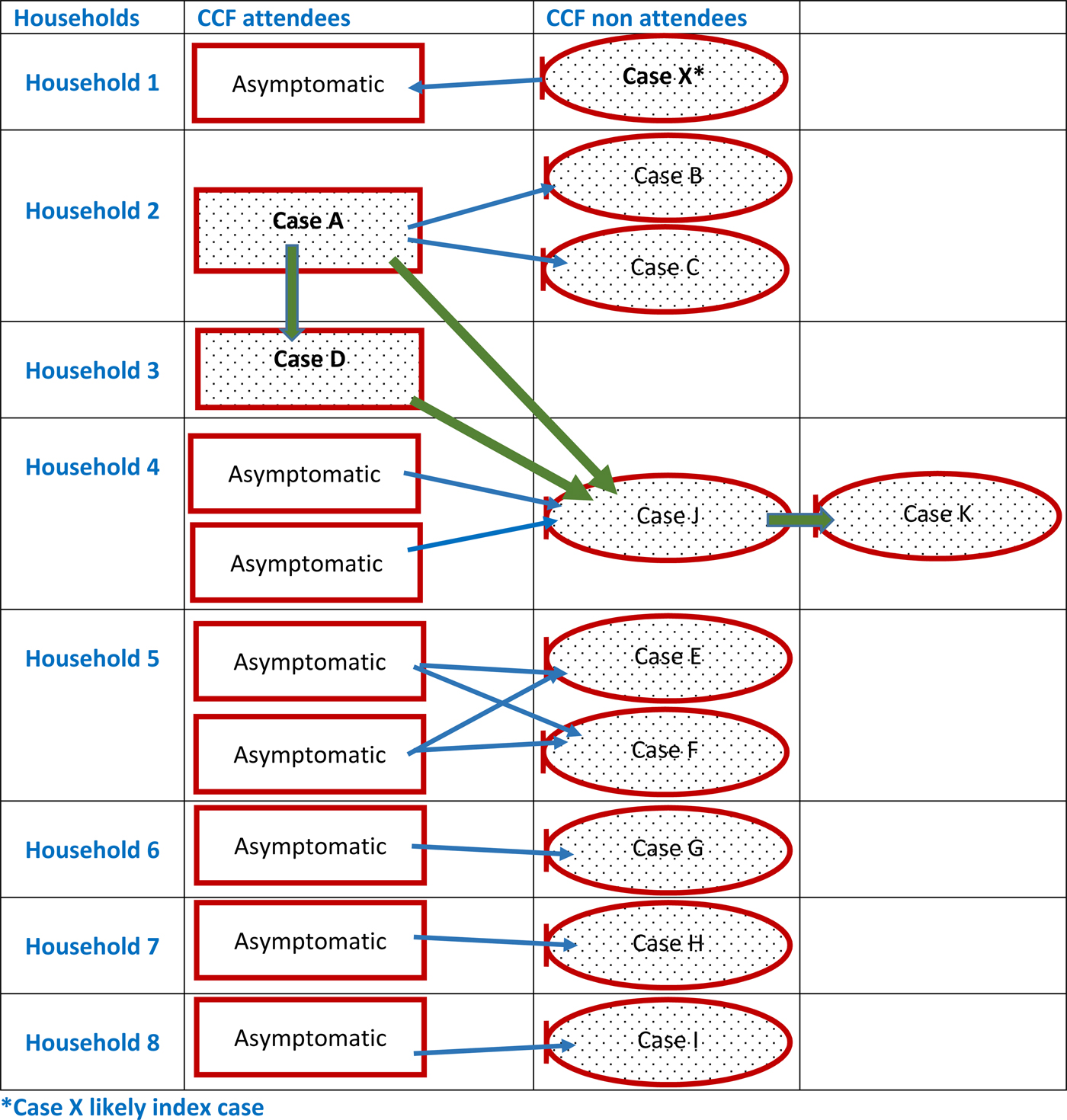
Fig. 1. Links between symptomatic cases and possible asymptomatic cases identified as part of the outbreak investigation.
Microbiological results
The four cases that were genotyped were genotype 1B. Comparison of the outbreak cases to 53 hepatitis A cases, sequenced in Ireland contemporaneously with the outbreak, using reference sequences was performed on a 293-bp fragment of the HAV VP1/2A gene (nucleotides 2939–3232, compared with the NC_001489 HAV whole genome reference sequence). A neighbour-joining tree was constructed using the Kimura-2-parameter model of evolution with a gamma distribution. Bootstrap values of 75% and over are shown on the corresponding nodes. The scale bar indicates an evolutionary distance of 9.0 nucleotide substitutions per site. Reference sequences are annotated with their GenBank accession number or HAVNet database ID, and confirmed genotype.
Comparison of the outbreak sequence against records in the HAVNet and GenBank databases identified a 100% similarity between these sequences and those from a UK outbreak in 2015. Sequences from cases X, A, B and D are highlighted in red and show 100% identity across the region sequenced (Fig. 2). Subsequently, it was decided that where an epidemiological link existed between cases further genotyping was not required.
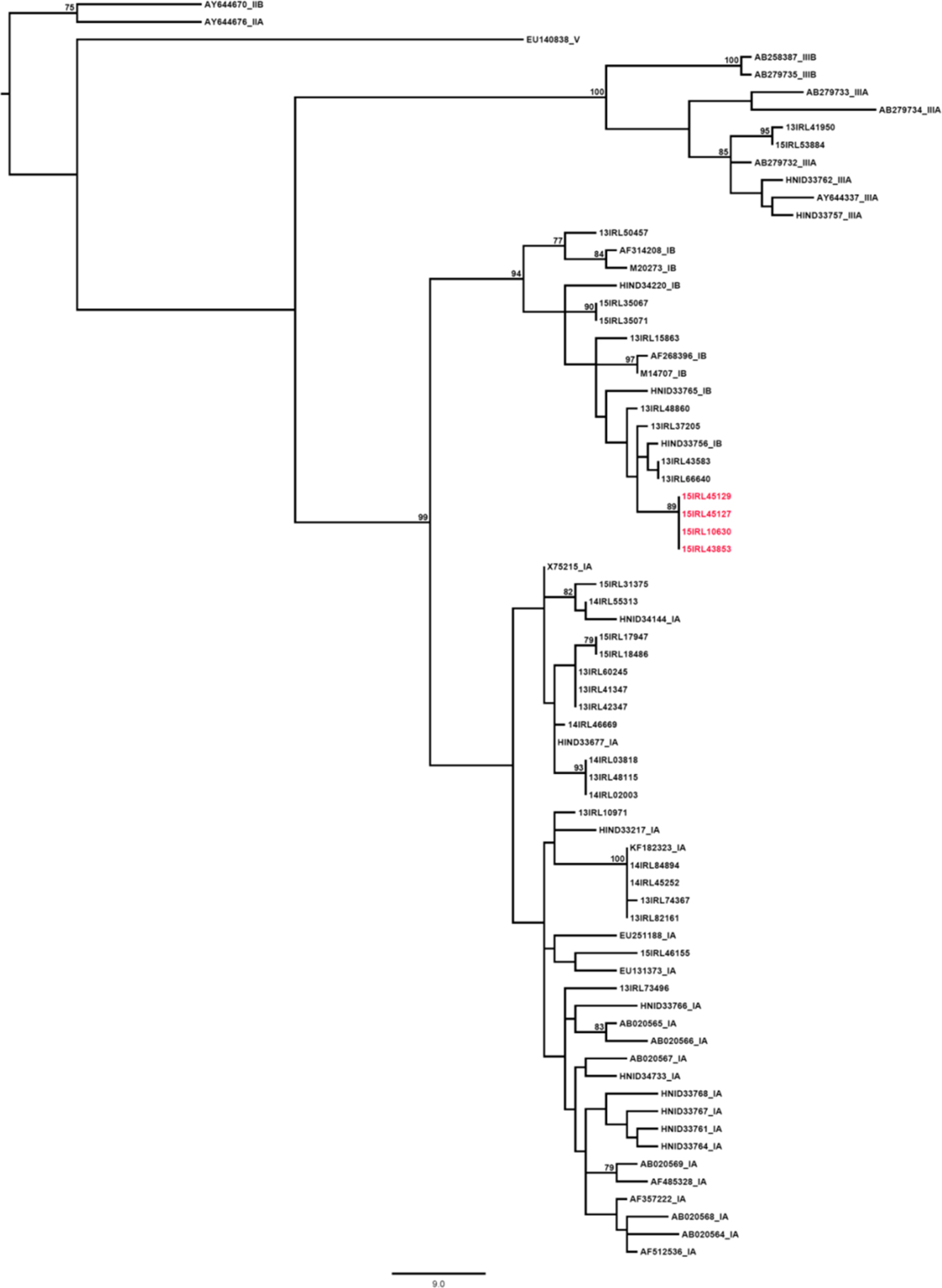
Fig. 2. Phylogenetic analysis results of cases X, A, B and D compared with other hepatitis A cases from a similar time, Ireland 2015.
Environmental results
A pre-school inspection report that preceded this outbreak highlighted deficiencies in the hand-washing practices of staff. An infection control audit, undertaken as part of this outbreak's management, identified a number of deficits, including CCF equipment in poor repair, inadequate hygiene measures during nappy changing, lack of foot-operated bins and the use of cloth covers on furnishings rather than waterproof material. A subsequent inspection by EHOs reported that adequate infection control measures were in place in the CCF.
EHO investigations of food premises identified on enhanced surveillance of cases did not link the outbreak to any food premises.
Outbreak control measures
Cases, once notified, were followed-up as a priority by the Dept. PH. All cases and their relevant contacts were informed of enteric and hygiene precautions and were offered post-exposure hepatitis A vaccine.
Repeated requests by Dept. PH staff were required to secure a complete list of all the CCF staff members and registered children. Within the CCF, all staff, and attendees’ parents received information on hepatitis A, hygiene and enteric precautions and were advised to seek medical advice if they or household members became unwell. Post-exposure hepatitis A vaccine was offered, in order to interrupt the circulation of hepatitis A infection, to the following groups, if aged between one and 50 years: (a) household contacts of cases, (b) CCF attendees and their household contacts and (c) CCF staff. A total of 554 people were offered hepatitis A vaccine. Hepatitis A vaccine was administered by the contact's GP. In total 350 contacts accepted Hepatitis A vaccine. Consideration was given to using oral fluid hepatitis A tests to test all CCF attendees and staff but this test was not available in Ireland at the time. Serological testing was considered too invasive to be used. Staff from the Dept. PH met with the principal of case 0's school to ensure there were no additional cases or unusual absenteeism in the school attended by case 0. Following a review of the CCF by public health, the CCF voluntarily closed for 2 days to facilitate deep cleaning and a staff education session on infection prevention and control measures. After two case-free incubation periods (60 days) the outbreak was declared over.
Outbreak control costs
Table 2 [12–14] details the costs of outbreak control measures related to medical care, laboratory investigation and vaccine provision. Costing was conducted from a health service perspective. A fee of €28.50 was paid to each GP per dose of hepatitis A vaccine administered. In total, 350 vaccines were administered which represented a 63.2% uptake rate. Complete diagnostic serology costs were unavailable and therefore costs included refer only to costs reported by the reference laboratory. Human resource costs to the health service were not included in cost estimations
Table 2. Outbreak control costs including medical care, laboratory investigation and vaccine provision

Discussion
Twelve symptomatic hepatitis A cases were identified in this outbreak. Six adult cases required hospitalisation, demonstrating the seriousness of hepatitis A infection. It is likely that the total number infected was larger as hepatitis A is frequently asymptomatic, particularly in children. Whilst the initial source of the hepatitis A was not identified, the subsequent transmission of infection suggested a pattern of person to person spread. Management of the outbreak required inter-sectoral and multi-agency collaboration and utilised substantial staff resources. Public health interventions brought this outbreak under control.
Asymptomatic hepatitis A is more likely in young children. The distribution of cases in this outbreak suggests that child-to-child transmission occurred in the CCF and asymptomatic CCF attendees likely infected their adult household contacts, some of whom developed symptomatic disease. This pattern replicates that seen in previous childcare associated hepatitis A outbreaks and highlights that symptomatic cases of hepatitis A only represent a proportion of the cases in an outbreak [Reference Galmes-Truyols15, Reference Arce Arnaez16]. It is suspected that case X was the index case in this outbreak and that infection of a younger sibling who attended the CCF occurred. The hypothesis is that this sibling attended the CCF with asymptomatic but infectious hepatitis A. A source for case X's infection was not identified.
A previous inspection of the CCF highlighted deficiencies in hand-washing practices. An infection prevention audit during the outbreak highlighted deficiencies in equipment and hygiene practices, including nappy changing practice, at the CCF. It is likely that if adequate hand hygiene and infection control precautions had been followed in the CCF such an extensive outbreak would not have occurred.
The incidence of hepatitis A in Ireland has reduced substantially since the year 2000, as a result, medical professionals may not frequently include it in their list of possible differential diagnoses [3]. Hepatitis A infection should be out ruled in all cases of jaundice in a previously healthy child. However, hepatitis A was not diagnosed when case A presented to a Paediatric ED with jaundice. As a consequence case A was not diagnosed until 3 weeks later by which time an adult household contact (case B) had developed hepatitis A. Case A was subsequently notified to the local Dept. PH, by which time post-exposure hepatitis A vaccine prophylaxis was no longer a treatment option. Post-exposure hepatitis A vaccine is recognised as being up to 79% effective when administered within 14 days of exposure [Reference Li, Penrice and Gunson17]. Had case A been diagnosed and notified at the time of presenting to ED, public health interventions including post-exposure vaccination of contacts might have curtailed the spread of hepatitis A.
Genotyping was useful in confirming the link between cases and in linking case X with the outbreak. It also identified that the outbreak cases were genotypically different to other hepatitis A cases that occurred around the same time. Currently, not all hospital laboratories send serologically positive specimens to the NVRL. To facilitate linkage of apparently sporadic hepatitis A cases, genotyping all serologically positive hepatitis A specimens should be considered.
Once the outbreak was identified, control measures were implemented, including the provision of hepatitis A vaccine. Despite delays in obtaining a complete list of the CCF staff and registered children, efforts were made to ensure all identified contacts were offered hepatitis A vaccine in a timely manner. Four vaccinated contacts developed symptomatic disease. It is impossible to predict when these vaccinated cases were exposed to hepatitis A and it is likely that they were incubating the illness at the time of vaccination.
Control measures, including standard hygiene precautions, are key to prevention and control of hepatitis A outbreaks, especially in CCFs. Demonstration of knowledge and implementation of these precautions should be a major component of the inspection of CCFs by regulatory authorities. Prior to 2014, the HSE preschool inspection teams inspected and monitored CCFs. In 2014, a Child and Family Agency ‘Tusla’ was created and inspectors from this new agency have a responsibility to inspect CCFs to ensure compliance with childcare legislation. EHOs from the HSE continue to inspect CCFs for compliance with environmental health legislation. However, as this activity focuses principally on food preparation activities, processes related to hygiene within the CCF are not being assessed in a standardised manner. Following notification of cases within this CCF, EHOs conducted an inspection and deemed the CCF review satisfactory. However, person–person spread was the most likely mode of Hepatitis A transmission within this CCF. A system of regulation and inspection, that ensures adequate precautions to mitigate against future similar infection control incidents in the child care setting, must be developed as a priority.
The management of this outbreak was logistically challenging. The option of providing hepatitis A vaccination clinics, staffed by Dept. PH, in the locality of the CCF was deemed unfeasible as the geographical spread of CCF attendees’ and their household contacts were considerable. The co-operation of GPs and their prompt vaccination of contacts were key to bringing this outbreak under control. Unlike many other jurisdictions, the HSE does not have a formal agreement with GPs to facilitate vaccination in the event of an outbreak. Such a contract between the HSE and GPs would facilitate a more streamlined response to similar outbreaks in the future.
Considerable resources, both human and financial were required to control this outbreak. These costs could have been avoided with early recognition of initial cases and instigation of timely control actions. Such actions would also have prevented costly adult hospital care.
Conclusion
Despite a considerable reduction in hepatitis A incidence in recent years in Ireland, outbreaks continue to occur and associated morbidity can be considerable. In CCFs, it can be difficult to recognise and prevent the spread of infection, due to the asymptomatic nature of hepatitis A infection in young children. Therefore, high standard infection prevention and control measures and implementation of inspection recommendations must be ensured.
The delayed notification to public health case A probably contributed to the extent of this outbreak. Medical professionals should be aware that, while uncommon, hepatitis A continues to occur in the population. Prompt recognition and notification of cases to public health facilitate initiation of immediate public health control measures. These measures should help mitigate against the significant morbidity associated with this infectious disease especially in adults.
Acknowledgements
The authors wish to thank all members of staff at the Department of Public Health, HSE East who were involved in the investigation and control of this outbreak. In addition, we wish to thank the staff of the National Immunisation Office who provided advice and practical support on vaccine-related matters during the outbreak. The authors also wish to thank all the general practitioners and their staff who facilitated vaccination of outbreak-related contacts. We also acknowledge and thank the staff at local hospital-based Departments of Microbiology and Dr Suzie Coughlan and Dr Joanne O'Gorman at the National Virus Reference Laboratory.
Declaration of interest
None declared.
Financial support
This research received no specific grant from any funding agency, commercial or not-for-profit sectors.
Ethical standards
The authors assert that all procedures contributing to this work comply with the ethical standards of the relevant national and institutional committees on human experimentation and with the Helsinki Declaration of 1975, as revised in 2008.