INTRODUCTION
Campylobacteriosis is the most common cause of bacterial enteritis in people in Canada, with about 9500 laboratory-confirmed cases each year [1]. The most commonly recovered species is Campylobacter jejuni, followed distantly by C. coli and C. lari [1, 2]. Campylobacter usually causes mild to severe gastrointestinal infection in humans, including nausea, vomiting and watery diarrhoea, but potentially life-threatening sequelae can occur (e.g. Guillain–Barré syndrome) [Reference Blaser and Fauci3, Reference Altekruse and Tollefson4]. The majority of human cases are sporadic and believed to be foodborne; however, since the early 1980s, several studies have investigated the role of companion animals as potential sources of human infections (e.g. [Reference Blaser5, Reference Fox, Moore and Ackerman6]). Many studies have identified having a household pet, especially a puppy, or a dog with diarrhoea, as a risk factor for Campylobacter infection in people [Reference Adak7–Reference Stafford12]. Dogs have also been suspected as the source of transmission in several cases of campylobacteriosis [Reference Goossens13–Reference Wolfs15].
Domestic dogs have long been recognized as potential sources of zoonotic enteric pathogens like Salmonella, Campylobacter and Giardia [Reference Shane, Beran and Steele16, Reference Acha, Szyfres, Acha and Szyfres17]. The prevalence of Campylobacter carriage in clinically healthy pet dogs has been estimated to be between 15% and 58% [Reference Baker, Barton and Lanser18–Reference Parsons24], but can be as high as 87% in stray animals [Reference Acke25]. Campylobacter can cause both clinical and non-clinical infections in dogs, with the most severe sequelae occurring in young and immunocompromised dogs; often associated with C. jejuni infection [Reference Fox, Moore and Ackerman6, Reference Fox and Greene26]. The most commonly isolated species of Campylobacter in dogs has varied in studies due to microbiological methods, but in recent work, C. upsaliensis has been the most frequently recovered species in dogs [Reference Acke21, Reference Westgarth22, Reference Parsons24, Reference Koene27].
It is estimated that there are about six million dogs in Canada, with 32% of households owning at least one dog [Reference Perrin28]. In North America, a strong human– animal bond means that dogs are often considered family members rather than simply ‘pets’. It is this close relationship that causes concern with respect to the potential transmission of zoonotic infections from dogs to humans. Several studies have identified young age, the presence of diarrhoea, season, and high-density housing, like kennels and shelters, as significant risk factors for the carriage of Campylobacter in dogs [Reference Wieland20–Reference Parsons24, Reference Hald29]. Due to a lack of detailed investigations examining pet-related management factors and their association with Campylobacter carriage in dogs in North America, investigations are required to identify these factors, and determine which management practices may potentially put pet-owners at increased risk of infection from their pets. The purpose of this study was to determine which pet-related management factors, including type of diet fed to the dog, the dog's exposure/access to other pets and livestock, the dog's involvement in group activities (e.g. obedience), and veterinary treatments, are associated with the carriage of Campylobacter. In addition, human-related factors, including the presence of children in the home, household members' exposure/access to other animals and livestock, whether household members have visited or worked in a hospital, and any household members experiencing vomiting or diarrhoea in the previous week, have also been examined. This study will be used to explore the epidemiology of carriage of Campylobacter and specific Campylobacter spp. in a population of client-owned pet dogs from the Region of Waterloo, Ontario, Canada.
METHODS
Recruitment
Between July 2008 and May 2009, dogs visiting seven veterinary clinics in the Region of Waterloo, Ontario were recruited to participate in a study to investigate the occurrence of Campylobacter, Salmonella, Giardia, and antimicrobial resistance in generic Escherichia coli in client-owned pet dogs. This paper contains only the Campylobacter results. Veterinarians from all 44 veterinary clinics in the Region of Waterloo, Ontario were sent letters inviting them to participate in the study, with nine clinics responding and seven clinics agreeing to participate. Once a clinic agreed to take part, the primary author (E.L.) visited each of the clinics every 7–14 days for 10 months to recruit client-owned pet dogs for the study. Any dog visiting the clinic was eligible to participate, including those with signs of gastrointestinal disease and those being treated with antimicrobials; however, only one dog per household was included in the study and dogs were only eligible to participate in the study once. Dog-owners visiting the veterinary clinics were asked by their veterinarian or the primary author to participate in the study. Those who agreed to participate then spoke with the primary author, the owner questionnaire was administered, and the owner was provided with a faecal collection kit to collect and return a single faecal swab per day for two consecutive days. The study was approved by the University of Guelph Research Ethics Board.
Samples
The faecal kit provided to the dog-owners contained an instruction guide, tongue depressors, disposable gloves, biohazard bags, sterile specimen containers, two sterile Cary–Blair agar swabs (CultureSwab™ Cary–Blair agar; Becton Dickson and Company; USA), and pre-addressed, postage-paid cushioned envelopes for mailing the samples. Faecal swabs were used for Campylobacter isolation because the samples had to travel in the mail, and due to the fastidiousness of Campylobacter, it was felt the agar in the Cary–Blair swabs would provide better recovery. In a small trial completed by our laboratory using faecal samples spiked with Campylobacter, the faecal swabs remained positive after being mailed, whereas the full faecal samples did not (unpublished results). The fecal swab was plunged into the freshly passed faeces collected in the sterile specimen containers, and then placed in the Cary–Blair agar tube. Proper use of the Cary–Blair swabs was demonstrated for each owner at the time of recruitment. The two Cary–Blair swabs were tested for Campylobacter spp. only.
Microbiological analysis
All samples were received via express post at the University of Guelph. Upon arrival, information pertaining to the faecal swabs was documented and swabs were immediately sent to the Laboratory Services Division (LSD), University of Guelph for Campylobacter isolation. The faecal swab was streaked directly on to modified cefoperazone charcoal deoxycholate agar (mCCDA) plates [Campylobacter selective blood-free agar (CM0739) and CCDA selective supplement (SR0155), Oxoid, Canada] and the swab was then inserted into 5 ml Bolton broth (Oxoid). A 1-ml aliquot of the inoculated Bolton broth was then added to 9 ml new Bolton broth for further enrichment. The plates and broth were incubated for 48 h at 42°C in a micro-aerophilic atmosphere, based on standard Campylobacter spp. isolation methods at LSD. The mCCDA plate with the direct streak was then read and both Bolton broth dilutions were plated onto mCCDA and incubated for another 72 h. Controls were used at every stage of the procedure. All mCCDA plates were observed for Campylobacter based on the presence of grey colonies. If present, colonies were re-streaked for purity, and tested for oxygen tolerance and growth at 25°C. Additionally, dark-field microscopy, catalase and oxidase tests, and antibiotic sensitivity tests for cephalothin and nalidixic acid, were conducted on all suspect colonies to confirm the presence of Campylobacter. All Campylobacter isolates were frozen in glycerol at −70°C to allow for future molecular typing. A dog was considered positive for Campylobacter if at least one swab tested positive.
Polymerase chain reaction (PCR) species identification
A series of PCR assays were performed targeting the 16S rRNA encoding genes to determine the species of Campylobacter. A loopful of the glycerol frozen broth containing the isolate was inoculated onto Columbia agar and incubated with CampyGen™ (Oxoid), an atmosphere generation system, at 37°C for 2 days. This culture was then subcultured on another Columbia agar plate and incubated with CampyGen™ at 37°C for 1 day. Once the isolate had been purified, the DNA was extracted using InstaGene™ (Bio-Rad Laboratories, USA) and the remaining culture was stored on Cryostor™ beads (Oxoid) at −70°C. If the culture was catalase positive from previous biochemical testing performed at LSD, PCR methods previously described were used to identify the isolate [Reference Denis30]. If the catalase-positive culture was negative for both C. jejuni and C. coli based on the above PCR methods, a second PCR method was used to identify C. lari [Reference Linton, Owen and Stanley31]. Finally, if the culture was catalase negative, a previously described PCR method for C. upsaliensis and C. helveticus was used to identify the isolate [Reference Linton, Owen and Stanley31]. The primers and targets used for Campylobacter spp. identification can be found in previously published work [Reference Denis30, Reference Linton, Owen and Stanley31].
Questionnaire
Each questionnaire was administered by the primary author to the primary caregiver of the recruited dog during their visit at the veterinary clinic. The questionnaire included questions concerning the following: the dog's main diet and whether additional animal products were added to the diet; the presence of other pets in the home; the dog's activities; the occurrence of vomiting and diarrhoea in the previous month; veterinary care, including de-worming; any contact with livestock; and the use of antibiotics in the previous month. Breed, age, sex and neuter status were also collected for all dogs. The variables investigated in this study can be found in Table 1 and the questionnaire is available upon request.
Table 1. List of pet-related management variables evaluated for an association with Campylobacter spp. carriage in client-owned pet dogs in the Region of Waterloo, Ontario 2008–2009 (n=240)

a Small, medium, large/giant breed or mixed breed.
b Participation in activities like obedience, flyball, agility.
c Livestock includes cattle, sheep, goats, pigs, or horses.
d Diagnosed with Salmonella, Campylobacter, Giardia or C. difficile.
Statistical analysis
Data from the questionnaires were entered into EpiData© version 3.1 (EpiData Association, Denmark) and analysed in Intercooled Stata/MP® 11.0 for Windows (USA). All tests were two-tailed with a statistical significance level of 5%. Univariable logistic regression models were used to screen all variables from the questionnaire for an association with Campylobacter spp. carriage and for each species of Campylobacter isolated if sufficient data were available (e.g. C. upsaliensis, C. jejuni). Significant continuous variables were evaluated for linearity with the log odds of the outcome using lowess curves and categorical linear trends (lintrend plots) [Reference Dohoo, Martin and Stryhn32]. Pair-wise correlations between significant variables from the univariable analysis (P⩽0·20) were examined using Spearman's correlation test. Variables with correlation values >0·7 were investigated and the variable that was more biologically plausible, or had the least number of missing values, was included in the model [Reference Dohoo, Martin and Stryhn32].
Multivariable models were constructed for the dogs Campylobacter spp. status (positive/negative) and for individual Campylobacter species where data were sufficient. The main-effects models were created with the significant variables from the univariable analysis (P⩽0·20). A manual backwards step-wise procedure was used to construct the multivariable model. Likelihood ratio (LR) tests were used to assess the significance of each model as variables were removed. Confounding was evaluated by examining the effect of the removed variables on the coefficients of the remaining variables. A variable was determined to be a confounder if the log odds of the other independent variables changed by ⩾20%, and it was not an intervening variable [Reference Dohoo, Martin and Stryhn32, Reference Thrusfield33]. The potential confounding effects and interactions of breed (mixed, pure small, pure medium, pure large); age (years); sex (male, female); and neuter status (intact, neutered) were examined regardless of statistical significance due to the suspected impact of these demographic characteristics on management-related risk factors. Interaction terms were examined for all remaining variables in the final model. To assess clustering, clinic was modelled using two approaches. In the first, clinic was modelled as a random effect. Clinic was also modelled as a fixed effect because of concerns that due to the limited number of clinics, the random effects would not be properly estimated. The significance of clinic both as a random effect and fixed effect was assessed based on a LR test. If the clinic variables were not significant and did not confound any measures of association, the simpler multivariable models were reported. For the multilevel models, the normality of best linear unbiased predictors (BLUPs) were assessed with normal quantile plots to determine model fit [Reference Dohoo, Martin and Stryhn32]. For standard logistic regression, residuals and Hosmer–Lemeshow goodness-of-fit tests for the final models were assessed. A P value ⩽0·05 from the goodness-of-fit test indicated that the model did not fit the data [Reference Dohoo, Martin and Stryhn32].
RESULTS
In total, 240 client-owned pet dogs were recruited for the study. From the seven participating veterinary clinics, 492 pet-owners were approached to participate in the study, 279 dogs were recruited for the study, and complete samples were received for 240 dogs. Both faecal swabs were received from 97·5% [234/240, 95% confidence interval (CI) 94·64–99·08] of the dogs, and only one swab was received from 2·5% (6/240, 95% CI 0·92–5·36) of the dogs. The median number of dogs recruited from the clinics was 35, with a minimum of three and maximum of 80. Questionnaires were completed for all dogs recruited for the study. Of the participating dogs, 52·9% (127/240, 95% CI 46·39–59·37) were female and 16·7% (40/240, 95% CI 12·18–21·99) were aged <1 year, with the average age of all participating dogs being 4·9 years (95% CI 4·39–5·39). Demographic information of the participating dogs can be found in Table 2.
Table 2. Demographic information of the client-owned pet dogs sampled in this study from the Region of Waterloo, Ontario 2008–2009 (n=240)
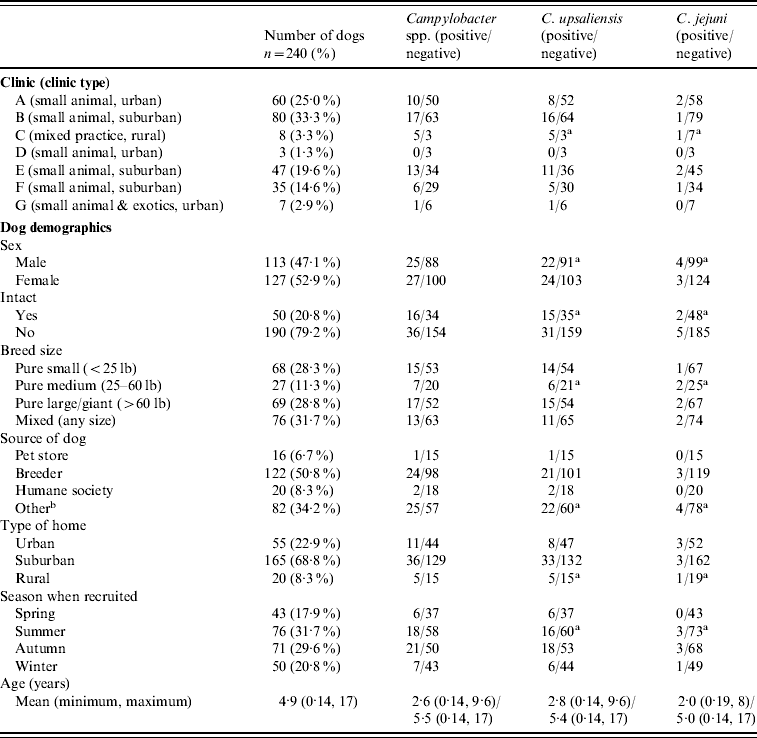
a Total is greater than total number positive because one dog had both C. upsaliensis and C. jejuni.
b Included farms, friends, rescue groups, etc.
About 21·7% (52/240, 95% CI 16·63–27·42) of the dogs enrolled in this study had at least one faecal swab positive for Campylobacter. The predominant species of Campylobacter recovered was C. upsaliensis, which was shed by 19·2% (46/240, 95% CI 14·39–24·73) of the dogs in the study. Of the Campylobacter-positive dogs, 88·5% (46/52, 95% CI 76·56–95·65) carried C. upsaliensis, and 13·5% (7/52, 95% CI 5·59–25·79) carried C. jejuni. One dog had both C. upsaliensis and C. jejuni and no other species of Campylobacter were recovered.
In total, 81 variables relating to the dogs' health, diet, and common exposures were examined in univariable models (Table 1). The variables found to be significant at the 20% level in the Campylobacter spp. and C. upsaliensis univariable logistic regression models are given in Table 3. There were no statistically significant associations found in any of the models between Campylobacter carriage and vomiting or diarrhoea, or Campylobacter carriage and season. Moreover, clinic was not found to be significant as a random effect or fixed effect in any multilevel logistic regression models, with P values >0·99 and variance <0·001, and insignificant LR tests. Age (years) was statistically significant and was found to be linearly associated with carrier status on the lowess and lintrend plots, and was kept as a continuous variable in all of the models. Univariable and multivariable logistic regression models were not examined for C. jejuni carriage because only seven dogs were found to be carrying C. jejuni resulting in a very small effective sample size.
Table 3. Descriptive statistics and significant associations (P⩽0·20) from univariable logistic regression analysis of pet-related management factors and Campylobacter spp. and C. upsaliensis carriage in client-owned pet dogs, recruited through veterinary clinics in the Region of Waterloo, Ontario, 2008–2009 (n=240)
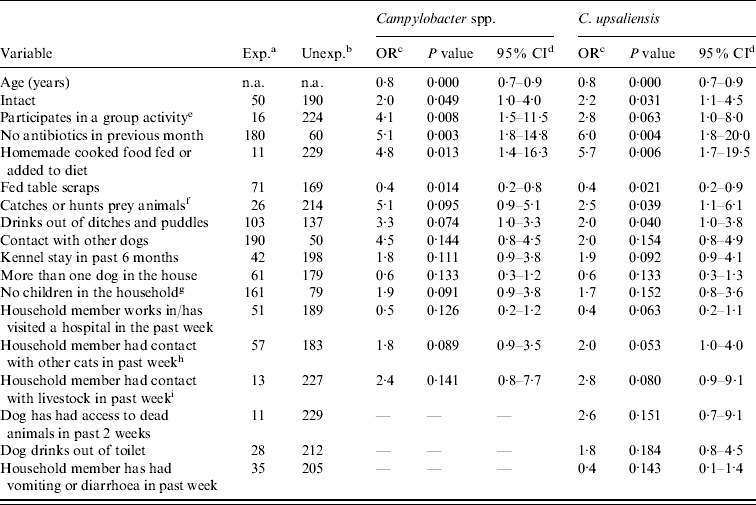
OR, Odds ratio; CI, confidence interval.
Dashes (–) signify that the variable was not significant at the 20% level in that univariable model.
a Exposed dogs (i.e. those that were positive for the risk factor).
b Unexposed dogs (i.e. those that were negative for the risk factor).
c Odds ratio calculated in Stata/MP 11.0.
d 95% confidence interval of the odds ratio calculated in Stata/MP 11.0.
e Included obedience, flyball and agility classes.
f Included birds, small rodents and other small prey.
g Children aged <18 years that live in the home on a regular basis.
h Someone who lives in the home had contact with a cat that was not their own in the previous 7 days.
i Someone who lives in the home had contact with livestock (cattle, sheep, goats, pigs, or horses) in the previous 7 days.
From the multivariable model for Campylobacter spp. carriage, not being treated with antibiotics in the previous month, not having children in the home, and having homemade cooked food as the dog's diet or added to the dog's diet increased the odds of carriage. The odds of Campylobacter spp. carriage decreased as the age of the dog increased (Table 4).
Table 4. Significant risk factors (P⩽0·05) from multivariable logistic regression analysis of pet-related management factors and Campylobacter spp. and C. upsaliensis carriage for client-owned pet dogs recruited through veterinary clinics in the Region of Waterloo, Ontario, 2008–2009 (n=240)
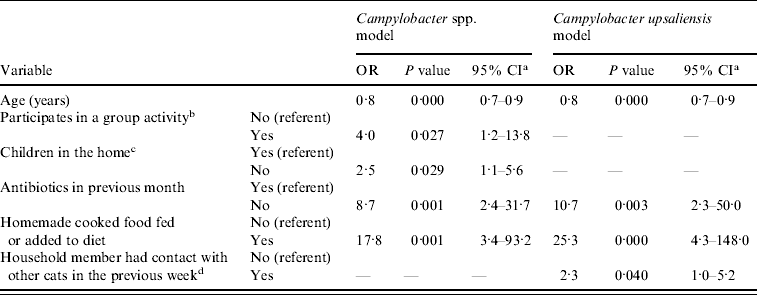
OR, Odds ratio; CI, confidence interval.
Dashes (—) signify that the variable was not significant at the 5% level in that multivariable model.
a 95% confidence interval of the odds ratio.
b Participation in activities like obedience, flyball, agility.
c Children aged <18 years that live in the home on a regular basis.
d Someone who lived in the home had contact with a cat that was not their own in the previous 7 days.
In the multivariable model for C. upsaliensis carriage, not being treated with antibiotics in the previous month, a household member having contact with a cat that was not their own in the previous week, and having homemade cooked food as the dog's diet or added to the dog's diet increased the odds of carriage. The odds of C. upsaliensis carriage decreased as the age of the dog increased (Table 4).
In the multivariable models for Campylobacter spp. and C. upsaliensis carriage, interactions between the significant variables were not found to be statistically significant (P>0·05). Residuals from both final multivariable models were examined for outliers and influential covariate observations. There were several observations with large residuals; however, the data were examined and found to be correct, and therefore all observations were kept in the final models. The final models for Campylobacter spp. and C. upsaliensis were not significant at the 5% level with the Hosmer–Lemeshow goodness-of-fit tests (P=0·64 for both models), indicating that the models fit the data.
DISCUSSION
This study offers a detailed investigation of pet-related risk factors for Campylobacter carriage in client-owned pet dogs in North America. Previous risk-factor research has been completed mostly in Europe and Australia. The occurrences of Campylobacter, C. upsaliensis, and C. jejuni found in this study were consistent with the estimated prevalences previously reported for household dogs [Reference Acke21–Reference Parsons24]. A number of the investigated pet-related management factors (i.e. age and antibiotic use) associated with the carriage of Campylobacter, were consistent with those found in previous studies [Reference Wieland20–Reference Westgarth22, Reference Parsons24]. The potential role of adding cooked human food to a pet dog's diet as a risk factor for C. upsaliensis carriage needs to be investigated further, as a similar association has also been demonstrated in a recent study [Reference Westgarth22]. Our study also demonstrated that C. upsaliensis was much more common in this population of pet dogs than C. jejuni, which is in agreement with several earlier studies [Reference Acke21, Reference Westgarth22, Reference Parsons24, Reference Koene27]. This study highlights the fact that pet dogs may be an important source of Campylobacter, especially C. upsaliensis, and exposure to dogs must be considered in human cases of campylobacteriosis. Moreover, Campylobacter from positive dogs should be speciated in order to determine the risk for human infection and any species-specific control methods that may be necessary.
This study has the following limitations that need to be considered to avoid over-interpreting our results: the subjects were not recruited randomly; the response rates by clinic and client were poor; and the exploratory nature of the study resulted in many variables being examined. Without a random sample, the reader should be cautious about extrapolating the prevalence in our study population to the Region of Waterloo or Ontario. However, similar prevalences of Campylobacter carriage in pet dogs have been found in several recent studies [Reference Acke21–Reference Parsons24]. In terms of poor response level, non-response is a form of selection bias that could have altered the size and direction of the odds ratios estimated from our models. However, for this selection bias to occur, non-participation (non-response) by dog-owners or veterinary clinics needs to relate to both the examined pet-related risk factors and Campylobacter carriage [Reference Dohoo, Martin and Stryhn32]. Considering that few animals were showing clinical signs, and no association was found between diarrhoea or vomiting and Campylobacter carriage, it is unlikely that owners' willingness to participate was related to both the outcome and the exposures of interest. Further, no association was found between clinic and Campylobacter carriage, therefore it is unlikely that clinics' willingness to participate was related to both the outcome and the exposures of interest. Finally, like many exploratory studies, a large number of variables were examined, so the possibility of type I errors should be noted. Where we have identified novel risk factors for Campylobacter carriage, we suggest these variables be examined in future studies. Also, in view of the fact that this study was cross-sectional in nature, we cannot determine which factors cause Campylobacter carriage and which factors prolong carriage since prevalence is a function of incidence and duration [Reference Dohoo, Martin and Stryhn32, Reference Rothman, Greenland and Lash34]. However, controlling management factors related to prevalence itself would be useful for protecting public health.
Feeding homemade cooked food was found to increase the odds of Campylobacter spp. and C. upsaliensis carriage in dogs in our study. Previously, C. jejuni-contaminated food has been associated with infection with Campylobacter in humans and animals [Reference Lee, Billington and Joens35, Reference Karenlampi36]. To date, dogs and cats have been assumed to be the only reservoir for C. upsaliensis [Reference Bourke, Chan and Sherman37]. However, a recent study by Westgarth et al. [Reference Westgarth22] also found an association between feeding leftover human food and C. upsaliensis carriage in community dogs. In our study, only one participating dog was fed a raw food diet; therefore, the association with feeding human food may be due to poor food-handling practices rather than direct exposure from raw food. Nonetheless, these findings of an association between feeding homemade cooked food and leftovers, and the presence of C. upsaliensis in canine faeces, may warrant the inclusion of C. upsaliensis in food safety surveillance programmes in the future. Microbiological testing of the foods fed to the dogs in this study was not done, so a direct connection cannot be made. However, sample size needs to be taken into account with this association in our study, as only 11/240 dogs were fed homemade cooked food, either as their main diet or added to their diet.
Similar to previous studies, a significant difference in Campylobacter and C. upsaliensis carriage was observed based on age [Reference Wieland20–Reference Westgarth22, Reference Parsons24, Reference Modolo and Giuffrida38], and with every year increase in age, the odds of Campylobacter and C. upsaliensis carriage decreased by 0·8 (Table 4). This is probably due to the inexperienced immune systems of the younger dogs; as dogs mature the occurrence of Campylobacter carriage decreases [Reference Fox, Moore and Ackerman6, Reference Hald29].
Lack of exposure to antibiotics in the month prior to sample testing was found to increase the odds of Campylobacter carriage in our study. A similar finding has been discussed in previous studies, but, unlike in our study, the association was not found to be statistically significant [Reference Westgarth22, Reference Parsons24]. The association between lack of antibiotic use and an increase in the risk of Campylobacter carriage is logical given the antibacterial function of most antibiotics; however, treatment with antibiotics is controversial and only recommended in severely ill animals [Reference Fox and Greene26]. Consequently, the use of antibiotics to prevent the carriage of Campylobacter in clinically healthy dogs is not normally recommended.
Interestingly, two previously unreported findings, not having children living in the home, and a household member having contact with a cat that was not their own, were associated with an increase in the odds of carriage of Campylobacter spp. and C. upsaliensis in pet dogs, respectively. In previous studies, having a dog or puppy was associated with an increase in the risk of Campylobacter carriage in children [Reference Tenkate and Stafford8, Reference Carrique-Mas10]; however, current research has not studied the association in the opposite direction. For C. upsaliensis carriage and cat contact, it is possible that these owners were experiencing a greater deal of contact with other animals and may have been acting as a vector of Campylobacter for their pets. Cats have been found to carry Campylobacter, including C. upsaliensis [Reference Bender39] and have been identified as a significant risk factor for C. jejuni infection [Reference Deming40]. It is also possible that these variables are acting as proxies for other statistical associations, or could simply be due to chance because of the large number of variables investigated. Nonetheless, these associations should be investigated in future studies.
Finally, given that only 52 dogs were found to be shedding Campylobacter spp., caution should be taken when interpreting the non-significant results in this study. Potential risk factors for carriage of Campylobacter spp. may have been missed due to the large effect and/or small amount of variation that is often needed to observe statistical significance in small studies [Reference Dohoo, Martin and Stryhn32]. Weaker associations could have been disguised by the small sample size.
This study identified several novel risk factors for Campylobacter spp. carriage in pet dogs, including lack of antibiotic exposure, not having children in the home, exposure to cats and other pets, and including homemade cooked food in the dog's diet, that require further investigation. These results may warrant a change in the current surveillance of Campylobacter spp. in food sources, specifically in the case of C. upsaliensis. Recent changes in laboratory methods for processing canine faecal samples have given rise to an increased prevalence of C. upsaliensis in dogs [Reference Byrne41, Reference Kulkarni42]. It is possible that C. upsaliensis is more common in food sources and in human cases of campylobacteriosis than is currently appreciated, since it may be missed as a result of using laboratory methods designed to detect C. jejuni and C. coli (i.e. catalase-positive Campylobacter spp.). Current laboratory methods used for isolation of Campylobacter spp. from human faecal samples and food samples often involve the use of agar plates and broth suspensions that contain cefaperazone, nalidixic acid, and cephalothin at levels that prevent the growth of C. upsaliensis [Reference Bourke, Chan and Sherman37]. Using a previously described filtration method, Lastovica & Le Roux [Reference Lastovica and Le Roux43] found that almost 25% of campylobacteriosis cases in humans in South Africa were due to C. upsaliensis. A study from the USA has suggested that C. upsaliensis is the second most commonly isolated Campylobacter spp. in humans, after C. jejuni [Reference Labarca44]. A Belgian study also found that C. upsaliensis was recovered more often than C. coli in humans, indicating that C. upsaliensis may be of greater importance than previously thought [Reference Goossens45]. C. upsaliensis is certainly capable of causing disease in humans and may be more common than believed in Canadian human infections [Reference Bourke, Chan and Sherman37, Reference Taylor, Hiratsuka and Mueller46]. Further research into the prevalence of C. upsaliensis in human gastrointestinal disease and the potential sources of C. upsaliensis is warranted. The information collected from this study and similar future studies, is crucial for the development of evidence-based guidelines for safe dog-ownership and to protect the public through responsible pet management.
ACKNOWLEDGEMENTS
Sample collection and testing for this study were supported by the Public Health Agency of Canada and the Ontario Veterinary College Pet Trust Fund. The infrastructure for statistical analyses was supported through a grant to D. L. Pearl from the Canada Foundation for Innovation and the Ontario Research Fund. The primary author was supported through the Blake Graham Fellowship from the Ontario Veterinary College. Isolation of Campylobacter was completed by Laboratory Services Division, University of Guelph (Dimitrinka Oke and Susan Lee). PCR was completed by Joyce Rousseau of Dr J. S. Weese's laboratory in the Department of Pathobiology, University of Guelph.
DECLARATION OF INTEREST
None.








