INTRODUCTION
Symptoms due to Shiga toxin-producing Escherichia coli (STEC) infection in humans range from asymptomatic or uncomplicated diarrhoea to more severe symptoms like bloody diarrhoea and haemolytic uraemic syndrome (HUS) [Reference Nataro and Kaper1, Reference Mead and Griffin2]. Children and the elderly are more likely to develop severe disease. STEC O157 is a zoonotic agent with ruminants as the main reservoir [Reference Nataro and Kaper1]. Inevitably, direct and indirect contact with ruminants, especially cattle, is implicated as a major transmission route [Reference Voetsch3, Reference O'Brien, Adak and Gilham4]. Incidence of STEC O157 infection follows a seasonal pattern, with most cases being reported during the late summer months [Reference Eklund5–Reference Slutsker7]. This pattern seems to run parallel to the seasonal pattern in excretion of STEC O157 in ruminants [Reference Caprioli8]. Furthermore, higher incidence of human STEC infection in rural areas has been found compared to urban areas, with a spatial association between the incidence of human STEC infection and cattle density [Reference Michel6, Reference Valcour9, Reference Frank10].
Since January 1999, all medical microbiological laboratories and public health services in The Netherlands have been requested to participate in an enhanced surveillance of STEC O157 infection. Notification of ‘disease due to enterohaemorrhagic E. coli’ became mandatory in December 1999. The aim of the current study was to evaluate the spatial association between reported incidence of human STEC O157 infections in The Netherlands and livestock density (cattle, pigs, poultry) in relation to age and season.
METHODS
For enhanced surveillance, all medical microbiological laboratories in The Netherlands were requested to report a positive result for STEC O157 to the local public health service. This could be a presumptive isolate from a faecal sample or a positive serological test for E. coli O157 antibodies. In addition, they were requested to send an isolate to the RIVM for O- and H-typing, and for testing for genes encoding Shiga toxin 1 (stx1), stx2, E. coli attaching-and-effacing (eae) gene and the EHEC haemolysin (e-hly) gene by PCR.
Cases are defined as patients reported between April 1999 and December 2008 with an STEC O157 isolate confirmed at the RIVM. In this period, two national outbreaks were registered; one in 2005 involving 21 cases where the most likely cause is thought to have been filet américain [Reference Doorduyn11], and one in 2007 involving 41 cases probably caused by lettuce [Reference Friesema12]. The latter outbreak was also linked to an outbreak in Iceland. In 2007, a regional outbreak of seven cases was registered. All seven cases reported consumption of filet américain, and all had bought it at one regional supermarket chain. Cases linked to these three outbreaks were excluded.
A spatial regression analysis was performed to assess possible associations between STEC O157 and densities of cattle, pigs, and poultry in the period 1999–2008. Information on population counts and livestock densities (animals/km2) for each municipality were obtained from the Statistics Netherlands database [13]. Every year, all farmers are requested to report their number of animals to the Ministry of Agriculture, Nature and Food Quality (response 96%), which is converted to number of animals per municipality before being made available to the public. Therefore, STEC O157 cases and population were also aggregated by municipality (496 in total). In The Netherlands, a median municipality comprises 19 250 inhabitants (range 1000–750 000 inhabitants), and a municipality covers a median area of 52 km2 (range 2–472 km2). Besides municipality, cases and population were aggregated by age group (<5, 5–9, 10–49, ⩾50 years), sex, and season [‘winter’ (December–May), ‘summer’ (June–November)]. Cases and population numbers were summed over the period 1999–2008. Person-years were defined as the summed population numbers over this period. Livestock densities were averaged over these 10 years.
Next, a model was built to describe the number of cases as a function of person-years (the population denominator corresponding to the number of cases), age, sex, season, and livestock density (categorized into five classes). Since it is expected that there is a higher risk in the summer season for young children, an interaction between season, age, and livestock density was added. Together, these terms are the so called fixed-effect terms. Two extra terms were added to account for extra variation due to spatially structured and unstructured variation. These terms are the so-called random-effect terms. Schematically, the model can be written as:
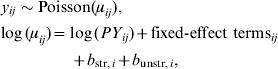
where y ij is the number of cases in municipality i and stratum j, with each stratum representing a subgroup of individuals with the same characteristics (age, sex, region, community, season of infection). The intensity μij is the expected number of cases, y ij is therefore Poisson-distributed with this mean. The log of the intensity is a summation of log person-years PY ij (the offset of the Poisson model), fixed effects and random effects.
The spatially structured variation b str,i represents the effect that neighbouring municipalities are more alike, perhaps due to a possible common unobserved (risk) factor. This term is modelled by the intrinsic Conditional Autoregressive Model [Reference Besag, York and Mollié14]. The unstructured term b unstr,i represents possible unobserved variation within municipalities and is modelled by independent and identically distributed Gaussian noise [Reference Lawson15].
Coefficients of the fixed effects are used to calculate relative rates. Relative rates of the spatially structured and unstructured effects are given for each municipality. Here the reference is total fixed effect for each municipality.
The model was fitted by the recently developed technique called the integrated nested Laplace approximation (INLA) [Reference Rue, Martino and Chopin16]. INLA is specifically developed for quick statistical inference of latent Gaussian Markov random fields like the random-effect terms in the model above. The advantage of INLA over the standard Markov chain Monte Carlo techniques using WinBUGS [Reference Lunn17] is that it has much shorter computing times: seconds or minutes instead of hours or days. Data pre- and post-processing, inference and visualization were done in the statistical software environment R [18].
RESULTS
Between April 1999 and December 2008, 409 symptomatic cases of STEC O157 infection were reported, with a median number of 40 cases/year (range 32–57 cases/year). These cases represent an annual incidence rate of 1·9–3·5 cases/1 000 000 inhabitants. The gender ratio of cases was 43% male and 57% female. The cumulative incidence rate (summed over 10 years) for sex and age groups is given in Table 1. Note the higher rates for young children and females in higher age groups. Lowest incidence is seen during December–May, and the highest incidence between July and September. The incidence of reported human STEC O157 infections varies greatly between municipalities and appears to be higher in the northern and eastern regions of The Netherlands (Fig. 1). Two neighbouring areas showing particularly low incidence of STEC O157 are in the mid- and north-west of The Netherlands.
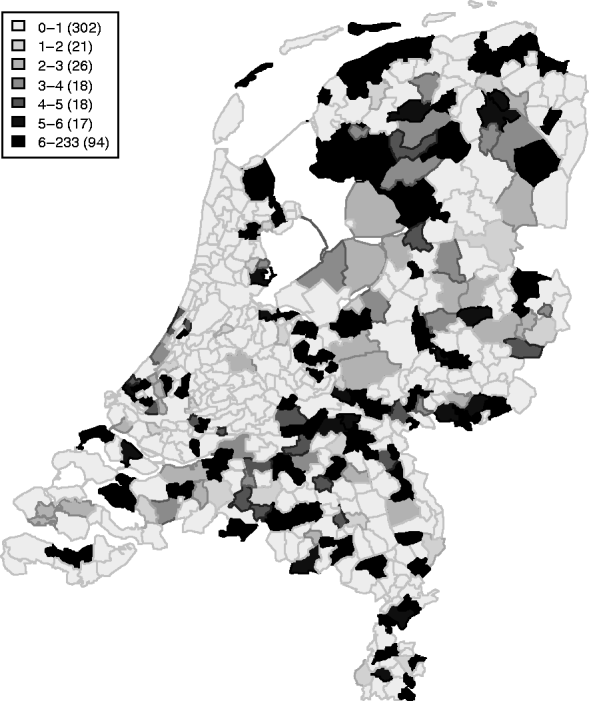
Fig. 1. Cumulative incidence rate (×1 000 000 person-years) (1999–2008) of STEC infections in The Netherlands.
Table 1. Cumulative incidence rates per 1 000 000 person-years by age group and gender in The Netherlands, 1999–2008
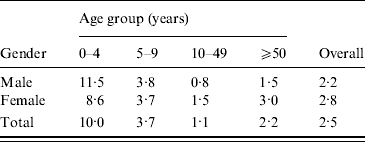
Cattle density is highest in the centre and central north of the country (Fig. 2). Pig density is highest in the east and south-east. Poultry density is comparable to pig density, but with higher densities also in the north. Figures 1 and 2 suggest an association between cattle density and STEC O157 cases.
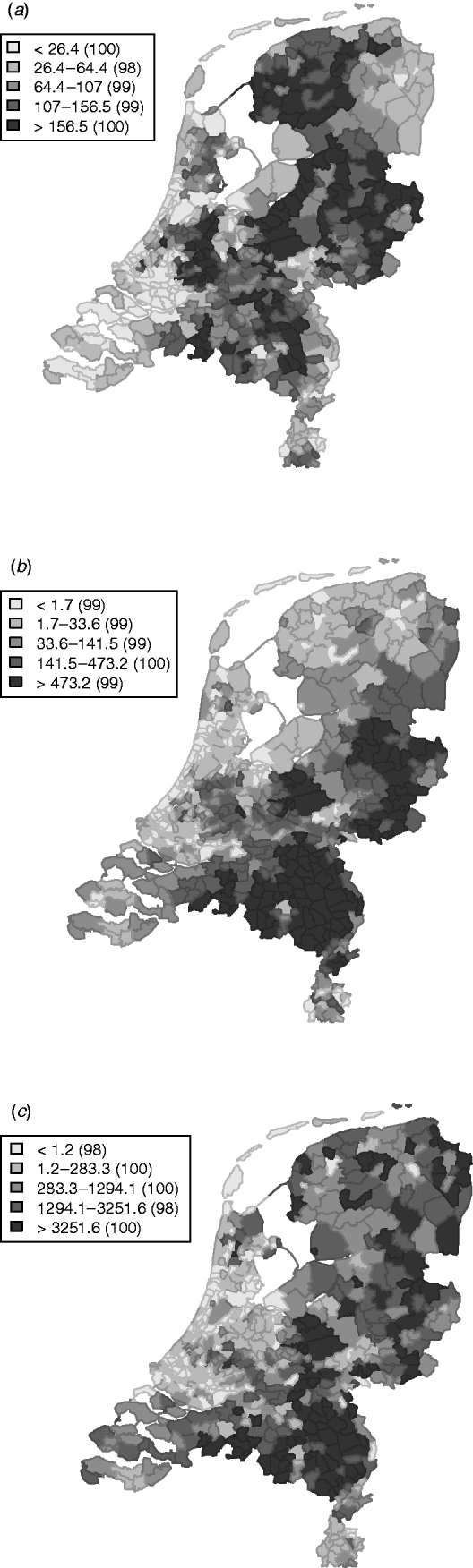
Fig. 2. Average livestock densities (/km2) (1999–2008) in The Netherlands for (a) cattle, (b) pigs, (c) poultry.
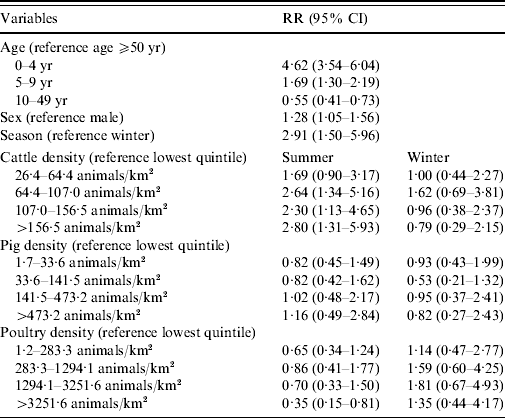
Living in areas with >64 cows/km2 increases the risk of reporting STEC O157 infection in summer by more than twofold of that of areas with <26 cows/km2 (Table 2). No association with STEC O157 infections was found for pig density and a decreased risk was found for living in the highest quintile regarding poultry density. Males had a lower risk of developing a STEC O157 infection. Young children, especially those aged <5 years, showed the highest chance of developing a symptomatic STEC O157 infection compared to adults aged ⩾50 years. The 10–49 years age group had almost half the risk for a reported STEC O157 infection compared to older adults.
Table 2. Relative risk (RR) estimates with 95% confidence intervals (CI) for STEC O157 infection in The Netherlands, 1999–2008
Adding an interaction term between season, age, and livestock density in the model showed only a seasonal effect for children aged <5 years and only with cattle. The results for season and cattle density for this age group are given in Table 3. Living in areas with >26 cows/km2 increases the risk of STEC O157 infections in young children in summer compared to the lowest quintile of cattle density.
Table 3. Relative risk (RR) estimates with 95% confidence intervals (CI) for STEC O157 infection in children aged 0–4 years in The Netherlands, 1999–2008
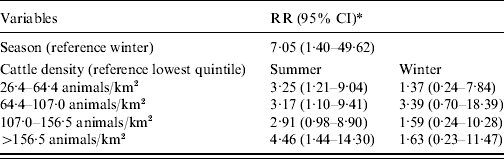
* Adjusted for the other three age groups, sex, pig density, poultry density.
The variation in the spatially structured residual risks of the main model showed a clear dependence to region (Fig. 3), with an increased residual risk (higher than expected) for STEC O157 infection in the northern and south-eastern regions of The Netherlands, and a lower residual risk (lower than expected) in the mid-west to north-western region.
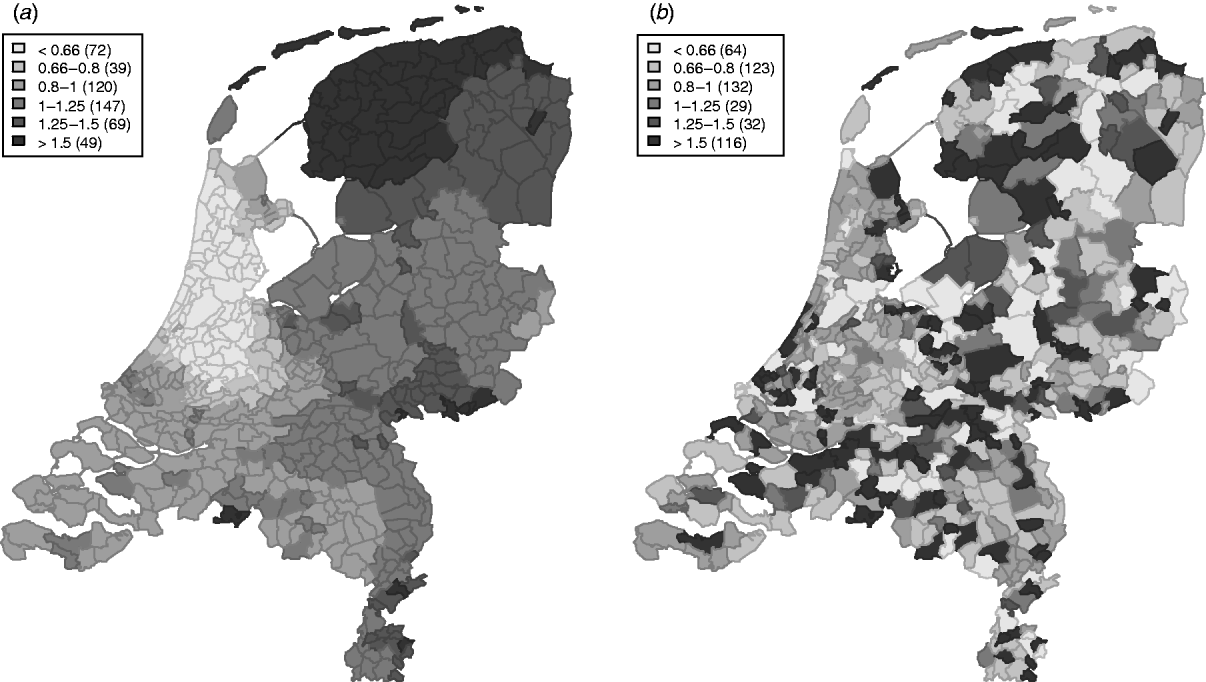
Fig. 3. Relative rates for the spatially (a) structured and (b) unstructured residual effects.
DISCUSSION
In the current study, a relationship was found between cattle density and incidence of reported STEC O157 infections in summer. Others have also reported associations for STEC O157 cases with cattle density [Reference Michel6, Reference Valcour9, Reference Frank10, Reference Kistemann19]. Valcour and colleagues [Reference Valcour9] have also examined livestock indicators other than cattle, and reported highest associations for cattle density and manure application, and a negative association between pig density and human STEC infection. In the Dutch situation no positive relationship between pig or poultry density and STEC O157 infections was found. Furthermore, campylobacteriosis was found to be related to living in rural areas, especially in children, in Denmark [Reference Ethelberg20]. It could be argued that the risk for cattle only is caused by pigs and poultry being kept inside large barns, but environmental emission may still be high. For example, in 2003, after large numbers of poultry were culled (almost all layer flocks) in The Netherlands, the incidence of campylobacteriosis immediately dropped [Reference Olson, Nachamkin, Szymanski and Blaser21]. This decrease was most pronounced in areas where the culling took place indicating that environmental emission must have been substantial. The association found in the current study between cattle density, but not for pigs or poultry, with the incidence of reported STEC O157 infections suggests that this is more likely due to direct or indirect contact with cattle rather than just living in a rural area.
The number of human STEC O157 infections varies during the year with highest incidence in summer or late summer. Faecal excretion of STEC in cattle shows a parallel seasonality with human infections in The Netherlands [Reference Kistemann19, Reference Valkenburgh22, Reference Schouten23]. This could explain the finding that cattle density is only related to human STEC O157 infections in summer. The chance of contact with animals is also greater in summer, as more animals are on pastures and humans spend more time outside when the weather is pleasant, or they are on holiday.
Incidence of reported STEC O157 cases was highest and the relationship with cattle density strongest in children aged <5 years. This is compatible with a German case-control study where the highest odds of disease for children aged <3 years was found for touching a ruminant [Reference Werber24]. Another German study also showed a spatial relationship with cattle density, age and seasonality comparable to the findings in our main model but did not study their interaction or the eventual role of other livestock [Reference Frank10]. A serosurvey among children (1–17 years) in Wisconsin showed an increase in seropositivity for O157:H7 lipopolysaccharide with increasing age [Reference Belongia25]. Highest seropositivity (25%) was found in children aged 15–17 years living on a farm. Contact with manure and contact with sheep were risk factors for seropositivity. This suggests that ruminants and manure are a common source for STEC O157, and that the chance of encountering STEC O157 at a young age may be considerable. If the course of the first infection is more likely to be symptomatic than later infections this might explain the apparent lack of relationship between cattle density and infection at older ages.
Overall, females appeared to have an increased risk of infection with STEC O157 compared to males. According to the cumulative incidence rates, this is primarily the case above the age of 10 years. The study of Kistemann et al. [Reference Kistemann19] showed the same sex ratio among STEC O157 patients as the current study. However, almost all age groups consisted of more females than males in the Swedish study.
Reporting of human STEC infections is mandatory in The Netherlands. However, the reported infections will often be the more severe infections. A Dutch study revealed that laboratory tests were selectively requested by general practitioners, and only done for 12% of the patients visiting a general practitioner with gastroenteritis [Reference van den Brandhof26]. Main reasons for testing a stool sample were duration and severity of complaints. In 2009, all Dutch laboratories received a questionnaire about their STEC diagnostics (response 85%). Three laboratories (6%) stated not reporting a positive STEC result as standard. This could partly explain the low number of cases in the north-west, but not in the mid-west area with high cattle density. A more inspiring hypothesis for the low incidence in the latter region could be a lower incidence of STEC O157 in cattle due to cattle feeding practices [Reference Callaway27] or other regional farm settings. This is worthy of further investigation.
Since 2007, the enhanced surveillance has been extended to also include STEC of other serogroups, because of the availability of a new real-time polymerase chain reaction (rt-PCR) method [Reference Schuurman28, Reference Van Duynhoven29] and commercial kits for determination of Shiga toxins. In the current study, only STEC O157 was considered, as reports of STEC non-O157 are not yet available nationally. Prior to 2007, laboratory methods for detecting STEC non-O157 infections were rarely used in The Netherlands and in 2008 still no more than 25% of the laboratories are using these methods. Other studies have found similar relationships between STEC or STEC non-O157 and cattle density as was found for STEC O157 [Reference Frank10, Reference Haus-Cheymol30].
In conclusion, the results of the current study indicate that cattle, but not pigs or poultry, are an important source for human STEC O157 infections. Besides geographical differences due to cattle density, a seasonal association was also seen, with a relationship between human STEC O157 infections and cattle density in summer, but not in winter. Furthermore, small children especially appear to be at higher risk of symptomatic STEC O157 infection when living in an area with a high density of cattle.
DECLARATION OF INTEREST
None.










