INTRODUCTION
Norovirus, the leading cause of non-bacterial gastroenteritis in all age groups worldwide, is a non-enveloped, single-stranded RNA virus and a member of the Caliciviridae family [Reference Glass, Parashar and Estes1]. A low infectious dose can easily be transmitted by the faecal–oral route or through environmental contamination to establish acute infection. Resistance to surface disinfectants and the ability to remain viable in the environment for up to 12 days, as demonstrated by an outbreak where two carpet fitters became ill after removing a contaminated carpet, contributes to the large nosocomial outbreaks reported globally [Reference Cheesbrough, Barkess-Jones and Brown2].
Hospitals can be viewed as confined communities composed of individuals, many of whom are immunocompromised for a variety of reasons, making them more susceptible to infectious disease. Norovirus can be introduced into the hospital setting by new admissions who are infected with the virus and are either symptomatic or asymptomatic, visitors, healthcare workers (HCWs) or food sources. Although in the community setting, norovirus infection is usually self-limiting, in immunocompromised individuals and the elderly significant morbidity and mortality may result [Reference Fretz3].
The purpose of this study was to review documented noscomial outbreaks of norovirus infection, published in the last 10 years and to identify modes of transmission, the number of patients affected, morbidity and mortality patterns, and recommendations to control outbreaks. Understanding the epidemiology of norovirus outbreaks in hospitals may result in early case identification by hospital staff and the application of effective infection control measures decreasing the burden of illness.
METHODS
Study inclusion criteria
The literature was reviewed to identify norovirus outbreak reports in hospital settings published or that had occurred between 1 January 2000 and 31 December 2010. The review was not limited to any geographical area. The reports fell into one of three categories: (1) published in peer-reviewed scientific journals, (2) published on the internet by government organizations, or (3) internal reports from public health agencies.
Study exclusion criteria
The following types of studies were excluded:
• community-acquired illness or outbreaks associated with outpatient settings;
• reports not written in English;
• long-term retrospective studies, burden-of-disease studies, surveillance summaries, and reviews.
Search strategy
Computer-aided searches of Scopus, CAB Global Health and CINAHL® (Cumulative Index to Nursing and Allied Health Literature), from 1 January 2000 to 31 December 2010, were completed in order to identify relevant outbreak reports. Population search words included: hospital(s) or infirmary(ies) or sanitorium(s) or sanitoria or sanitarium(s) or sanitaria or ‘medical center(tre)(s)’ or ‘health care facility(ties)’ or ‘medical institution(s)’ or nosocomial or ‘cross infection(s)’. Outcome search words included: enteric or gastrointestinal or gastroenteritis or diarrhoea or diarrhea or vomiting as well as ‘norovirus’ and ‘Norwalk’. The term ‘outbreak(s)’ was included as a required term in all searches. The Worldwide Database for Nosocomial Outbreaks (Institute for Hygiene and Environmental Medicine, 2010) was also searched using keywords ‘enteric’, ‘gastro’.
The following journals do not submit abstracts to the above databases and so were hand-searched for relevant reports:
• Canada Communicable Disease Report, 2000–2010
• Public Health Epidemiology Report Ontario (PHERO), 2000–2004
• Minnesota State Foodborne Outbreak Summaries, 2000–2006
• New South Wales Public Health Bulletin, 2000–2010
• New Zealand Public Health Surveillance, 2003–2010
• Environmental Health Reviews, 2000–2010
• Ontario Branch News, Canadian Institute of Public Health Inspectors News, 2000–2010
• Communicable Disease Corner (Capital Health, Edmonton, Alberta), 2000–2008
• Communicable Diseases Monthly Report (Northern Ireland Edition), 2001–2007
• Victoria (Australia) Infectious Disease Bulletin, 2000–2010
• EpiNorth (Europe), 2000–2010
• Communicable Disease Monthly Report (Northern Ireland), 2001–2009
To validate the electronic search methodology bibliographies and reference lists were hand-searched. Public health and government websites were searched for nosocomial norovirus.
Abstracts were reviewed and a relevance tool applied by two individuals to determine if the inclusion criteria were met: (1) published between 2000 and 2010, (2) a nosocomial norovirus outbreak, and (3) the report was published in the English language. The report was excluded if the three criteria were not met. Reports were reviewed to ensure the information was not duplicated; should duplication occur, the published manuscript was used as the reference source.
Data extraction
The following information was extracted for each outbreak: country and year of outbreak; number exposed, laboratory-confirmed, ill; symptoms and major sequelae; mode of transmission; if foodborne, the food vehicle and if prepared on-site; preventive strategies implemented at the time of the outbreak; investigators' recommendations to prevent future outbreaks; and the source of the report.
Level of evidence
Norovirus was identified by laboratory confirmation from cases or the food consumed.
RESULTS
Description of reports
Figure 1 shows the flow of citations through the review process. Sources of the 54 outbreaks that met the inclusion criteria: 48 (88·9%) peer-reviewed journals, five (9·3%) government publications, and one (1·9%) non-peer-reviewed publication. The following geographical areas were represented in the review: Europe (28), North America (10), Asia (9), and Australia and New Zealand (7). The type of ward/hospital was reported in 38/54 reports (70·4%). A tertiary-care hospital was identified (9/38), psychiatric ward/hospital (7/38) and geriatric ward/hospital (5/38). The remaining 16 reports simply referred to ‘hospital’.
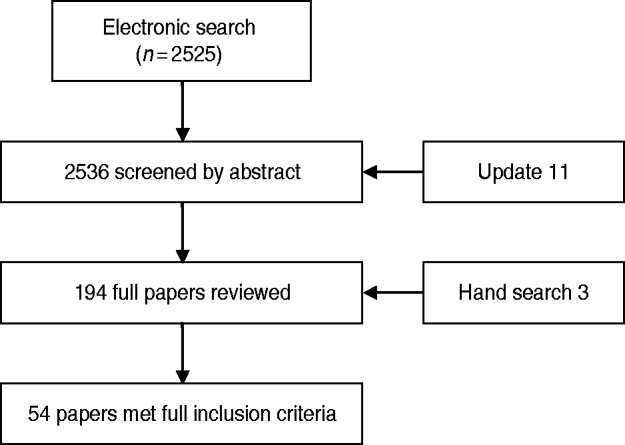
Fig. 1. Flow of citations through the review process.
Description of outbreaks
Genotype GII.4 was reported in 16/21 (76%) investigation reports that identified genotype. The 54 outbreaks reported 2033 cases (605 confirmed, 29·8%) and 16 deaths. The mean duration of nosocomial outbreaks was 32.5 days (range 8–120 days). The most commonly reported route of transmission was person-to-person 10/54 (18·5%), only two outbreaks were reported as transmitted via food while the majority (77·8%) did not report the means of transmission or it was unknown. The mean number of cases per outbreak was 37·6 (range 2–295). When reported, the three most frequent symptoms were recorded for each outbreak (46/54); these were diarrhoea (45/46, 97·8%), nausea and/or vomiting (30/46, 65·2%) and fever (7/46, 15·2%). The clinical attack rate was included in only 11 outbreak reports; these included five psychiatric hospitals (mean 15·4%, range 4–24%), three geriatric hospitals (mean 50·7%, range 39–57%), and three others listed as hospitals (mean 32·3%, range 22–44%).
Mortality
Nine deaths occurred within 30 days of diagnosis during an outbreak in patients in a Finnish University hospital. Norovirus infection could have exacerbated the already potentially fatal conditions of these individuals [Reference Kanerva4]. Two norovirus outbreaks in preterm infants resulted in five deaths associated with the development of necrotizing enterocolitis, a condition with a poorly understood pathophysiology [Reference Turcios-Ruiz5, Reference Stuart6]. Two other reports each reported deaths in individuals with underlying serious conditions.
Risk factors for norovirus outbreak
Several reports included specific risk factors associated with the outbreaks but it is recognized that the majority of hospitalized individuals are in an immunocompromised state due to disease, infection or recuperation from surgery. Newborns have immature immune systems and those most at risk for severe sequelae resulting from norovirus infection included those of younger gestation ages, lower birth weight, and lower Apgar score [Reference Turcios-Ruiz5].
Norovirus was introduced through the transfer of patients from another ward [Reference Schmid7], admission of new patients who shortly became ill [Reference Weber8], or from exposure to patients with day passes [Reference Gilbride9] in six outbreak reports. Transmission from community outbreaks to hospitalized patients was also frequently reported [Reference Fretz3, Reference Kanerva4, Reference Beersma10, Reference Koek11]. Four outbreaks reported exposure to environmental contamination resulting from close contact in the hospital (shared bathrooms and common areas) as a risk factor [Reference Weber8, 12–Reference Simon14].
Four outbreaks reported symptomatic food handlers or HCWs handling patients' food or administering tube feedings [Reference Verbelen15], while two studies identified prolonged/chronic shedders [Reference Sukhrie16, Reference Godoy17]. An outbreak in an Austrian neonatal intensive care unit reported prolonged viral shedding for >2 weeks in 27% of cases with a maximum of 39 days although there was no follow-up after discharge [Reference Sommer, Mueller and Resch18]. Asymptomatic norovirus shedding has been noted in infants aged ⩽6 months, probably related to maternal immunity and the child's immature immune status [Reference Murata19].
Two studies reported a lack of staff knowledge of outbreak policy [Reference Conway20] and slow reaction to the outbreak which contributed to the spread of the outbreak [Reference Lynn21].
Control actions during an outbreak
Actions taken to control the ongoing outbreak were stated in 41/54 (75·9%) reports (302 control measures in total) and are summarized in Table 1. The restriction of movements of individuals is frequently reported as a measure to control the spread of an outbreak (68/302, 22·5%). Placing patients in isolation (21/302, 7%) is difficult during an outbreak due to lack of private rooms and staffing shortages due to illness in staff members. Cohorting of ill patients and restricting staff to working on only one ward in one hospital (25/302, 8·3%) is often reported as a control measure since it minimizes potential cross-contamination between patients and/or staff and patients. Frequently an entire unit would be treated as an isolation section or unused areas in the hospital were cleaned and equipped for isolation. Many reviewed reports suggested that medical and health professionals who move from ward to ward should be reminded of the importance of hand hygiene and should visit the affected wards last (hand hygiene 31/302, 10·3%). Movement of patients was sometimes restricted (17/302, 5·6%) by patients having therapy or meals in their rooms rather than in communal areas. Often visitors were allowed only if not ill, while in other outbreaks visitors were prohibited (5/302, 1·7%).
Table 1. Reported actions taken in 41/54 outbreaks to control infection
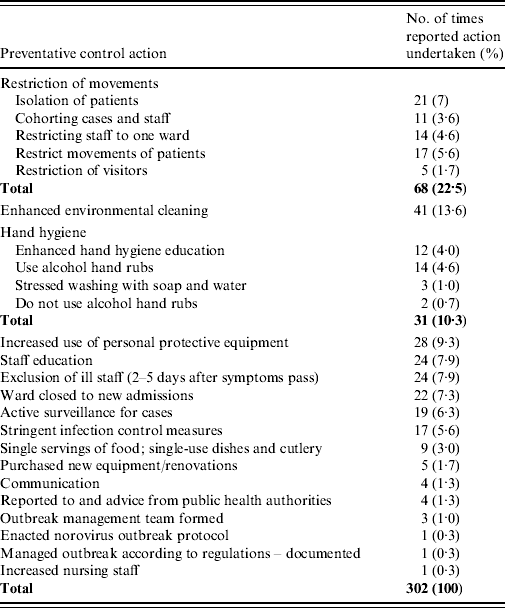
Enhanced environmental cleaning was the second most reported control measure (41/302, 13·6%). ‘Enhanced hand hygiene compliance’ is a term frequently used in investigation reports, which can be interpreted as a reinforcing of the hand hygiene message. Emphasizing this message was listed as a control measure in 31/302 (10·3%) of the reports reviewed. Twelve reports stated that enhanced hand hygiene education was an important control measure and needed to be directed at staff, patients and visitors.
Personal protective equipment (gloves and gowns) were recommended in isolation areas and for patient contact (28/302, 9·3%). Although five reports recommended wearing masks there is no evidence to support such a recommendation [Reference Chadwick22].
The need for education sessions was noted in 24/302 (7·9%) control measures taken and topics included norovirus infectivity, how to minimize transmission, hand hygiene and infection prevention, and control policies. These sessions were directed at staff, patients, volunteers and visitors.
Symptomatic staff were immediately excluded from work for between 2 and 5 days after symptoms of illness were past (24/302, 7·9%). The majority of reports recommended that staff be assigned to cohorts of ill patients upon their return.
Closing the ward to new admissions (22/302, 7·3%) was stated as essential to stop the transfer of infection.
Other control measures noted included active surveillance for new cases (19/302, 6·3%) and stringent control measures (17/302, 5·6%) although these were not defined. Exposed food such as fruit should be discarded. Cutlery sets should be individually wrapped, or single use (9/302, 3·0%); disposable dishes were suggested. In five outbreaks purchase of new equipment was reported following the outbreak and renovations were made to the existing ward (5/302, 1·7%). Specific types of communication were reported: staff-specific information, voice mail messages were left for staff concerning not reporting to work if ill, infection control officer had developed question-and-answer sheets as well as information for outpatients and visitors (4/302, 1·3%). Daily meetings were considered important to discuss infection management and new cases (4/302, 1·3%); early constant communication with public health officials was important (4/302, 1·3%). An outbreak protocol was followed in two reports and three others developed an outbreak management team. Nursing staff was increased for one outbreak.
Recommendations made retrospectively by investigators to prevent a re-occurrence of outbreaks
Recommendations for the control of norovirus outbreaks in hospital settings were recorded in 37/54 (69%) of investigation reports (161 recommendations in total), although their effectiveness was not statistically evaluated in any hospital norovirus outbreak report (Table 2). Rapid outbreak detection allowing immediate implementation of control measures was noted in 30/161 (18·6%) recommendations for outbreak control while isolation and/or cohorting of infected patients and their caregivers was recommended in 23/161 (14·3%). Enhanced handwashing compliance was recommended in 22/161 (13·7%) recommendations for outbreak control while 16/161 (9·9%) stressed implementation of infection control measures and 14/161 (8·7%) reported enhanced cleaning. The development of an outbreak management plan with specific standard operating procedures for the management of infectious diseases was recommended in nine reports (5·6%). Although only two reports cited the source of infection as foodborne, nine (5·6%) reports gave recommendations on safe handling of food. Exclusion of ill staff and visitors, closure of the affected ward and use of barrier precautions, stressing the maintenance of necessary supplies were each recommended in eight (5·0%) reports. Education of staff on effective infection control measures and of patients on the severe potential effects of norovirus infection was recommended in seven (4·3%) reports. Using molecular methods to better understand the epidemiology of norovirus in hospitals and the genetic factors associated with increased risk of infection, especially in high-risk populations, was suggested in three reports. Other recommendations included safe disposal of waste materials (two reports), prompt treatment of cases and effective communication (one report each).
Table 2. Recommendations to control or prevent future outbreaks made in 37/54 reports following outbreak investigations
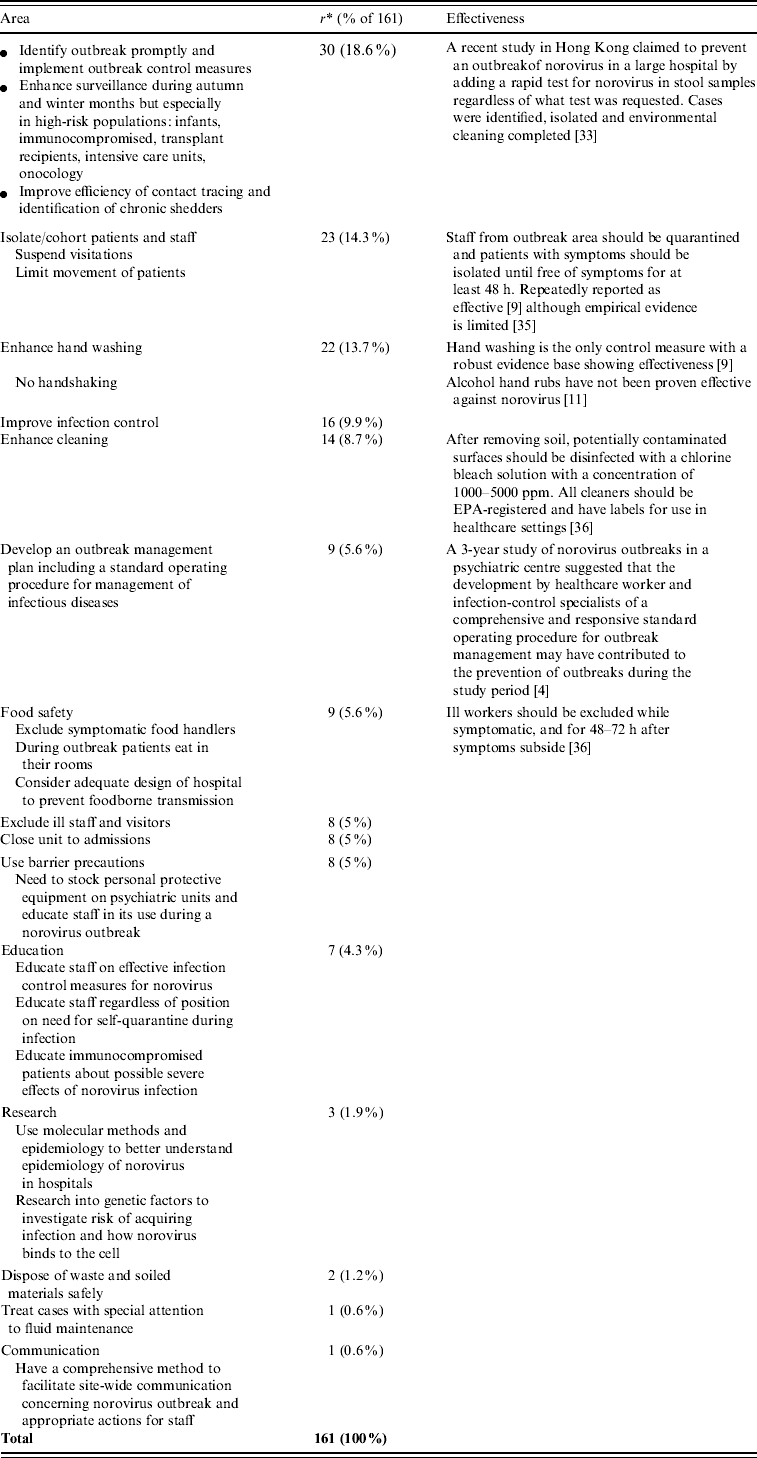
* r=number of recommendations.
DISCUSSION
Norovirus infections continue to occur globally because the virus genome easily mutates resulting in antigenic shift and recombination [Reference Glass, Parashar and Estes1]. In this review GII.4 was specifically reported in 14/54 (25·9%) reports. Since 2001 the majority of norovirus outbreaks have been associated with genogroup II, genotype 4 (II.4). A review of norovirus strains over the last 20 years show emergent strains replacing those previously dominant resulting in new global epidemics every 2–4 years. This results in a lack of cross-protection between strains and long-term immunity [Reference Glass, Parashar and Estes1]. Primary studies have shown that protection against the same strain may last from 8 weeks to 6 months [Reference Johnson23, Reference Parrino24]. These factors ensure a constant susceptible population to the norovirus.
Transmission of norovirus infection is facilitated by a low estimated infectious dose of 18 virons [Reference Teunis25]. Asymptomatic infection and viral shedding occur in 30% of individuals [Reference Chiba26]. Prodromal shedding is estimated to occur in 30% of exposed persons with peak shedding 2–5 days after infection [Reference Glass, Parashar and Estes1]. The virus can be shed for up to 8 weeks in previously healthy people but in the immunocompromised or those who have undergone transplantation the virus may be shed for a year [Reference Nilsson27]. Identification of norovirus infection is vital in this population as gastrointestinal symptoms may be an early indication of rejection or a side-effect of medication.
Although illness associated with norovirus infection is usually self-limiting, mortality is associated with the very young, the elderly and immunocompromised. Sequelae associated with norovirus infection include necrotizing enterocolitis in neonates, benign infantile seizures, chronic diarrhoea in immunodeficient individuals; post-infectious irritable bowel syndrome has also been associated with norovirus infection [Reference Turcios-Ruiz5, Reference Marshall28]. Four outbreaks that occurred during a 3-year period (duration ranged from 14 to 28 days) in an in-patient psychiatric unit in Taiwan noted that the longer the illness the more likely the individual would experience abdominal pain, the younger the case the more likely they would experience abdominal pain and decreased frequency of vomiting was associated with increased frequency of diarrhoea [Reference Tseng29].
The review identified measures taken by personnel during the outbreak considered to have contributed to the control of the infection (Table 1). Restriction of patient and staff movements and isolation of cases were considered by investigators to be effective control measures. Norovirus can survive in the environment for long periods, up to 12 days, therefore, enhanced environmental cleaning of common surface areas, washrooms and terminal cleaning of patient units were considered by investigators to be effective control measures. Effective hand hygiene was identified as a means of outbreak control but there was conflicting advice concerning the use of alcohol hand sanitizers (AHRs). Todd et al. state that AHRs are not effective against norovirus and if staff do not use soap and water but instead rely on AHRs they might actually spread the virus [Reference Todd30]. However, other authors claim an outbreak was contained in Hong Kong using a World Health Organization formulation of an alcohol-based hand rub with ethanol (80% v/v) with 30 s contact time [Reference Cheng31] combined with other infection control measures; no attempt was made to evaluate the contribution of the other control measures to the containment of the outbreak. An adequate number of properly functioning hand sinks with liquid soap and paper towels is a necessity. One report noted that antiquated sinks were replaced following an outbreak in a neonatal intensive care unit [Reference Turcios-Ruiz5]. Increased use of personal protective equipment incorporated into ‘universal barrier nursing’ and education of staff on the pathogen and its control were frequently reported as control measures. Closing affected wards and excluding ill staff for 2–5 days following final symptoms were reported as effective in controlling transmission of infection. During an outbreak most reports cited an increase in active surveillance for patients with symptomatic infection and their subsequent isolation. Communication played an important role in infection control including reporting to public health authorities and frequent meetings of the outbreak management team.
Following an outbreak, investigative teams will complete a report with recommendations to prevent outbreaks in the future (Table 2). Unlike intervention strategies in other sectors, it has not been possible to conduct trials and statistically evaluate the effectiveness of one recommendation compared to another. Recommendations to control enteric outbreaks are seldom made from the results of evaluative studies because few exist. Rather, recommendations are seen as ‘expert opinion’ garnered from investigations of outbreaks where indications and epidemiological analysis highlight a problem and provide guidance to prevent future outbreaks. Although effective handwashing has a robust evidence base showing a decrease in diarrhoeal episodes [32], the reviewed reports presented conflicting recommendations as to whether soap and water or AHRs were the most effective. Identification of an outbreak was the most frequent recommendation, but this can be difficult considering the range of conditions present in a hospital. A hospital in Hong Kong has added a rapid test to all stool sample requests to rapidly identify those shedding the virus and aid in rapid isolation and outbreak control [Reference Cheng33]. Isolating cases and cohorting them with the same staff was frequently recommended in reports. Preventing staff from working on other wards or hospitals prevents transmission to uninfected wards. Suspension of visitors was also frequently recommended, especially when the level of norovirus outbreaks in the community is elevated. This can be difficult to enforce with paediatric and elderly patients or those with life-threatening illness.
The most commonly reported route of transmission was person-to-person, as to be expected in often crowded conditions with the sharing of bathrooms. Close contact by staff administering care and visitors can introduce the virus from the community. Recommendations included having a cleaning crew for the isolation unit who did not move to other wards and additional cleaning staff to cope with the additional demands caused by outbreak. Cleaning equipment should be designated for the outbreak area. Chadwick et al. made several recommendations including daily disinfection of hard surfaces and more frequent cleaning of bathrooms and toilets. They also recommended that surfaces first be cleaned with a detergent solution followed by a 0·1% hypochlorite solution (1000 ppm) with particular attention to frequently touched areas such as toilets and door handles. Following the outbreak the ward and any equipment that will be reused should be cleaned thoroughly before reopening; bed curtains should be changed, and carpets and soft furnishings steam cleaned or cleaned with hot water and detergent [Reference Chadwick22]. Cheesbrough et al. did not recommend vacuuming due to the possibility of airborne transmission of viruses that survive well in the environment [Reference Cheesbrough, Barkess-Jones and Brown2].
Development of an outbreak management plan including a standard operating procedure (SOP) for management of infectious diseases was recommended in several reports. This is particularly important in settings such as psychiatric hospitals where staff are not accustomed to dealing with infectious diseases and appropriate equipment is not available. Seven outbreaks occurred on psychiatric wards or hospitals and reports identified unique features for control in these settings. Implementation of control measures can be very challenging as demonstrated by a norovirus outbreak in a Canadian psychiatric unit [Reference Gilbride9]. Many of the treatment interventions were based around social interaction so it was very difficult to isolate the patient population in their rooms. In the report, the investigators also stressed the importance of hand washing, environmental cleaning, discouraged the use of communal hospital areas and treatment of symptomatic day patients to contain such outbreaks. Staff education on isolation precautions was required immediately since this is not common practice on this type of unit and personal protective equipment had to be procured [Reference Gilbride9]. Some patients refused to remain in their rooms and attempts at hand washing education had limited results due to the patients' level of functioning [Reference Gilbride9]. Therapies frequently centre on group activities and patient care can be adversely affected during an outbreak. As part of their treatment programme, patients frequently share common areas and eat meals together, increasing the risk of person-to-person transfer and environmental contamination.
Although only two reviewed reports identified a foodborne source of infection, nine reports provided recommendations concerning the safe handling of food and exclusion of infected food workers for 48–72 h after symptoms have ceased.
Education of staff on effective norovirus infection control and the need for self-quarantine during infection was identified. Immunocompromised patients should be educated on the possible severe effects of norovirus infection.
This review is limited because only a fraction of outbreaks occurring during the timeframe of the study are included due to underreporting, lack of publication and the English-language requirement.
CONCLUSIONS
Norovirus outbreaks frequently occur in the general population but outbreaks in hospitals pose a significant risk of serious or even life-threatening illness for those in an immunocompromised state. Moreover, norovirus outbreaks can have a significant financial impact on the affected hospital; it was noted that financial losses due to bed closures were greater than the amount spent on increased laboratory testing and the cost of infection control [Reference Zingg34].
The most frequent recommendations from the studies reviewed included rapid identification of the outbreak with immediate implementation of infection control measures, isolation/cohorting of infected individuals, enhanced handwashing, and implementation of infection control measures.
Studies are needed which evaluate the effectiveness of infection control recommendations using statistical methodology to develop an evidence base for practice. Identification of best practices would improve patient care, possibly decrease the extent of the outbreak and allow the best allocation of human and financial resources. Improved reporting of the details of norovirus outbreaks would increase the body of knowledge; frequent shortcomings in reporting were noted in a recent study [Reference Harris, Lopman and O'Brien35].
ACKNOWLEDGEMENTS
The authors thank Mrs Janet Harris of the Public Health Agency of Canada for her kind assistance in the computer-aided searches of the literature and article procurement.
DECLARATION OF INTEREST
J.G. is an employee of the Public Health Agency of Canada. M.L. is an employee of Ryerson University.







