INTRODUCTION
Foodborne outbreaks of human salmonellosis are frequently associated with poultry, cattle, or pigs and about 20% of cases are due to contaminated pork and pork products [Reference Steinbach and Kroell1]. An increased risk of pork carcass contamination during slaughter and processing is linked to high levels of Salmonella spp. in pig herds [Reference Rajic and Keenliside2]. Salmonella-infected pigs, even after being successfully treated, may become carriers and serve as the major source of contamination at slaughter.
Salmonella contamination at slaughter is generally unavoidable because a significant proportion of apparently healthy market pigs shed Salmonella in manure in response to stresses such as transport and holding with different herds [Reference Hurd3, Reference Larsen4]. Rapid infection during transport, and particularly during holding, is another major reason for increased Salmonella prevalence in market pigs [Reference Hurd3].
Wiping out Salmonella completely in pig herds is not yet successful in most countries, but curbing the number of infected and carrier market pigs is achievable by pre-slaughter surveillance and monitoring Salmonella in herds followed by proper management of infections. Denmark's nationwide control programme of Salmonella in pork [Reference Wegener5] is one proven example.
Emergence of antimicrobial-resistant human and animal pathogens is regarded as an ominous complication of antimicrobial abuse in livestock. To ensure responsible and prudent use of antimicrobials in livestock and veterinary medicine and to monitor emergence of antimicrobial resistance, the major livestock-producing countries have established their own national monitoring system, e.g. the National Antimicrobial Resistance Monitoring System (NARMS) of the United States [6], the European Antimicrobial Resistance Surveillance System (EARSS) [7] and the Japanese Veterinary Antimicrobial Resistance Monitoring (JVARM) system [8]. However, not much research has been carried out with regard to epidemiology of Salmonella in Japanese pigs.
This study reports the prevalence, serotype diversity, and antimicrobial resistance of Salmonella isolated from healthy pigs on Japanese farms.
MATERIALS AND METHODS
Farms, herds and animals
As of February 2004, there were 7420 pig farms in 47 prefectures of Japan, with 1120 farms housing 1000–2000 pigs and 897 farms housing >2000 pigs. Pig farms in this study were selected using the following selection criteria: (1) farms with no history of a Salmonella outbreak within the past 6 months, (2) a minimal herd size of 1000 pigs, (3) the willingness of farm owners to participate in this project and (4) the availability of veterinarians for on-site sampling.
Seventy-three pig farms in 24 prefectures meeting our selection criteria were investigated over two separate periods, 1998–1999 and 2004–2005. A total of 6771 clinically healthy pigs (n=2980 on 31 farms in 1998–1999 and n=3791 on 42 farms in 2004–2005) of different growth stages, including sows (aged 8 months to 3 years), weaned pigs (aged 3 weeks to 2 months), fattening pigs (aged 2–4 months) and finishing pigs (aged 4–7 months), were randomly enrolled in this study.
Bacterial isolation, identification and serotyping
One gram of fresh faecal sample (one sample per animal) was inoculated into Hajna tetrathionate broth or selenite-cystine broth and cultured at 37°C for 18–20 h. The enriched culture was subcultured onto desoxycholate hydrogen-sulfide lactose (DHL) and mannitol lysine crystal violet brilliant green (MLCB) or brilliant green agar plate. At least five H2S-producing colonies from each faecal sample, grown overnight, were selected for Salmonella identification by standard biochemical tests. One representative isolate from each sample was used in serotyping by slide and tube agglutination following the Kauffmann–White scheme. All isolates were stored at −70°C until used.
Antimicrobial susceptibility testing
All Salmonella isolates were examined for their susceptibility to a panel of 22 common antimicrobials, belonging to nine different antimicrobial classes: penicillins (benzyl penicillin, ampicillin, amoxicillin), first-generation cephalosporins (cefazolin, cephaloridine), aminoglycosides (gentamicin, kanamycin, streptomycin, fradiomycin), polymixins (colistin), tetracyclines (tetracycline, chlortetracycline, oxytetracycline), phenicols (chloramphenicol, thiamphenicol), sulfonamides (sulfadimethoxine, sulfamethoxazole, sulfamethoxypyridazine), dihydrofolate reductase inhibitors (trimethoprim), sulfamethoxazole/trimethoprim and second-generation quinolones (norfloxacin and ofloxacin). The antimicrobials were obtained from Sigma Chemical Co. (St Louis, MO, USA) or Wako Pure Chemical Industries Ltd (Tokyo, Japan). The minimal inhibitory concentration (MIC) range of <0·125 to >512 mg/l was tested by the broth macrodilution method following CLSI recommendations [9]. MIC breakpoints used and interpretation of MIC results were as described by the CLSI unless otherwise stated. Escherichia coli JCM5491 and Staphylococcus aureus JCM2413 were used as quality control strains.
Statistical analysis
Significance of differences between the variables was tested with the χ2 test or Fisher's exact test using Microsoft Excel Statistics 2006 for Windows (SSRI Co. Ltd, Tokyo, Japan).
RESULTS
Prevalence
Non-typhoidal Salmonella spp. were recovered from 35·5% (11/31) and 35·7% (15/42) of the pig farms (data not shown) or 2·2% (67/2980) and 3·3% (126/3791) of pigs tested in 1998–1999 and 2004–2005, respectively, with an overall prevalence of 35·6% (26/73) at the farm level or 2·9% (193/6771) at the animal level (Table 1). The prevalence by growth stage was 2·4% (87/3574) for sows, 3·3% (36/1102) for weaned pigs, 2·7% (26/950) for fattening pigs and 3·8% (44/1145) for finishing pigs (Table 1).
Table 1. Prevalence of Salmonella enterica by investigation period and growth stage in healthy pigs
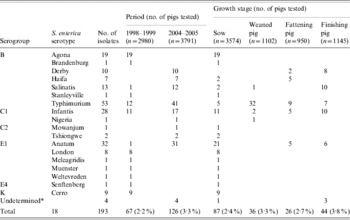
* Isolates of serogroup C2 with undertermined H antigen.
Serodiversity
Six serogroups or 18 serotypes (13 in 1998–1999 and nine in 2004–2005, with four overlapping serotypes) were identified among the isolates, of which 76% (147/193) belonged to serogroups B and E1. The predominant serotypes observed were Agona (28·4%, 19/67), followed by Typhimurium (17·9%, 12/67) and Infantis (16·4%, 11/67) in 1998–1999, and Typhimurium (32·5%, 41/126), followed by Anatum (24·6%, 31/126) and Infantis (13·5%, 17/126) in 2004–2005 (Table 1). The isolation rates of serotypes Typhimurium and Anatum in 2004–2005 were significantly higher compared to that in 1998–1999 (17·9% vs. 32·5%, P<0·05 for Typhimurium; 1·5% vs. 24·6%, P<0·01 for Anatum).
Antimicrobial resistance
Table 2 shows the susceptibility of Salmonella isolates to antimicrobials commonly used for humans, veterinary medicine and livestock (as feed additives) in Japan. No isolates were resistant to gentamycin, cefazolin, norfloxacin, ofloxacin and colistin. All isolates in 2004–2005, regardless of their serotype, were resistant to at least one antimicrobial, in comparison to only 38% (26/67) of the 1998–1999 isolates. In comparison with the 1998–1999 isolates, the isolates in 2004–2005 exhibited significantly higher resistance to several antimicrobials (P<0·01 to P<0·05) (Table 2). Of note, sulfadimethoxine resistance increased from 14·9% (10/67) in 1998–1999 to 100% (126/126) in 2004–2005. Multi-antimicrobial resistance (MAR; resistance to ⩾2 antimicrobials), was observed in 32·6% (63/193) of total isolates or 19·4% (13/67) in 1998–1999 and 39·7% (50/126) in 2004–2005. Penta-resistance phenotype, R-type ACSSuT (ampicillin, chloramphenicol, streptomycin, sulfonamides, tetracycline), was found in 47·6% (30/63) of the MAR isolates, represented by 23% (3/13) of the MAR isolates in 1998–1999 and 54% (27/50) of the MAR isolates in 2004–2005, or 15·5% (30/193) of total isolates, represented by 4·4% (3/67) in 1998–1999 and 21·4% (27/126) in 2004–2005. All Typhimurium isolates, which accounted for 90% (27/30) of penta-resistant isolates, were of R-type ACSSuT+, showing additional resistance to cephaloridine.
Table 2. Antimicrobial resistance of Salmonella enterica isolates (n=193) from healthy pigs
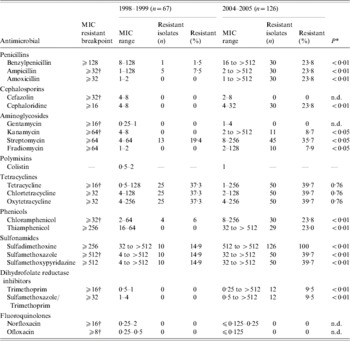
Minimum inhibitory concentration (MIC) values and breakpoints are in mg/l; n.d., not determined. Breakpoints were defined as the midpoint between the peaks of each MIC distribution for the antimicrobials for which no Clinical and Laboratory Standards Institute (CLSI) breakpoints are currently available.
* P values account for the difference in the number of resistant isolates observed between 1998–1999 and 2004–2005.
† Resistant breakpoints taken from the CLSI.
Simultaneous resistance to all antimicrobials in the same class was observed for penicillins in 100% (30/30), tetracyclines in 100% (50/50), phenicols in 96·7% (29/30) and sulfonamides in 39·7% (50/126) of the 2004–2005 isolates, and tetracyclines in 100% (25/25) and sulfonamides in 100% (10/10) of the 1998–1999 isolates.
DISCUSSION
Prevalence
With the view that Salmonella carriage in a healthy pig population (the asymptomatic carriers) has a greater impact on herd health and public health, this study has focused on healthy pigs. We examined 6771 pigs on 73 farms (about 90 pigs per farm), covering most major pork-producing farms or 51% (24/47) of the prefectures in Japan and therefore the results presented herein can be considered representative of Salmonella prevalence in healthy pigs of different growth stages in Japan.
Due to differences in sampling approach, isolation/identification strategy and reporting method, epidemiological data of Salmonella in pigs reported from various countries are considerably varied and rather difficult to compare. Nevertheless, the consensus evidence from these reports has shown that even though pre-slaughter prevalence of Salmonella in pigs may be considerably low (e.g. 2·2–3·3% in this study), prevalence at slaughter can be significantly high (up to 50%) [Reference Padungtod and Kaneene10–Reference Vieira-Pinto, Temudo and Martins13].
The farm-level prevalence of Salmonella (35·7%) in this study is also relatively low compared to a previous report of 57·3% on US pig farms [Reference Rodriguez14]. Salmonella carriage is reportedly varied among pigs at different growth stages [Reference Korsak11, Reference Rowe15], but no significant difference was observed in this study (Table 1). It is worth mentioning that although there were no significant differences in the overall prevalence of Salmonella at both farm and animal levels between the two investigation periods or between different growth stages, significantly higher isolation rates were observed for serotypes Typhimurium and Anatum in 2004–2005, while Agona, the most isolated serotype in 1998–1999, was not detected in 2004–2005. Moreover, nearly 90% of isolates recovered from the weaned pigs were Typhimurium, indicating the potential of young pigs to become carriers or act as a reservoir of the serotype, which is of public and animal health concern.
Antimicrobial resistance
High rates of resistance to the antimicrobials used in veterinary medicine and animal feeds have been documented for the animal isolates [Reference Gebreyes16–Reference Agustin19]. With the exception of tetracycline, cephaloridine and norfloxacin, the antimicrobials tested in this study are either approved or not forbidden for use in Japanese animals. Further, oxytetracycline, chlortetracycline and colistin are commonly used as feed additives in Japan; tetracycline compounds share 43% of animal antimicrobial sales, followed by sulfa-containing compounds (16%), penicillins and erythromycin (10% each) and aminoglycosides (6%) [Reference Takahashi20]. The reasons described above may explain why our isolates exhibited considerably high rates of resistance to most antimicrobials tested, although other factors such as transmission of resistant clones through imported animal feeds cannot be ruled out. Interestingly, however, no colistin resistance was observed despite its use as a feed additive.
Susceptibility testing customarily comprises only one representative of a particular antimicrobial class, but bacteria may show variable susceptibility to the derivatives in the same spectrum class. Therefore, we tested more than one antimicrobial for a class to see the overall susceptibility pattern. As a result, we identified a very high frequency of simultaneous resistance by our isolates to all the derivatives in the same class for the commonly used antimicrobials, particularly, penicillins, tetracyclines, phenicols and sulfonamides. However, most isolates were still not resistant to one or more antimicrobials in other classes.
Serotype Typhimurium strains with antimicrobial resistance profiles similar to those prevalent worldwide, including quinolone-resistant strains, are increasingly recovered from humans and animals in Japan [Reference Izumiya21, Reference Izumiya22]. Similarly, in this study, the occurrence of Typhimurium on pig farms increased sevenfold after the first investigation period, and these strains also exhibited similar antibiograms, albeit no quinolone resistance was present. Furthermore, all Typhimurium isolates with R-type ACSSuT were positive for definitive phage-type DT104 (K. Futagawa-Saito, unpublished data). Of note, the isolates belonging to a particular serotype recovered from the same pig farm showed identical or very similar resistance profiles (data not shown), suggesting that only a limited number of clones are responsible for Salmonella prevalence on Japanese pig farms.
In summary, despite the relatively similar overall prevalence, there is a twofold difference in resistance rates of Salmonella strains to several antimicrobials and the number of multiresistant and/or penta-resistant strains between the two investigation periods. These differences are obviously caused by the higher isolation rate of Typhimurium in 2004–2005. As exemplified by the low prevalence of healthy carriers in this study, shedding of Salmonella by asymptomatic carrier pigs is intermittent and difficult to detect [Reference Malorny and Hoorfar23], suggesting a relatively large number of animals in each farm (e.g. at least 50 pigs per farm in this study) are required for testing in order to observe Salmonella infection. Furthermore, since a high proportion of isolates with multi-antimicrobial resistance profiles is indicative of increased human health risks, sustained monitoring and a reduction of Salmonella-carrying pigs on farms should receive priority attention.
ACKNOWLEDGEMENTS
We thank the veterinarians and workers at the 73 pig farms for their kind assistance in collecting the samples. This study was supported by Azabu University.
DECLARATION OF INTEREST
None.






