1. Introduction
Neurodevelopmental and eating disorders, such as autism and avoidant/restrictive food intake disorder (ARFID), have well defined impact on dietary variety [Reference Martins, Young and Robson1, Reference Williams and Seiverling2]. In children with ARFID, habitual diet is comprised of a few preferred and accepted items that often do not meet their nutritional needs. Researchers interested in atypical development of paediatric eating behaviour have typically focused on single disorders, often comparing them to typically developing (TD) children, despite indications that similar underlying mechanisms, such as sensory hypersensitivity [3–Reference Nederkoorn, Jansen and Havermans8], may be prevalent in all forms of feeding difficulties and related disorders. Direct comparisons of different groups of children with feeding problems are necessary to understand the development of dietary variety and how it deviates into a disorder.
One of the difficulties in distinguishing between clinically relevant food avoidance and typically developing eating behaviour is that most children transverse a food neophobic stage [Reference Dovey, Staples, Gibson and Halford9]. Food neophobia is defined by a child's refusal to accept novel foods into their habitual diet. Food refusal in healthy children is transient, and they will learn to accept foods through repeated exposure [Reference Wardle, Herrera, Cooke and Gibson10, Reference Williams, Paul, Pizzo and Riegel11]. Children with clinically relevant food avoidance behaviours, however, do not respond to repeated exposure in the same manner as TD children [Reference Schmid, Schreier, Meyer and Wolke12, Reference Wolke, Schmid, Schreier and Meyer13]. Without intervention, the diet of children with ARFID remains restricted into adulthood and can be used to diagnose a large proportion of individuals attending an eating disorder service [Reference Norris, Robinson, Obeid, Harrison, Spettigue and Henderson14]. Children who fall into this stable category of food avoidance, which will affect their weight status, nutrient-replete profile and/or psychosocial functioning, are diagnosed with ARFID [Reference Bryant-Waugh, Markham, Kreipe and Walsh15]. In addition to this clinically diagnosable group of children with ARFID, it is known that many parents in epidemiological studies report feeding problems with their children [Reference Dahl and Sundelin16, Reference Carruth, Ziegler, Gordon and Barr17]. This feeding difficulty is commonly referred to as picky eating (PE) - defined as unwillingness to eat familiar foods or try new foods, and, according to recent epidemiological data, is present in at least 35% of the children [Reference Taylor, Wernimont, Northstone and Emmett18]. At present, there is no consensus whether PE requires clinical attention with some researchers arguing for [Reference McCormick and Markowitz19] and others against [Reference Kerzner, Milano, MacLean, Berall, Stuart and Chatoor20] intervention. Therefore, the forced split between TD children and those with clinically relevant feeding problems actively leads to exclusion of a large number of children who experience feeding difficulties.
Family mealtimes including children with feeding difficulties are described as exceptionally frustrating and stressful, with children engaging in problem behaviours [21–Reference Martin, Dovey, Coulthard and Southall23]. These problem behaviours include spitting out food, hand-batting food away and packing (holding food in the mouth and refusing to swallow it) [Reference Carruth and Skinner24] and are maintained by successfully escaping from the mealtime environment [Reference Piazza, Patel, Gulotta, Sevin and Layer25]. Although true in some cases, recent quantitative evidence from children with ARFID during mealtimes suggest that they engage in more subtle behaviours indicative of general restlessness [Reference Aldridge, Dovey, el Hawi, Martiniuc, Martin and Meyer26]. Food fussiness (a synonym of PE) and food-responsiveness (repetitively requesting food and responding to food cues in the environment) have been associated with hyperactivity [Reference Blissett, Church, Fergusson, Lambie, Langley and Liberty27] and anxiety [Reference Farrow and Coulthard28]. This suggests that children with feeding problems, both clinically diagnosed and parental reported, are anxious, restless or hyperactive during mealtimes, but to date, direct comparisons of eating and broader behaviour between children with PE and ARFID are noticeably absent within the literature, possibly due to the very recent inclusion of ARFID in diagnostic manuals [Reference American Psychiatric Association29].
The aim of the present study therefore was to examine parent-reported food avoidance, eating behaviour, behavioural problems and sensory hypersensitivity in groups of children with [Reference Martins, Young and Robson1] ARFID [Reference Williams and Seiverling2], autistic spectrum disorders (ASD) and [Reference Coulthard and Blissett3] PE, relative to each other and to a group of TD children. We hypothesised that children with a clinical diagnosis, either ARFID or ASD, would have feeding difficulties, behaviour problems and hypersensitivity, compared to TD children; and that those reported to have PE would have a profile more similar to those with ARFID than TD children, or occupy an intermediate position.
2. Methods
2.1 Sample, design and procedure
The study was designed in collaboration with Parenting Science Gang, a user-led citizen science project supported by the Wellcome Trust, and following initial discussions with the Mealtime Hostage Parenting Science Gang group, to explore child-related factors involved in food avoidance using a set of psychometric questionnaires. The selected questionnaires were made available through an online platform (Qualtrics) following approval of a Brunel University London Ethics Committee (Ref: 12219-MHR-Aug/2018- 13949-3). A link to Qualtrics was placed on the forum and, following informed consent, one parent, self-defined as the primary caregiver, completed a set of informant-report measures (see further), for one of their children, in their own homes on personal computers or mobile phones at their leisure. None of the participants received any remuneration for taking part. In total, 1235 people clicked on the link. Of these, 473 did not consent to the study and 100 did not complete at least 90% of the questionnaire items.
The group allocation of children was determined by reported diagnosis provided to the parent by a medical or psychological professional. The groups were created following positive answers to the following questions– ‘Do you think your child has feeding difficulties (yes/no)’, ‘Has your child received a diagnosis from a professional’ with a selection of disorders including ‘Not applicable, ARFID, Autism, Other developmental disorder, Any eating disorder, any physical disorder (tick all that apply)’, ‘Does your child have any other medical or psychological diagnoses (please name it here)? All questions were answered through a tick box response format. Children deemed typically developing were those who had no reported feeding difficulties or diagnoses. To create distinct groups of children, those who with co-morbid/multiple diagnoses were excluded (n = 25). Data from parents of further 151 children could not be included as their children had a variety of medical conditions, intellectual and physical disabilities.
The final sample (N = 486) constituted 39.4% of the total population that were potentially available from initially clicking on the link. The use of an opportunistic sampling strategy meant varying numbers (in line with known prevalence rates of ARFID, ASD and PE) in the final four groups of children: [Reference Martins, Young and Robson1] ARFID (n = 29) [Reference Williams and Seiverling2], ASD and reported feeding difficulties (n = 56) [Reference Coulthard and Blissett3], feeding difficulties but no clinical diagnosis (PE; n = 143), and [Reference Dovey, Aldridge, Dignan, Staples, Gibson and Halford4] TD (n = 259).
2.2 Psychometric measures
2.2.1 Behavioral pediatrics feeding assessment scale (BPFAS)
The 35-item frequency subscales of the BPFAS [Reference Crist and Napier-Philips30, Reference Crist W McDonnell, Beck, Gillespie, Barrett and Mathews31] was used to measure food avoidance. Twenty-five-items assessed frequency of problematic feeding behaviour in the target child and 10-items assessed how the parents' feel about mealtimes. Examples of questions that referred explicitly to the child's behaviour were 'takes longer than 20 min to finish a meal; enjoys eating; has problems chewing foods' and parents were 'I feel that my child's eating pattern hurts his/her general health'. Questions were answered on a 5-point Likert scale (anchored with 1 never-to-5 always). The BPFAS has been implemented for a heterogeneous sample attending feeding clinic [Reference Crist W McDonnell, Beck, Gillespie, Barrett and Mathews31], shown to discriminate between children with ARFID [Reference Dovey, Aldridge, Martin, Wilken and Meyer32, Reference Dovey, Jordan, Aldridge and Martin33] and ASD [Reference Allen, Smith, Duku, Vaillancourt, Szatmari and Bryson34] from the general population, and is sensitive to successful psychological interventions [Reference Dovey and Martin35].
2.2.2 Children’s eating behaviour questionnaire (CEBQ)
The CEBQ [Reference Wardle, Guthrie, Sanderson and Rapoport36] is a 35-item, valid and reliable measure of children’s eating behaviour. It has eight subscales: food-responsiveness (e.g. My child is always asking for food), food fussiness (e.g. My child is difficult to please with meals), emotional over-eating (e.g. My child eats more when worried), enjoyment of food (e.g. My child loves food), desire to drink (e.g. My child is always asking for a drink), satiety-responsiveness (e.g. My child gets full up easily), slowness in eating (e.g. My child eats slowly) and emotional under-eating (e.g. My child eats less when angry). Parents record their responses on a 5-point scale for each item (never-to-always). Higher scores reflect more extreme eating behaviours. The CEBQ shows good correspondence with children’s energy intake making it an excellent proxy measure for children's eating behaviour [Reference Carnell and Wardle37].
2.2.3 Strengths and difficulties questionnaire (SDQ)
The SDQ [Reference Goodman38] was used to index behavioural problems. It is a 25-item questionnaire that can be completed by parents or teachers regarding children between 3 and 16 years. It contains five subscales: emotional symptoms (e.g. ‘many worries, often seems worried’), conduct problems (e.g. ‘often fights with other children or bullies them’), hyperactivity (e.g. ‘restless, over-active, cannot stay still for long’), peer problems (e.g. ‘rather solitary, tends to play alone’) and prosocial behaviour (e.g. ‘considerate of other people’s feelings’). Each item has three response options (not true, somewhat true, always true), scored 0–2 or 2–0 depending on the item phrasing. Higher scores reflect more dysfunctional behaviour except for the prosocial subscale, for which high scores reflect less dysfunctional social interaction. Its factor structure, reliability and validity have been confirmed, and it is suitable for parental report with both clinical and non-clinical groups [Reference Vostanis39]. In addition to the original analytic method, we also included the internalising and externalising subscales of the SDQ [Reference Goodman, Lamping and Ploubidis40]. It has been advised that when using novel or population samples, the new formulation may be preferable.
2.2.4 The sensory experiences questionnaire (SEQ)
The SEQ [Reference Baranek, David, Poe, Stone and Watson41] is a 21-item questionnaire which contains four subscales intended to measure sensory hypo- and hypersensitivity in social and non-social domains. For this study, only the sensory hypersensitivity social (e.g. distressed during grooming) and non-social (e.g. sensitive to lights) subscales were included. Parents completed a five-point Likert ('0′ almost never to '4' almost always) based on the frequency with which a specific behaviour related to hypersensitivity occurs. The SEQ has favourable reliability and validity metrics and can identify sensory responsiveness in children with ASD [Reference Little, Freuler, Houser, Guckian, Carbine and David42].
2.3 Data screening and analysis
There were notable ceiling effects in the CEBQ food fussiness, with the maximal scores for many children in the ARFID, ASD or PE groups. The SDQ prosocial behaviour subscale had the ceiling effect while the SDQ peer problems subscale had a floor effect, with the maximum/minimum scores for many TD children. All other subscales exhibited either normally distributed or slight positive skew distributions. For several of the variables, violations in homogeneity of variance were present. For such variables, we conducted a Welch's F test followed up by Games-Howell post hoc tests. In situations where no violations in parametric assumptions were uncovered, a standard one-way analysis of variance (ANOVA) with Tukey's-b post hoc for variable group sizes was performed. All post-hoc analyses were corrected for multiple comparisons. All statistical tests were performed on IBM SPSS v25.0 for Windows, with statistical significance maintained at p ≤ 0.05.
3. Results
3.1 Sample characteristics
Overwhelmingly, participants reported their ethnicity to be a variant of European decent (90.2%); 6.8% reported being of a mixed heritage and the remaining 3% comprised of people of African, Asian, South Asian, South-East Asian or Oceanic decent, or left the question unanswered. The majority (85.7%) of parents reported that their children were born at term with no significant differences between the groups [Welch's F(3,98) = 1.25; p = 0.30; ω2 = 0.003]. Birth weight [F(3,467) = 0.5; p = 0.66; η2 = 0.001] and parent's average age at the child's birth was comparable for the four groups (F(3,479) = 2.23; p = 0.08; η2 = 0.01] (see Table 1). The average number of children living in the households of the families that took part was similar across the groups (roughly 1.8–1.9 children per family). The vast majority of the questionnaires were completed by the mother (mother: 97%; father: 2.5%) of the child in question, and 73.4% had a university degree or higher qualification.
Table 1 The means and ± 1 standard deviations of the age and population age-corrected body mass index (z scores) for children in the current sample.
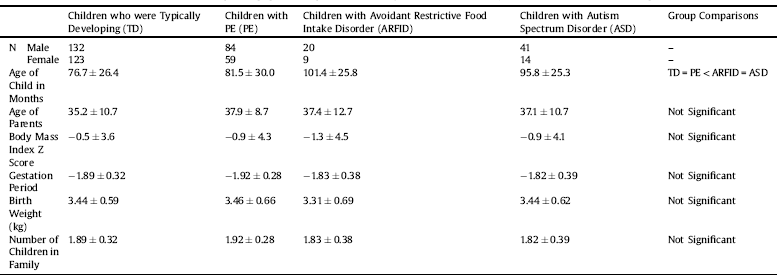
Children with ARFID had the lowest population age-corrected body mass index (zBMI) but did not differ significantly from the other groups [F(3,469) = 0.66; p = 0.58 η2 = 0.003] (Table 1). The ARFID and ASD groups were, on average, 1.5 year older than the TD and PE groups [Welch's F(3,110) = 14.6; p < 0.001 ω2 = 0.07] (see Table 1).
3.2 Group differences in eating behaviour, behavioural problems and sensory hypersensitivity
As expected (described below), there were significant group differences in feeding difficulties, eating behaviours, behavioural problems and sensory hypersensitivity (Table 2, Fig. 1).
Table 2 Means and standard deviations for the four questionnaires.
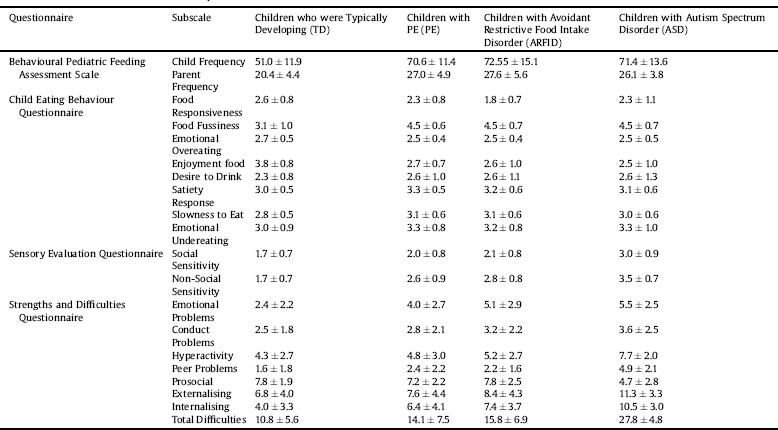

Fig. 1. Feeding difficulties, eating behaviour, sensory hypersensitivity and behavioural problems in the four study group (Em Overeat = Emotional Overeating; Em Undereat = Emotional Undereating).
3.2.1 Feeding difficulties
The BPFAS scores revealed highly significant group differences for both the child frequency [Welch's F(3,96) = 108.6; p < 0.001; ω2 = 0.4] and parent frequency [F(3,482) = 83.8; p < 0.001; η2 = 0.34]. Follow-up analyses showed that the ARFID, ASD and PE groups had greater food avoidance than the TD group (all p < 0.001) but the PE, ARFID and ASD groups did not differ from one another (p > 0.89).
3.2.2 Eating behaviour
We found significant group differences in all CEBQ scales: food-responsiveness [Welch's F(3,98) = 15.5; p < 0.001; ω2 = 0.08], food fussiness [Welch's F(3,106) = 110.7; p < 0.001; ω2 = 0.40], emotional over-eating [Welch's F(3,102) = 5.40; p = 0.002 ω2 = 0.08], enjoyment of food [Welch's F(3,96) = 80.2; p < 0.001; ω2 = 0.33], desire to drink scores [Welch's F(3,93) = 4.4; p = 0.006; ω2 = 0.02], satiety-responsiveness [F(3,482) = 9.6; p < 0.001; η2 = 0.06], emotional undereating [F(3,481) = 5.75; p = 0.001; η2 = 0.03] and slowness to eat [F(3,482) = 8.2; p < 0.001; η2 = 0.05].
For food-responsiveness, the PE and ARFID groups had lower scores than the TD group (p values<0.001), and the ARFID group also had lower scores than the PE group (p = 0.02). The ASD group did not differ significantly from the TD (p = 0.06), PE (p =.99), or ARFID groups (p = 0.16). For food fussiness, the PE, ARFID and ASD groups had higher scores than the TD group (p < 0.001), but they did not differ from one another (p >.84). For enjoyment of food, the PE, ARFID and ASD groups had lower scores compared to the TD group (p<0.001), but they did not differ from one another (p > 0.80). For desire to drink, the PE group (p = 0.013), but not the ARFID (p = 0.40) and ASD groups (p = 0.16), had higher scores than the TD group. However, the PE, ARFID and ASD groups did not differ significantly (p > 0.95). For satiety-responsiveness, the PE group (p < 0.001), but not the ARFID (p = 0.90) or ASD group (p = 0.11), had higher scores than the TD group. Again, the PE, ARFID and ASD groups had comparable scores (p > 0.10). For slowness to eat, the PE (p < 0.001) and ARFID (p = 0.025) groups, but not the ASD group (p > 0.59), were significantly slower at eating than the TD group. However, as seen earlier for most other CEBQ subscales, the PE, ARFID and ASD groups did not differ from one another (p > 0.35). Lastly, for emotional undereating, the PE (p = 0.002) and ASD groups (p = 0.02), but not the ARFID group (p = 0.19), had higher scores than the TD group. Again, the PE, ARFID and ASD groups were not different from one another (p > 0.63).
3.2.3 Behaviour problems
There were significant group differences in all SDQ subscales: emotional problems [Welch's F(3,96) = 35.8; p < 0.002; ω2 = 0.19), conduct problems [F(3,481) = 4.9; p = 0.002; η2 = 0.03], hyperactivity [F(3,481) = 24.7; p < 0.001; η2 = 0.13], peer problems [Welch's F(3,101) = 41.0; p < 0.001; ω2 = 0.20], and prosocial behaviours [Welch's F(3,95) = 21.5; p < 0.001; ω2 = 0.11].
For emotional problems, the PE, ARFID and ASD groups scored higher compared to the TD group (all p < 0.001). The ASD group, with the highest score of all groups, also had significantly higher score than the PE group (p = 0.002), but did not differ from the ARFID group (p = 0.92). For conduct problems, the ASD group had higher scores than the TD group (p = 0.002). The PE (p =.67) and ARFID (p = 0.41) groups did not differ significantly from the TD group. There was a trend for the PE to score lower than the ASD (p = 0.09), but the ARFID and ASD groups were similar (p = 0.85). For hyperactivity, the ASD group scored higher than the other three groups (p < 0.001) who did not differ from one another (p > 0.18). For peer problems, the ASD group scored higher than the other three groups (p < 0.001); PE children also scored higher than the TD group (p = 0.002), but other group comparisons were non-significant (p > 0.18). Lastly, for prosocial behaviours, the ASD group scored lower than the other three groups (p < 0.001). The PE group also scored higher than the TD group (p = 0.02), but other group comparisons were non-significant (p > 0.52).
For the externalising subscale, a significant group effect [F(3,480) = 18.9; p < 0.001; η2 = 0.11] was observed, indicating high scores in the ASD group than the PE (p < 0.001), ARFID (p = 0.01) and TD (p < 0.001) groups. There was also a significant group effect in the internalising subscale [Welch's F(3,100)=72.5; p < 0.001; ω2 = 0.31]. This was due to children with ASD, ARFID and PE all scoring higher than TD children (all p < 0.003). The ARFID and PE groups were similar (p = 0.59) and both scored lower than the ASD group (p < 0.002).
3.2.4 Sensory hypersensitivity
There was a significant group effect in SEQ hypersensitivity [social (Welch's F(3,96) = 49.1; p < 0.001; ω2 = 0.23, non-social [Welch's F(3,98) = 120.4; p < 0.001; ω2 = 0.43]. For the social hypersensitivity subscale, the ASD group had higher scores compared to other three groups (all p < 0.001) who did not differ significantly from one another (p > 0.058). For the non-social hypersensitivity, the ARFID, ASD and PE groups scored significantly higher than the TD group (all p < 0.001); the ASD group also scored significantly higher than the PE (p < 0.001) and ARFID (p < 0.001) groups but the ARFID and PE groups had comparable scores (p = 0.83).
4. Discussion
The current study aimed to uncover similarities and differences between children with ARFID, ASD and PE. Our findings revealed more similarities than differences between children with ARFID and PE in their eating difficulties, behavioural problems and sensory hypersensitivity, relative to TD children. There were, however, some quantitative differences between the groups. Specifically, the ARFID group showed the lowest food-responsiveness and differed significantly from the PE and TD (but not from ASD) groups, while the ASD group showed pronounced and significantly greater behavioural problems, social and non-social hypersensitivity than all other groups.
Considering children with ARFID versus those with PE, it was difficult to discriminate between these two groups on most of the subscales of the four questionnaires employed. Scores on only food-responsiveness were significantly lower in children with ARFID in comparison to those who were PE or typically developing. High scores on the food-responsiveness subscale of the CEBQ has been shown to represent a susceptibility to weight gain [Reference Wardle, Guthrie, Sanderson and Rapoport36]. Although a heterogeneous condition, one of the diagnostic criteria for ARFID is a significant weight loss or failure to achieve expected weight gain. Food-responsiveness may be a particularly useful assessment of children’s eating behaviour risk, with greater risk of ARFID at the lower end of the scale, and susceptibility to obesity at the higher end [Reference Carnell and Wardle37, Reference Fulkerson, Hannan, Rock, Smyth, Himes and Story43]. One possible interpretation of normal range food-responsiveness in PE children is perhaps that they are engaging with, requesting and eating preferred foods, while children with ARFID are not engaging with food irrespective of its subjective preference. Although satiety-responsiveness was higher in PE children than typically developing children, their scores were similar to those with ARFID or ASD. Heightened satiety-responsiveness is believed to be related to being less responsive to food cues and, in this context, potentially terminating the meal early [Reference Brown and Lee44].
Our study replicated and extended several previous studies. For example, we observed more emotional eating behaviours or emotional problem behaviour in children with feeding difficulties [Reference Haycraft, Farrow, Meyer, Powell and Blissett45]. Furthermore, children who were more sensory hypersensitive tended to be in one of the three feeding difficulty groups [Reference Coulthard and Blissett3, Reference Dovey and Martin5, Reference Nadon, Feldman, Dunn and Gisel7, Reference Nederkoorn, Jansen and Havermans8]. Although some evidence in adults suggests that sensory hypersensitivity can differentiate ARFID from PE [Reference Zickgraff, Franklin and Rozin46], using child-related sensory hypersensitivity measures we were unable to differentiate children with ARFID and PE. There may be two possible explanations. First, the approach to measurement for sensory hypersensitivity was not sensitive enough in children. Second, and perhaps more interesting, there may be a developmental difference between ARFID and PE. This life course development to hypersensitivity allows individuals with PE to eventually desensitise to their environment, while those with ARFID do not. Further cross-sectional and sequential research would be required to investigate these potential explanations. Despite these differences, there was some clear similarities with the findings of the current study and those in adults with PE and ARFID [Reference Zickgraff, Franklin and Rozin46]. Zickgraff and colleagues [Reference Zickgraff, Franklin and Rozin46] were not able to discriminate between PE and ARFID on standard eating disorder inventories, similar to how we were unable to do so with the BPFAS. Equally, there was significant overlap in scores on most measures for ARFID and PE.
Concerning our observations in children with ASD, they had more behavioural problems [Reference Totsika, Hastings, Emerson, Lancaster and Berridge47], and specifically more externalising behavioural problems, which manifest in conduct problems, hyperactivity, peer problems and lack of prosocial behaviours. Children with PE, ARFID and ASD all had significantly higher internalised behavioural problems. These differences were predominantly accounted for by emotional behavioural problems. This would match the heightened anxiety observed in ARFID [Reference Aldridge, Dovey, el Hawi, Martiniuc, Martin and Meyer26] and PE [Reference Farrow and Coulthard28]. These results are also in keeping with findings of more generic assessments of paediatric disorders [Reference Glazbrook, Hollis, Heussler, Goodman and Coates48]. Caution is recommended in generalisation of these findings to all children with ASD. Only children with feeding difficulties and ASD were included in this study. Therefore, these findings relate only to ASD children with feeding difficulties rather than all children with the condition.
The present study had some limitations. First, the dataset was drawn from parents using a psychometric informant-report procedure that dominates paediatric research, due to difficulties in accessing and gathering reliable evidence from a large number of children. In defence of the method, however, many of our results replicate and extend previous findings and our data set is comparatively large and allowed direct comparisons between the four groups of interest. Another limitation worthy of note is that there was age difference with TD and PE children being slightly younger than those with ARFID or ASD. However, comparing the clinical samples to children who are younger, if anything, constitutes a conservative estimate of differences since children with ASD and ARFID will have developed a little more than the TD or PE children. At present, limited knowledge is available concerning children with ARFID and how they develop. Although, the children in the current sample were above the age range expected to start showing the signs of ARFID - namely 5 years of age - research has yet to report the severity, progression and stability of the behaviours associated with ARFID. It is possible that the children in the PE group may eventually go on to develop into ARFID. Emergent data indicates that PE and ARFID are similar in terms of their aetiological characteristics (Dovey, 2018). Thus, the diet of both PE and ARFID are similar in terms of limited variety; however, children with ARFID likely accept fewer foods and are more resistant to exposure techniques. Should these expectations hold in observational studies, logically ARFID children should show a reluctance to try new and familiar foods and thus have a low and stable dietary variety that does not sustain their nutritional needs in the long-term. A valid alternative explanation may be that a significant life event would be required to change a child with PE to further restrict their diet leading to a diagnosis of ARFID. Future research will be required to delineate between these two competing perspectives.
The children with ARFID were not screened for ASD in the current dataset. It is likely that the parents of children with diagnoses did not have additional co-morbid disorders. The reason for this assumption was that these children were already in support services and if the parents or their associated professionals suspected ASD then a referral would likely have taken place. Children who had multiple ASD and ARFID diagnoses were excluded for clear delineation of the groups. It must be noted that the children who were assigned to these groups may not have been free from other co-morbid disorders or did not have an undiagnosed ASD or ARFID condition depending on the group in question. Future research may wish to employ a more carefully controlled sample similar to experimental investigations [Reference Aldridge, Dovey, el Hawi, Martiniuc, Martin and Meyer26], consider direct observational and real feeding investigations, and investigate the underlying emotional components to feeding difficulties in children (that we identified in this study through parental reports) using objective experimental methods. This would extend and compliment the psychometric investigations that currently dominate the literature and provide definitive evidence as to some of the causes of eating disorders in children.
To conclude, this study has shown that there are more similarities than differences between children with PE, ARFID and ASD in their eating behaviour. The eating behaviour of children with PE differed from those with ARFID only in terms of food-responsiveness. Furthermore, all children (PE, ARFID and ASD) with feeding difficulties appear to exhibit more internalised behavioural problems, especially within the emotional domain. Despite researchers typically focusing on either PE or clinically relevant feeding difficulties (ARFID), it seems that these groups are dealing with similar behaviours. Therefore, it is likely that food avoidance, as it is currently conceptualised, is on a continuum and thus dimensional rather than discrete diagnostic categories.
Ethical standards
The authors assert that all procedures contributing to this work comply with the ethical standards of the relevant national and institutional committees on human experimentation and with the Helsinki Declaration of 1975, as revised in 2008
Financial support
This work was supported by the Wellcome Trust who funded the Citizen Science Project (http://parentingsciencegang.org.uk/; ref 360G-Wellcome-204786_Z_16_Z).
Acknowledgements
We would like to thank Ms Tamasin Greenough-Graham for her support with the preparation of the manuscript and facilitating the development of the project.







Comments
No Comments have been published for this article.