Bloodstream infection (BSI) outbreaks due to contamination of pharmaceutical solutions during manufacturing occur rarely.Reference O’Grady, Alexander and Burns 1 , Reference Maki and Mermel 2 Most nosocomial outbreaks of infusion-related BSIs have been derived from contamination of infusate by gram-negative bacilli (GNB) introduced during manufacturing. Among these microorganisms, Ralstonia pickettii has been reported to cause infection via contaminated solutions.Reference Yabuuchi, Kosako, Yano, Hotta and Nishiuchi 3 Solutions contaminated at the point of manufacture have also been associated with outbreaks of BSIs or catheter-related infections.Reference Labarca, Peterson and Bendaqa 4 , Reference Ryan, Pembroke and Adley 5
The R. pickettii bacterium is waterborne and can survive and grow in various water sources, including water supplies, sterile water, intravenous ranitidine, narcotics, skin disinfectants, blood culture bottles, saline solutions, magnesium vials, and other solutions used for patient care in hospitals.Reference Labarca, Peterson and Bendaqa 4 , Reference Boutros, Gonullu, Casetta, Guibert, Ingrand and Lebrun 6 – Reference Ryan, Pembroke and Adley 10 Those contaminated solutions can be administered by vascular injection, by intravenous drip infusion, or via wound cleaning, resulting in BSIs or other infections. Numerous additional outbreaks caused by R. pickettii contamination of saline solution have been reported by hospitals in several countries,Reference Maki and Mermel 2 and most of these contamination events occurred during the manufacturing stage.Reference Adley and Saieb 11
Although the pathogenicity of this bacterium is not strong, it has higher virulence for immunosuppressed patients and those who are debilitated in some way.Reference Vincenti, Quaranta and De Meo 12 – Reference Marroni, Pasticci, Pantosti, Colozza, Stagni and Tonato 14
In our hospital, a total of 4 blood specimens from 4 patients were culture positive for R. pickettii within 1 week. We conducted clinical epidemiological and microbiological investigations to identify possible sources of infection and to block the route of infection to end the outbreak.
MATERIALS AND METHODS
Setting and Patients
This study was conducted in a 3,000-bed tertiary referral medical center in Taiwan with >8,500 admissions during May 2015. Patients had been treated in the injection room or chemotherapy room at outpatient departments, emergency departments, or hospital wards. All patients who were culture positive for R. pickettii from May 3 to June 11, 2015, were eligible for the study. Hospitalwide infection surveillance was conducted.Reference Horan, Andrus and Dudeck 15 A case was defined using 2 criteria: (1) a positive R. pickettii culture using ≥1 samples of blood or fluid withdrawn via a catheter, and (2) injection-use 20-mL normal saline solution had been used to flush the patient’s catheter. This study and report were reviewed and approved, and an exemption was issued by our institutional review board.
Epidemiological Surveillance and Data Collection
The outbreak period, defined as positive culture for R. pickettii, occurred from May 3 to June 30, 2015. We investigated the associated epidemiological data, including patient data collection, and we conducted field visits and retrieved items with possible contamination. Initially, the first 4 patients were culture positive for R. pickettii during May 3–8, 2015. Although these cases were dispersed across 4 wards, the third and the fourth patient specimens positive for these rare bacteria occurred on the same day, and the infection control team immediately launched an investigation. Before May 14, cultures were ordered for 3 additional cases. On May 14, from analysis of epidemiological data, the infection control team highly suspected that injection-use 20-mL normal saline solution was the common source of the infections. Late on May 16, when the laboratory reported 2 positive cultures from unopened 20-mL normal saline ampules of the same lot number (which were suspected glucose-nonfermentative), we immediately stopped using the 20-mL normal saline ampules in all wards. Early on May 17, we confirmed that the bacterial cultures were R. pickettii, and we stopped using all 20-mL normal saline ampules throughout the entire hospital. The same day, we notified the Taiwan Centers for Disease Control (Taiwan CDC) and the Taiwan Food and Drug Administration (Taiwan FDA).
During the preoutbreak and outbreak periods, there were no changes in healthcare-associated infection surveillance, culture ordering practices, or intravascular therapy procedures, including intravascular catheter-related care and infusion-related care. We also reviewed laboratory procedures for processing blood cultures and identifying species to exclude the possibility of contamination during the culture process. The blood-culture bottles from clinical units were sent to the laboratory, received barcode scanning, and were placed directly inside the culturing instrument until the culture was complete. This procedure prevented contamination by personnel.
Data on demographic and clinical characteristics, therapeutic devices used, and infusion-related methods were recorded. We also retrieved samples of intravascular catheter- and infusion-related items from patient wards for culture, including unopened 20-mL normal saline ampules and other items (see Tables 1 and 2).
TABLE 1 Clinical Characteristics of the Affected Patients for Ralstonia pickettii
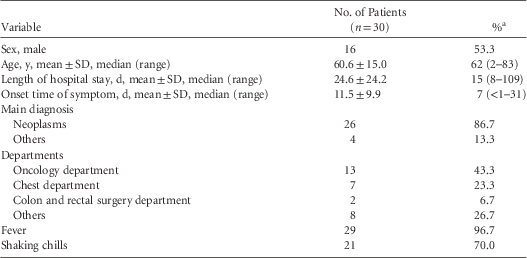
NOTE. SD, standard deviation.
a Or median and range, as indicated.
TABLE 2 Clinical Various Devices Used for the Affected Patients

NOTE. CVC, central venous catheter; IV, intravenous; BD, Becton Dickinson.
Culture-Positive Patient Discovery and Follow-Up
We conducted detection and monitoring of patients with possible colonization or infection who might have received the contaminated normal saline from the contaminated pharmaceutical batch between April 10 and the full retrieval of all related products on May 18.
All inpatients who met any of the following criteria were monitored and followed to the last discharged patient (July 20). (1) We followed all patients who had a Port-a-Cath (Bard, Reynosa, Tamaulipas, Mexico) or Hickman catheter (Bard, Reynosa, Tamaulipas, Mexico) implantable venous access device and visited the emergency department. If the patient had fever after the catheter was flushed, then 2 sets of blood cultures were performed and the infectious disease physicians were notified. (2) We followed patients who returned to the injection room as outpatients to have their Port-a-Cath or Hickman catheter flushed. These patients were required to have 3–5-mL fluid withdrawn from the catheter until blood appeared. Microbe cultures were then performed on these samples. (3) We followed all inpatients with Port-a-Caths or Hickman catheters during the outbreak. Physicians were urged to consider suspending the relevant treatment via these implants (ie, before the culture results from the implants were obtained).
Microbiological Identification
Our microbiology laboratory is certified internationally through the College of American Pathologists. Identification of all R. pickettii isolates was confirmed at the laboratory. The samples of the retrieved items were examined using the Bact/ALERT 3D method (bioMerieux, Durham, NC). Cultures of these retrieved items were processed in an aseptic laboratory room. All R. pickettii clinical isolates from blood cultures and withdrawn fluid from catheter lumens were identified using an automated method with identification cards in the VITEK 2 system and the VITEK-MS system (bioMerieux, Mercy L’Etoil, France). To determine their clonality, all confirmed isolates were subjected to pulsed-field gel electrophoresis (PFGE). The plug preparation and digestion were performed in accordance with a previous study.Reference Labarca, Trick and Peterson 16 Isolates with >85% similarity were grouped as the same clone.
RESULTS
Descriptive Epidemiology
During the outbreak period, 57 culture specimens from 30 case patients were identified as R. pickettii. Blood samples were used for 24 of these 30 cultures; 6 cultures used fluid withdrawn from Port-a-Caths. Interestingly, 14 of these cultures contained >2 specimens. Of these 30 culture-positive R. pickettii case patients, 16 were male (53.3%). The mean age of the overall cohort was 60.6±15 years, and the median hospital stay was 15 days. The most frequent diagnoses were neoplasms (26 cases; 76.7%). These case patients were admitted to 20 various medical wards in 12 departments, including the oncology department (13 cases; 43.3%). Furthermore, 20 case patients (66.7%) were undergoing chemotherapy. Overall, 29 case patients (96.7%) had fever; among them, 21 (72.4%) had shaking chills and 4 (13.8%) had symptoms within 24 hours (minimum 3 hours) after the catheter flushing. Other case patients presented symptoms up to 31 days after catheter flushing. A single patient died of another cause of disease (Table 1).
These 30 patients had all received intravascular device implants, including 23 patients (76.7%) with Port-a-Caths. All case patients had received 20 mL 0.9% normal saline via catheter flushing. Among them, monthly routine Port-a-Cath flushing was performed on 16 patients (53.3%). Table 2 lists the various reasons that the normal saline solution was used to perform a single catheter flushing.
The first case of BSI due catheter flushing with contaminated normal saline occurred April 29, and the last case occurred on May 16. The dates of catheter flushing that resulted in R. pickettii BSI were mostly centralized from May 5 to May 15 (23 cases; 76.7%). Catheter flushing in case patients occurred in 10 different locations: 2 injection rooms of outpatients, 6 wards, 1 anesthesia induction room, and 1 endoscopy center. The overall incidence of R. pickettii in the outpatient injection room was 18 cases (60%). For the 30 culture-positive cases, catheter flushing was performed by 24 nurses and 2 anesthesia personnel.
The culture-positive R. pickettii specimens were obtained from peripheral veins or/and catheters (18 cases, 60%). In 9 cases (30%), positive cultures were obtained during follow-up from peripheral veins, from catheters, withdrawn fluid, or the catheter tip (ie, therapeutic device–related BSI). In 3 cases (10%), positive cultures were obtained from fluid withdrawn from a Port-a-Cath. In 18 cases, the Port-a-Caths were removed, including cases in which the catheter tip was culture positive (9 cases, 50%), was culture negative (6 cases, 33.3%) cases, was culture positive for gram-negative bacilli or gram-positive cocci (2 cases, 11.1%), or was not cultured (1 case, 5.6%).
Microbiological Investigations
A total of 206 various catheter-related items from units where positive cultures were obtained were cultured. On day 7, bacteria culture confirmed R. pickettii in 2 unopened ampules of 20-mL normal saline solution (ie, the same batch but from 2 different wards). The Taiwan CDC sampled and tested 53 ampules of 20-mL normal saline from the same manufactured batch from the pharmaceutical company and also identified the contaminant strain as R. pickettii. PFGE indicated that all clinical isolates had similarity of >90%, confirming the outbreak of the same clone of R. pickettii.
DISCUSSION
Based on epidemiological data and microbiological and PFGE tests, we determined that the outbreak of R. pickettii BSIs occurred via contaminated 20-mL injection-use ampules of normal saline used to flush catheter lumens. Active and prudent epidemiological investigation is very important in detecting potential problems. In our epidemiological investigation, although laboratory testing results had not yet been confirmed, intervention strategies were implemented immediately, including retrieval of items suspected of contamination. We also agreed that a ward or department that yielded ≥2 or more rare species from blood cultures in a short period should be vigilant and should conduct an acuity investigation to determine whether it the contamination was internally or externally derived.Reference Ryan, Pembroke and Adley 5 , Reference Moreira, Leobons and Pellegrin 17
Suspicious Items and Plant Investigations
Several related R. pickettii contamination reports have indicated that contaminated sterile 0.9% saline was the source of outbreaks in 1983 and 1998.Reference Maki and Mermel 2 Later, Ryan et alReference Ryan, Pembroke and Adley 5 integrated 55 reports of R. pickettii infection distributed in Europe, America, and Asia, including 24 BSI reports (43.6%). The most common sources of contamination in these outbreaks were distilled water, physiological saline, and catheters.Reference Ryan, Pembroke and Adley 5 Recently, Ross et alReference Ross, Steinmann and Buer 9 also reported on R. pickettii BSI outbreaks caused by contaminated magnesium vials in an intensive care unit. To identify the source of the infections in our outbreak, we obtained the specimens from intravascular catheter- and infusion-related items for microbe culture. Among these retrieved items, we confirmed the same pathogen in 2 unopened 20-mL normal saline ampules in the same supply batch. The results of the PFGE tests were similar, demonstrating that this BSI outbreak event was caused by contamination of normal saline. No further cases occurred after the use of the implicated product was discontinued.
The Taiwan CDC tests for samples from our hospital, Taiwan FDA tests of samples from the pharmaceutical factory, and our hospital laboratory tests revealed that the unopened injectable normal saline ampules were contaminated with R. pickettii. Our hospital tests confirmed the same bacteria in the normal saline ampules from the same batch. This pharmaceutical company used automatic production models and product manufacturing processes for closed systems; however, several laboratory studies have shown that R. pickettii can pass through filters, and even low numbers inoculated into 0.9% sodium chloride solution can proliferate at high temperatures.Reference Labarca, Peterson and Bendaqa 4 , Reference Adley and Saieb 11 , Reference Ryan and Adley 13 Previous reports pointed out that was the product contaminated by R. pickettii may have been caused by either ineffective sterilization or contamination of equipment used in the production process.Reference Maki and Mermel 2
Patient Investigations
Although R. pickettii has low virulence, emergent antibiotic-resistant opportunistic pathogens, such as R. pickettii isolated from hospital environments, could be potentially more dangerous than other more well-known waterborne pathogens.Reference Vincenti, Quaranta and De Meo 12 Bacteremia is the most frequent clinical manifestation of R. pickettii .Reference Ryan, Pembroke and Adley 5 , Reference Ross, Steinmann and Buer 9 In particular, this pathogen is more likely to cause infection in patients with impaired immune systems.Reference Kismet, Atay and Demirkaya 7 , Reference Marroni, Pasticci, Pantosti, Colozza, Stagni and Tonato 14 , Reference Moreira, Leobons and Pellegrin 17 , Reference Coenye, Goris, De Vos, Vandamme and LiPuma 18 Among our 30 cases, the most frequent diagnoses were neoplasms (86.7%), and 20 of these patients were undergoing chemotherapy.
In our study, we identified 29 BSI case patients with fever or/and shaking chills; these patients presented with symptoms within 24 hours up to 31 days after the catheter was flushed with the contaminated normal saline. We postulate 2 reasons for this variation in the occurrence of symptoms. First, the contaminated normal saline administered directly through the catheter lumen flashing to the bloodstream resulted in symptoms presenting in a short period of time. Second, in patients undergoing monthly routine catheter flushing, the contaminated normal saline remained in their lumen, allowing R. pickettii colonies to gradually increase over time until they migrated into the bloodstream. Thus, onset of symptoms occurred later. We tested fluid withdrawn from the Port-a-Cath or Hickman catheters of outpatients or inpatients during the outbreak period, and we found 6 culture-positive cases (20%). We confirmed these speculative culture-positive cases using fluid withdrawn from catheters of asymptomatic patients. R. pickettii can grow in various solutions, and our findings are consistent with previous reports.Reference Maki and Mermel 2
Several studies have illustrated that R. pickettii also forms and maintains biofilms inside plastic catheters, making them more resistant to biocides and, consequently, more difficult to eradicate.Reference Adley and Saieb 11 , Reference Mijnendonckx, Provoost and Ott 19 , Reference Dombrowsky, Kirschner and Sommer 20 In light of our investigation results, 18 Port-a-Caths were removed, and 50% of them were culture positive for the catheter tip. Kismet et alReference Kismet, Atay and Demirkaya 7 reported 2 cases of R. pickettii bacteremia in patients with Port-a-Caths that could be treated only by removal of the catheters.
In conclusion, during the investigation and retrieval of 6 batches of normal saline ampules, our hospital and the Taiwan FDA confirmed that the R. pickettii were from the same batch and that the other batches were culture negative. We believe that the 20-mL normal saline ampules used for all 30 cases came from the same batch number. Hospital monitoring mechanisms are extremely important in identifying and ending BSI outbreaks such as this one.
ACKNOWLEDGMENTS
We thank the members of the infection control team for their hard work, and the Department of Nursing and Pharmacy for helping with patients and retrieving test items in Taipei Veterans General Hospital.
Financial support: No financial support was provided relevant to this article.
Potential conflicts of interest: All authors report no conflicts of interest relevant to this article.






