PURPOSE
This expert guidance document (EG) provides recommendations regarding discontinuation of contact precautions (CP) at the individual patient level in acute-care hospitals employing CP for 1 or more of the following organisms: methicillin-resistant Staphylococcus aureus (MRSA), vancomycin-resistant enterococci (VRE), Clostridium difficile, and multidrug-resistant Enterobacteriaceae (MDR-E), including carbapenem-resistant Enterobacteriaceae (CRE) and extended-spectrum β-lactamase (ESBL)–producing organisms. This document also provides a review of the role of molecular testing in guiding decisions pertaining to duration of CP for patients with these organisms. The guidance does not address decisions regarding the initiation of CP for any specific organism.
Previously published guidelines describe components of CP and identify situations in which CP should be used; currently, however, few publications address the issue of how long CP should be maintained. At the time of publication, decisions related to implementation of CP for select, endemic organisms are made by individual facilities based on factors such as institutional epidemiology, resources, organizational priorities, and previously published guidance, and these vary widely. The SHEA Guidelines Committee (GLC) selected this topic to address when CP should be discontinued for individual patients in acute-care settings that employ CP for the aforementioned organisms.
Although the organisms addressed are frequently encountered in other settings (eg, nursing homes, long-term acute-care facilities, rehabilitation centers, outpatient medical care settings), additional considerations may affect the application of these recommendations outside the acute-care hospital environment.
AUTHORS
The authors consist of current and past members of the SHEA Guidelines Committee (GLC), who serve as volunteers. All authors are involved at their respective institutions in the development of policies pertaining to CP, either directly or in an advisory role.
INTENDED USE
Special-topic EG documents are developed to address areas of relatively narrow scope that lack the level of evidence required for a formal guideline but are important for the provision of safe and effective healthcare. As such, systematic grading of evidence level is not provided for individual recommendations. Each EG is based on a synthesis of limited evidence, theoretical rationale, current practices, practical considerations, the opinion of the writing group, and consideration of potential harms where applicable. Within the EG, a summary list of recommendations is provided, along with their respective rationales. We also conducted a survey of the SHEA Research Network (SRN).
No EG can anticipate all situations and this EG is not meant to be a substitute for individual judgment by qualified professionals.
METHODS
Expert Guidance Development
This EG follows the process outlined in the Handbook for SHEA-Sponsored Guidelines and Expert Guidance Documents. 10 The topic of duration of CP was among those proposed and ranked highest by the GLC. A manuscript proposal was approved by the SHEA Publications Committee and the SHEA Board of Trustees. We developed PICO-style (ie, population, intervention, control, and outcomes) questions based on agreed-upon themes. These questions were used to define the scope of the EG and the development of search terms, which were voted on until unanimous approval was achieved. We identified the period during which articles would be collected as January 1, 1990, to April 1, 2016. Only English-language articles were included. The lists of articles generated by the searches were reviewed for inclusion by a primary reviewer and a secondary reviewer. The EG was also informed by a survey of the SHEA Research Network (SRN).
Expert guidance development includes a formalized process for reaching expert consensus. Recommendations are listed with rationale statements. The consensus around each recommendation was determined via an anonymous rating and comment period.
This EG was reviewed and approved by the SHEA Guidelines Committee, the SHEA Publications Committee, and the SHEA Board of Trustees. This EG was endorsed by the Association for Professionals in Infection Control and Epidemiology (APIC), the Society of Hospital Medicine (SHM), and the Association of Medical Microbiology and Infectious Disease Canada (AMMI Canada).
Multidrug-Resistant Enterobacteriaceae (MDR-E)
For purposes of this EG, we considered MDR-E to be Enterobacteriaceae described as ESBL-producing (ESBL-E), carbapenem-resistant (CRE), or MDR-E defined by resistance to multiple classes of antibiotics. This EG does not address additional epidemiologically significant multidrug-resistant Gram-negative organisms such as Pseudomonas aeruginosa or members of the Acinetobacter baumannii complex.
Microbiology and Testing
For this EG, we reviewed FDA-cleared microbiology tests. We recognize that other tests, including laboratory developed tests, are in use. These are not included. This document focused on MRSA, VRE, and MDR-E. Molecular testing for C. difficile is not addressed.
Colonization
Colonization was defined as the isolation of the organism from a nonsterile site in the absence of symptoms of infection.
GUIDANCE STATEMENT
This document does not provide recommendations regarding indications for the use of CP. It is intended for acute-care hospitals that already use CP, and it addresses when and under what circumstances CP may be discontinued.
Methicillin-Resistant Staphylococcus aureus (MRSA)
Recommendations
-
1. If a hospital uses CP for patients previously colonized or infected with MRSA, we recommend establishing a policy for the discontinuation of CP for MRSA.
-
2. For patients not on antimicrobial therapy with activity against MRSA, we recommend negative screening cultures to guide decisions about discontinuation of CP. The optimal number of negative cultures needed is unclear, though 1–3 negative cultures are often used. The anterior nares are a common site of culture sampling, though the literature is unclear regarding the optimal site and the role of extra-nasal sampling.
-
3. For high-risk patients, such as those with chronic wounds or patients from long-term care facilities, we recommend extending CP from the last MRSA-positive culture, prior to assessing for CP discontinuation.
-
4. Outside an outbreak setting, if a facility’s endemic rates of MRSA infection are low, the hospital may consider the alternative approach of using CP for patients with active MRSA infection for the duration of the index admission and discontinuing CP on hospital discharge. In adopting this approach, a hospital should monitor facility MRSA infection rates, maximize and consider monitoring use of standard precautions, and minimize patient cohorting to avoid intrafacility transmission. If the hospital’s MRSA infection rates increase, the hospital should transition to a screening-culture–based approach for discontinuation of CP.
Rationale
Wide variation exists in hospital practices for discontinuation of CP for MRSA,Reference Shenoy, Hsu, Noubary, Hooper and Walensky 11 and a policy for discontinuation can provide guidance to practitioners and infection prevention staff. A policy for CP discontinuation should address (1) inclusion and exclusion criteria, (2) laboratory testing and surveillance strategies, and (3) policy implementation and oversight. The studies utilized for this manuscript used various time periods during which an individual with a history of MRSA could be eligible for testing for discontinuation of CP. The SRN survey found that most institutions used 1–3 negative MRSA surveillance cultures to determine whether CP could be discontinued, though some providers report that their hospital utilizes CP indefinitely in patients with a history of MRSA. Published evidence indicates that most patients will remain negative for MRSA colonization if they have 3 consecutive negative weekly surveillance cultures,Reference Huckabee, Huskins and Murray 12 though the optimal number of and interval between cultures remains unclear in the literature. Patients with chronic wounds and those from long-term care facilities may be at higher risk for persistent MRSA colonization and recolonizationReference Scanvic, Denic, Gaillon, Giry, Andremont and Lucet 13 ; thus, for these patients, hospitals may extend CP. The optimal duration of extension is unknown, though a minimum of 6 months was used commonly by institutions in the SRN survey.
Vancomycin-Resistant Enterococci (VRE)
Recommendations
-
1. If a hospital uses CP when caring for patients colonized or infected with VRE, we recommend establishing a policy for discontinuation of CP for VRE.
-
2. We recommend that following treatment of VRE infection, the hospital use negative stool or rectal swab cultures to guide decisions about the discontinuation of CP. The optimal number of negative cultures needed is unclear, though 1–3 negative cultures, each at least 1 week apart if multiple cultures are obtained, are often used.
-
3. Hospitals should consider extending CP prior to assessing for CP discontinuation in VRE infected patients who are (1) highly immunosuppressed, (2) receiving broad spectrum systemic antimicrobial therapy without VRE activity, (3) receiving care in protected environments (eg, burn units, bone marrow transplant units, or settings with neutropenic patients), or (4) receiving care at institutions with high rates of VRE infection.
-
4. Outside an outbreak setting and if facility endemic rates of VRE infection are low, the hospital may consider the alternative approach of using CP for patients with active VRE infection for the duration of the index admission and discontinuation of CP on hospital discharge. In adopting this approach, hospitals should monitor VRE infection rates, maximize and consider monitoring use of standard precautions, and minimize patient cohorting to avoid intrafacility transmission. If institutional VRE infection rates increase, the hospital should transition to a screening-culture–based approach for discontinuation of CP.
Rationale
Wide variation exists in hospital practices regarding the duration of CP for patients with VRE infection or colonization, and a policy on discontinuation of can guide providers and infection control staff. A policy for CP discontinuation should address (1) inclusion and exclusion criteria, (2) laboratory testing and surveillance strategies, and (3) policy implementation and oversight. In the SRN survey, most institutions use 1–3 negative surveillance cultures to identify patients for which CP can be discontinued. Data on duration of VRE colonization indicate that colonization may be prolonged, with high rates of relapse after multiple consecutive negative surveillance cultures.Reference Baden, Thiemke and Skolnik 14 – Reference Patel, Allen and Manahan 16 Factors associated with prolonged VRE carriage include immunocompromising conditions and concomitant antibiotic exposure.Reference Patel, Allen and Manahan 16 – Reference Karki, Land and Aitchison 19 Additionally, patients with diarrhea and uncontrolled respiratory secretions and draining wounds may pose the highest risk for transmission in the healthcare environment. The sensitivity of surveillance cultures of stool or rectal swab samples for the detecting VRE colonization is not well established. Settings where VRE infection rates increase, particularly in outbreak settings, or settings where care is provided to patients who, if colonized, may be at high risk for invasive infection, may benefit from intensified infection prevention efforts to reduce VRE transmission. Most frequently, 3 consecutive negative cultures performed weekly have been studied, although hospitals may consider other strategies.Reference Baden, Thiemke and Skolnik 14 , Reference Byers, Anglim, Anneski and Farr 20 Given the limitations of sequential culture strategies in documenting VRE eradication, prolonging CP may be an effective enhanced measure in preventing VRE transmission in high-risk settings, though evidence does not exist to identify the optimal period of prolongation.
Multidrug-Resistant Enterobacteriaceae (MDR-E)
Recommendations
-
1. If a hospital uses CP for patients infected or colonized with MDR-E (ESBL-E and/or CRE), we recommend establishing a policy for discontinuation of CP for MDR-E that includes the following:
-
a. Maintaining CP for ESBL-E and CRE for the duration of the index hospital stay when infection or colonization with these bacteria is first detected.
-
b. Considering discontinuation of CP on a case-by-case basis, taking into account the following criteria: (1) at least 6 months have elapsed since the last positive culture; (2) presence of a clinical infection and ongoing antibiotic use, where discontinuation of CP should be discouraged in the setting of suspected or known infection with ESBL-E or CRE, and concurrent broad-spectrum antibiotic use that may select for these organisms; and (3) procurement of an adequate number of screening samples, with at least 2 consecutive negative rectal swab samples obtained at least 1 week apart to consider an individual negative for ESBL-E or CRE colonization.
-
2. We recommend that for extensively drug-resistant Enterobacteriaceae, such as carbapenemase-producing CRE, or Enterobacteriaceae with very limited treatment options (susceptible to ≤2 antibiotic classes used to treat that organism), hospitals should maintain CP indefinitely.
Rationale
Variation exists among hospital practices regarding duration of CP for patients with ESBL-E and CRE infection or colonization, with most respondents in the survey reporting that their hospital utilizes CP indefinitely in patients with a history of ESBL or CRE. If an institution elects to consider discontinuation of CP for MDR-E, we recommend a policy to guide practitioners and infection prevention staff that includes (1) inclusion and exclusion criteria, (2) laboratory testing and surveillance strategies, and (3) policy implementation and oversight. Nearly all studies described prolonged and persistent colonization with these organisms. Various risk factors are associated with persistent carriage with ESBL-E and CRE and include positivity on clinical (vs screening) cultures and exposure and re-exposure to healthcare facilities.Reference Bart, Paul, Eluk, Geffen, Rabino and Hussein 21 , Reference Cheng, Chan and Wong 22 Studies also have described variability in detection of these bacteria from the gastrointestinal (GI) tract of the same individual over time (ie, positive culture followed by negative culture then reverting to positive).Reference Bart, Paul, Eluk, Geffen, Rabino and Hussein 21 , Reference Vehreschild, Hamprecht and Peterson 23 Finally, certain extensively drug-resistant Enterobacteriaceae, particularly carbapenemase-producing bacteria, have no or limited treatment options, which makes the impact of even a single transmission event significant and provides the basis for a more conservative approach to duration of CP.
Clostridium difficile
Recommendations
-
1. We recommend that patients with C. difficile infection (CDI) receive care with CP for at least 48 hours after resolution of diarrhea.
-
2. Hospitals should consider extending CP through the duration of hospitalization if elevated rates of CDI are present despite appropriate infection prevention and control measures.
-
3. At this time, insufficient evidence exists to make a formal recommendation as to whether patients with CDI should be placed on CP if they are readmitted to the hospital.
Rationale
Currently, the Centers for Disease Control and Prevention (CDC) and the SHEA/IDSA Compendium of Strategies to Prevent Clostridium difficile in Acute-Care Hospitals recommend the discontinuation of CP 48 hours after the resolution of diarrhea among patients diagnosed with C. difficile colitis.Reference Yokoe, Anderson and Berenholtz 24 Patients that are C. difficile carriers shed the organism in their stools for weeks after cessation of diarrhea.Reference Sethi, Al-Nassir, Nerandzic, Bobulsky and Donskey 25 , Reference Jinno, Kundrapu, Guerrero, Jury, Nerandzic and Donskey 26 The shedding of C. difficile spores after resolution of diarrhea may contribute to the spread of this organism. Recent data suggest that isolation of asymptomatic carriers reduced the incidence of C. difficile in the hospital setting.Reference Longtin, Paquet-Bolduc and Gilca 27 Based on these findings, we recommend extending the duration of CP for the duration of hospitalization in settings in which control of C. difficile is not optimal despite the institution of the standard practices. At this time, evidence does not exist supporting repeat laboratory testing for C. difficile to guide decisions regarding discontinuation of CP for patients with CDI.
Microbiological Screening and Molecular Testing
Recommendation
At this time, insufficient evidence exists to make a formal recommendation supporting the use of molecular testing for the purpose of discontinuation of CP for MDROs.
Rationale
While we assume that polymerase chain reaction (PCR) tests perform with superior sensitivity compared to culture, due to lack of high-quality studies at this time, we cannot definitively ascertain the impact of molecular methods on informing the duration of colonization and guiding decisions about CP.
BACKGROUND
SHEA Research Network Survey
We surveyed SHEA Research Network (SRN) institutions to ascertain policies and practices for guiding duration and discontinuation of CP for MRSA, VRE, MDR-E, and C. difficile, as well as screening and testing methods (Table 1). On June 22,, June 28, and July 6, 2016, SHEA sent the survey to members of the SRN, which includes 134 individual institutions, 26% of which are outside the United States and Canada. Among them, 4 institutions opted out because they were not eligible to participate, and 7 indicated that they were unavailable to respond during the time the survey was open. The survey response rate was 70.7% (87 of 123).
TABLE 1 Survey of Institutions in the SHEA Research Network (SRN)
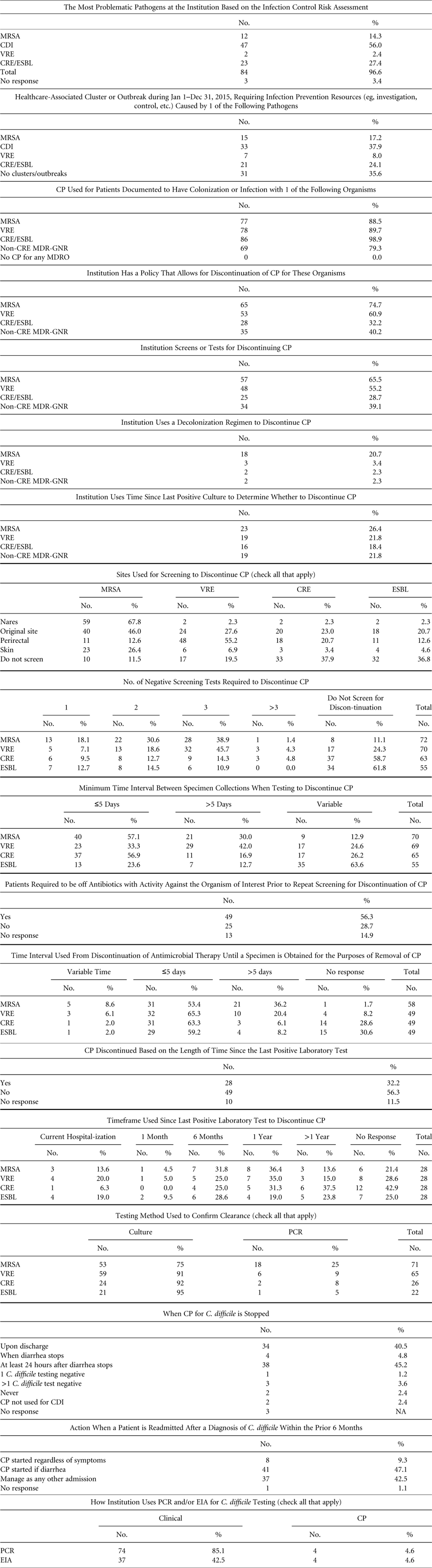
NOTE. CP, contact precautions; MRSA, methicillin-resistant Staphylococcus aureus; VRE, vancomycin-resistant enterococci (VRE); CDI, Clostridium difficile infection; MDR-GNR, multidrug-resistant Gram-negative rods; CRE, carbapenem-resistant Enterobacteriaceae; ESBL, extended-spectrum β-lactamase; EIA, enzyme immunoassay; PCR, polymerase chain reaction.
Of the responding institutions, 60.5% were academic medical centers, 18.4% were community teaching hospitals with academic affiliations, and 7.9% were community hospitals with no academic affiliations. The responding institutions varied in bed size and number of intensive care units (ICUs): 51–300 beds (29.7%), 301–600 beds (31.4%), 601–900 beds (16.3%), >900 beds (23.3%), and 1–3 ICUs (41.9%), 4–6 ICUs (30.2%), 7–9 ICUs (12.8%), ≥10 ICUs (14%). Overall, 94% of institutions had pediatric beds. On average, institutions reported having 1.4 hospital epidemiologists and 4.8 infection preventionists.
Individual respondents reported the following primary roles: healthcare epidemiologist (75.3%), infection committee chair (54.6%), and infection preventionist (26%). These individuals indicated that they are involved in patient care (40.3%), teaching (40.3%), clinical research (31.2%), and administration (18.9%).
More than half of the respondents identified C. difficile (56%) as the most problematic pathogen at their institution. Another 27% reported that multidrug-resistant Gram-negative rods (MDR-GNR), such as ESBL and CRE, were their highest concern. MRSA and VRE followed with 14% and 2%, respectively. Moreover, 38% of respondents reported a healthcare-associated cluster or outbreak related to C. difficile infection in calendar year 2015, followed by MDR-GNR (24%), MRSA (17%), and VRE (8%).
Most respondents reported using CP in patients colonized with MDRO: CRE (99%), MRSA (89%), VRE (90%), and other MDR-GNR (79%). All respondents reported using CP for at least 1 indication. In addition, 75% of respondents reported that their institution had a policy for discontinuing CP in patients colonized with MRSA; 61% for VRE; 32% for CRE; and 40% for other MDR-GNR. Furthermore, 25% of respondents reported using molecular methods of testing for detection of MRSA; 9% for VRE; 8% for CRE; and 5% for other MDR-GNR. The remainder reported the use of culture methods for screening.
Most respondents reported the use of screening tests for discontinuation of CP for MRSA (66%) and VRE (55%), but tests were less commonly used for discontinuing CP in patients with CRE and other MDR-GNR (29% and 26%, respectively). With regard to anatomic screening sites, the nares was the most commonly screened site for MRSA, followed by the rectum for VRE and CRE. In cases in which patients were infected with an MDRO, the original site of infection was screened frequently (45% for MRSA, 28% for VRE, 23% for CRE, and 21% for other MDR-GNR). Respondents reported using screening for discontinuing CP more frequently with MRSA and VRE (83% and 80%, respectively) than with CRE and other MDR-GNR (73% and 63%, respectively). Most commonly, 3 negative screens were required for discontinuing CP for MRSA (40%), VRE (46%), and CRE (14%). A large proportion of respondents indicated that >5 days need to elapse between screening specimen collections (30% for MRSA, 42% for VRE, 17% for CRE, and 12.7% for MDR-GNR).
The use of decolonization was reported by nearly a quarter of respondents for the purpose of discontinuing CP in MRSA (24%). A small number of institutions reported its use for eradication of VRE (3%), CRE (2%), and other MDR-GNR (2%).
Respondents reported that CP for C. difficile infection (CDI) is discontinued most frequently upon discharge (40%) or ≥24 hours after the cessation of diarrhea related to CDI (45%). Patients who are admitted with a history (within the last 6 months) of CDI are most frequently put on CP if they develop diarrhea (47%) or are managed as if they do not have a history of CDI (42%). In addition, 85% of respondents reported use of C. difficile toxin PCR for diagnosis of CDI, and 42% reported the use of enzyme immunoassay (EIA) for this purpose, suggesting that some institutions may be using both. Less than 5% of PCR and EIA users reported their use for discontinuing CP.
METHICILLIN-RESISTANT STAPHYLOCOCCUS AUREUS (MRSA)
Current Recommendations on the Discontinuation of CP
Several states have laws supporting screening programs to identify patients with MRSA colonization, 28 particularly patients viewed to be high-risk for MRSA colonization (eg, patients admitted to the intensive care unit). 29 Hospitals determine where and when active surveillance should be performed and which high-risk populations and body sites are tested for colonization.Reference Calfee, Salgado and Milstone 30 In addition, some hospitals perform surveillance cultures during hospitalization in predefined units to identify cases of MRSA acquisition.Reference Calfee, Salgado and Milstone 30 Because colonization with MRSA can be persistent,Reference Kluytmans, van Belkum and Verbrugh 31 especially in high-risk populations, some previously published guidance documents recommend automatically placing patients on CP during hospitalization if they have a history of MRSA colonization or infection during prior hospital admissions,Reference Morgan, Murthy and Munoz-Price 5 , Reference Calfee, Salgado and Milstone 30 thereby increasing the population in healthcare facilities who are on CP for MRSA.Reference Morgan, Murthy and Munoz-Price 5
There are no national guidelines pertaining to discontinuation of MRSA CP. The optimal duration of CP is unknown for patients previously colonized or infected with MRSA. Institutions often develop their own criteria for the discontinuation of CP.Reference Russell, Beekmann, Polgreen, Rubin and Uslan 9 , Reference Shenoy, Hsu, Noubary, Hooper and Walensky 11 , Reference Shenoy, Walensky, Lee, Orcutt and Hooper 32 Siegel et alReference Siegel, Rhinehart, Jackson and Chiarello 33 suggest that if a patient has not been on antibiotics for several weeks and has at least 3 negative surveillance cultures for MRSA over the course of 1–2 weeks, this may be sufficient for discontinuing CP.
Variation exists among hospital policies for the discontinuation of CP for MRSA. Shenoy et alReference Shenoy, Hsu, Noubary, Hooper and Walensky 11 conducted a survey of infection preventionists on the discontinuation of CP and found that 73% of respondents stated that their institutions had a policy for discontinuing CP for patients with a history of MRSA. However, the study found significant variation in the criteria used, and most institutions did not actively screen patients for the purpose of CP discontinuation. A survey of 55 hospitals administered by Hospital Corporation of America (HCA, Inc.), a large healthcare system in the United States, found that 68% of ICUs had a policy for discontinuing CP for MRSA.Reference Moody, Septimus and Hickok 34 Additionally, 18% of the respondents to a survey administered to physicians in the Emerging Infections Network (EIN) reported that the duration of isolation for MRSA at their hospital was indefinite once a patient was positive.Reference Russell, Beekmann, Polgreen, Rubin and Uslan 9
Shenoy et alReference Shenoy, Kim and Rosenberg 36 also conducted a randomized control trial of individuals with a history of MRSA colonization or infection at least 90 days prior to enrollment in the study and compared standard hospital practice of clinician-initiated screening using 3 sequential cultures (ie, “passive screening”) to study initiated screening for discontinuation of CP (ie, “active screening”). In the active screening arm, research staff obtained 3 sets of surveillance nasal cultures performed at least 24 hours apart, processed by both culture and PCR. Patients in both arms with 3 negative cultures who had not received antibiotics with activity against MRSA were eligible to have CP discontinued. The investigators found that in the passive screening arm, 31% of subjects had screening initiated and only 9.6% of subjects completed the series of swabs. In contrast, all subjects in the active screening arm had screenings initiated, and 74% had 3 swabs obtained to complete the series.Reference Shenoy, Kim and Rosenberg 36 The negative predictive value of the first nasal swab processed by PCR was 97% compared to 3 sequential cultures. Shenoy et alReference Shenoy, Lee and Cotter 37 also evaluated the use of a single PCR-based screening in an emergency department for patients with a history of MRSA prior to 90 days from that visit and who were not receiving antibiotics with activity against MRSA in the prior 48 hours. Of the patients eligible for the study, 65% were PCR negative and CP were discontinued. However, 69% of patients were not properly assessed for enrollment, demonstrating the significant challenges in implementing active screening programs for CP discontinuation, particularly in busy emergency departments.Reference Gase and Reese 38
Other studies evaluating screening for discontinuation of CP used longer time intervals since the last MRSA culture for study eligibility. In a study by Vikram et al,Reference Vikram, Dumigan, Kohan, Havill, Tauman and Boyce 39 individuals with no positive MRSA cultures in 6 months prior to hospital admission were screened for MRSA at multiple body sites (nares; wound if present; and if no wound, axillae and perineum) 3 consecutive times. The authors observed that only 21% of patients had 3 sets of surveillance cultures that were negative and were removed from CP. Goldsack et alReference Goldsack, DeRitter and Power 40 collected 2 sets of nares cultures from patients readmitted to the hospital with a history of MRSA at least 1 year prior to the admission and without receipt of antibiotics in the prior 72 hours to assess continued MRSA colonization. They found that 80% of patients studied were no longer colonized with MRSA; these patients were subsequently removed from contact isolation.Reference Goldsack, DeRitter and Power 40
Automated electronic health record systems with microbiologic data and electronic alerts may improve compliance with hospital policies for CP. In one system, discontinuation criteria are displayed to physicians when they attempt to discontinue CP, and only an infection preventionist can remove the visual alert for MRSA in the electronic health record when criteria are met per hospital protocol.Reference Quan, Cousins, Porter, Puppo and Huang 41 Carson et alReference Carson, Danford and Carson 42 evaluated outpatients who were not on long-term antibiotics and who had a MRSA infection at least 3 months prior to enrollment and therefore were “MRSA flagged” in the EMR. Eligible subjects had surveillance cultures obtained from the nares, axilla, inguinal area, and wound if present; if all cultures were negative, patients returned at least 7 days later for testing at these body sites a second time. They observed that 72% of outpatients in the study were no longer carriers of MRSA and were able to have the alert removed from their medical record.Reference Carson, Danford and Carson 42
Duration of Colonization
Staphylococcus aureus colonization may be persistent (always present), intermittent (sometimes present and sometimes absent), or rarely occurring.Reference Kluytmans, van Belkum and Verbrugh 31 Studies examining individuals following hospital discharge have suggested that over time, many individuals will clear MRSA colonization, although a proportion will remain long-term carriers.Reference Scanvic, Denic, Gaillon, Giry, Andremont and Lucet 13 , Reference Robicsek, Beaumont and Peterson 43 , Reference Sanford, Widmer, Bale, Jones and Wenzel 44 Long-term carriers of MRSA retain an increased risk of serious infection and even death due to MRSA compared to noncarriers.Reference Datta and Huang 45 The median time to clearance of MRSA colonization is 7–9 monthsReference Scanvic, Denic, Gaillon, Giry, Andremont and Lucet 13 , Reference Marschall and Mühlemann 46 , Reference Lucet, Paoletti and Demontpion 47 ; however, some individuals can have more prolonged carriage.Reference Robicsek, Beaumont and Peterson 43 In an evaluation of children in the community over a 1-year period, 18% of children who were colonized with MRSA in the nares at enrollment remained colonized 1 year later.Reference Fritz, Krauss and Epplin 48 In a retrospective assessment of known MRSA carriers who were readmitted to a large tertiary-care hospital, Sanford et alReference Sanford, Widmer, Bale, Jones and Wenzel 44 observed that a proportion of readmitted carriers remained colonized for >3 years and that all patients colonized for >12 months had at least 1 positive wound culture. Similarly, Scanvic et alReference Scanvic, Denic, Gaillon, Giry, Andremont and Lucet 13 examined patients who were previously identified as MRSA carriers who were readmitted to the hospital and reported that those with a break in the skin at readmission were more likely to have persistent carriage of MRSA. Other factors associated with prolonged carriage of MRSA include older age, household members with MRSA colonization, need for help with daily activities, indwelling devices, a recent ICU stay, less time since the last MRSA-positive culture, longer length of stay in a healthcare facility, or residence in a long-term care facility.Reference Carson, Danford and Carson 42 , Reference Sanford, Widmer, Bale, Jones and Wenzel 44 , Reference Lucet, Paoletti and Demontpion 47 , Reference Cluzet, Gerber and Nachamkin 49 – Reference Ridenour, Wong, Call and Climo 58 In addition, colonization with MRSA at multiple body sites has been associated with prolonged carriage among patients readmitted to a university hospital.Reference Mattner, Biertz, Ziesing, Gastmeier and Chaberny 59
Some studies have documented shorter duration of colonization. Ghosh et alReference Ghosh, Jiao, Al-Mutawa, O’Neill and Mertz 60 examined patients with prolonged hospital stays and used active surveillance to identify patients that lose MRSA carriage. They observed a clearance rate of 11% for MRSA with a median time to clearance of 23 days, although 4 of these 19 patients were later found to be recolonized with MRSA. Cluzet et alReference Cluzet, Gerber and Nachamkin 49 also documented a shorter duration of MRSA colonization of 21 days in individuals screened every 2 weeks following a skin infection due to MRSA. Notably, this population was younger, and most individuals received antibiotic therapy for skin infection, which could contribute to earlier clearance; 20% of individuals never cleared colonization during 6 months of follow-up. In a separate analysis, these investigators observed that 43.6% of individuals who had cleared MRSA colonization ultimately became recolonized with a median time to recurrence of 53 days.Reference Cluzet, Gerber and Nachamkin 61 In a study of patients at neurologic long-term care facilities, patients not colonized with S. aureus at admission were followed with weekly nasal surveillance cultures for a median duration of 20 weeks. The median time to clearance of MRSA colonization (acquisition of colonization until 2 consecutive nasal swabs negative for that strain) was 3 weeks.Reference Couderc, Thiébaut and Lawrence 62
Challenges exist to developing a standardized definition of clearance of MRSA. Due to the heterogeneity among study designs, it is difficult to draw overarching conclusions. Prior studies showed considerable variability with respect to follow-up time, frequency of screening cultures, and definitions of what constituted MRSA clearance. Some studies included potential confounding variables, such as receipt of concomitant antibiotics or the use of decolonization regimens.Reference Shenoy, Paras, Noubary, Walensky and Hooper 63 Because of variations in MRSA colonization duration, many hospitals wait at least 3 months prior to assessing an individual for clearance of MRSA colonization,Reference Shenoy, Kim and Rosenberg 36 with a large proportion of hospitals requiring 3 negative specimens to document clearance of MRSA.Reference Shenoy, Kim and Rosenberg 36 Published evidence indicates that most patients will remain negative for MRSA if they have 3 consecutive negative weekly surveillance cultures.Reference Huckabee, Huskins and Murray 12
Sites of Colonization
The anterior nares is the primary site of MRSA colonization,Reference Kluytmans, van Belkum and Verbrugh 31 although various extra-nasal sites are also colonized (eg, throat, axilla, inguinal area, perirectal area, and chronic wounds).Reference McKinnell, Huang, Eells, Cui and Miller 64 Most hospital surveillance programs screen for MRSA colonization solely in the anterior nares. However, this screening may result in unrecognized extra-nasal colonization.Reference Calfee, Salgado and Milstone 30 A review of 23 studies on testing for MRSA colonization estimated that extra-nasal screening increased MRSA detection by more than 33% compared to nares screening alone.Reference McKinnell, Huang, Eells, Cui and Miller 64 It is unclear whether evaluation of extra-nasal MRSA colonization is beneficial for hospital surveillance programs or if extra-nasal colonization should be considered in a decision about discontinuation of CP. Some hospitals use nares cultures alone to confirm clearance, whereas others use additional sites of cultures to guide protocols for discontinuing CP.Reference Shenoy, Hsu, Noubary, Hooper and Walensky 11 Further research is warranted in the role of extra-nasal surveillance cultures for the purpose of discontinuing CP.
VANCOMYCIN-RESISTANT ENTEROCOCCUS (VRE)
Current Recommendations on the Discontinuation of CP
Early guidance suggested that CP for VRE should be discontinued after 3 negative stool cultures obtained at least 1 week apart. 65 Subsequent guidelines from CDC and HICPAC supported this practice in the absence of uncontrolled respiratory secretions, draining wounds, or the involvement of the patient in an institutional outbreak.Reference Siegel, Rhinehart, Jackson and Chiarello 1 Sequential testing revealed that after 3 sequential negative cultures, 35 of 37 patients (95%) remained culture negative.Reference Byers, Anglim, Anneski and Farr 20 One institution, which defined VRE clearance as 3 negative stool cultures more than 3 weeks apart, found in subsequent, prospective surveillance testing that VRE recolonization occurred in 5 of 21 patients (24%) who had “cleared.”Reference Baden, Thiemke and Skolnik 14 The same authors identified 12 patients who had at least 2 VRE-positive cultures more than 1 year apart, supporting concern for prolonged colonization.Reference Baden, Thiemke and Skolnik 14
Donskey et alReference Donskey, Hoyen, Das, Helfand and Hecker 15 demonstrated that individuals who had cleared VRE colonization with 3 negative stool cultures collected at least 1 week apart had a high recurrence rate (62%) if they were exposed to antibiotics in the following year. Individuals with risk factors for VRE colonization have demonstrated recurrence,Reference Patel, Allen and Manahan 16 and recent studies suggest that a strategy of 3 weekly consecutive surveillance cultures to identify VRE clearance results in recurrence rates of 11%.Reference Henard, Lozniewski, Aissa, Jouzeau and Rabaud 66
Duration of Colonization
Multiple studies evaluated the duration of VRE colonization and the predictive value of VRE-negative stool cultures in predicting VRE clearance. Studies on this subject have been largely retrospective and have been focused in hospital settings. A retrospective analysis of a large, multicenter study revealed that among 394 patients with VRE colonization, 76% of those admitted within 50 days of initial VRE detection remained positive for VRE, and among 126 patients admitted over 300 days from the last positive VRE culture, 20 (15.9%) remained positive in screening surveillance cultures.Reference Huang, Rifas-Shiman and Pottinger 67 In a single-center study, 35 of 210 VRE-colonized patients cleared colonization at a mean of 49 days. Using a logistic regression model the study predicted that 40% of colonized patients would clear colonization at 100 days.Reference Goetz, Rihs, Wagener and Muder 68 In another study, 22 of 105 VRE colonized patients remained colonized for more than 100 days.Reference Byers, Anglim, Anneski and Farr 20 A recent systematic analysis pooled data on the subject from 13 studies, identifying a range of VRE clearance from 1 to 43 weeks. A logistic regression model estimated that 50% of subjects cleared colonization at 25 weeks after an initial VRE-positive stool test.Reference Shenoy, Paras, Noubary, Walensky and Hooper 63 Limitations associated with most of these studies include inconsistent reporting of concomitantly administered antibiotics, variation in testing modalities (culture vs molecular methods), limited duration of follow-up, and retrospective nature of the study designs.
Several factors may influence the duration of VRE colonization and the likelihood of VRE transmission in the hospital setting. Prolonged hospitalization and intensive care unit stay have been associated with prolonged VRE colonization.Reference Byers, Anglim, Anneski and Farr 20 Immunosuppressed patients may remain colonized with VRE for longer periods of timeReference Sohn, Peck and Joo 17 , Reference Henning, Delencastre and Eagan 18 , Reference Singh, Esparza, Patterson, Vogel, Patel and Gornick 69 and may have a high rate of recurrent VRE colonization after multiple negative VRE cultures.Reference Patel, Allen and Manahan 16 Antibiotic use, specifically vancomycinReference Karki, Land and Aitchison 19 , Reference Yoon, Lee and Lee 70 and fluoroquinoloneReference Sohn, Peck and Joo 17 , Reference Karki, Land and Aitchison 19 use, have been associated with prolonged VRE colonization. Older patients and patients receiving fluoroquinolones were more likely to transmit VRE to roommates.Reference Zhou, Moore, Eden, Tong and McGeer 71 Prior guidance suggests consideration of concurrent antibiotic use, particularly antibiotics with activity against VRE, when making decisions regarding discontinuation of CP.Reference Siegel, Rhinehart, Jackson and Chiarello 1
Sites of Colonization
The gastrointestinal tract is the main site of VRE colonization, though VRE has been identified on the skin of patients colonized with VRE with diarrhea.Reference Beezhold, Slaughter and Hayden 72 Most studies investigating the duration of VRE colonization duration have used gastrointestinal tract samples,Reference Shenoy, Paras, Noubary, Walensky and Hooper 63 stool samples,Reference Baden, Thiemke and Skolnik 14 , Reference Karki, Land and Aitchison 19 and perirectal and perineal swabs,Reference Byers, Anglim, Anneski and Farr 20 , Reference Huang, Rifas-Shiman and Pottinger 67 , Reference Goetz, Rihs, Wagener and Muder 68 , Reference Haverkate, Derde, Brun-Buisson, Bonten and Bootsma 73 or both.Reference Huckabee, Huskins and Murray 12 , Reference Ghosh, Jiao, Al-Mutawa, O’Neill and Mertz 60 Some studies have included surveillance cultures obtained from other sites including throat and urine samples.Reference Patel, Allen and Manahan 16 While limited, data comparing the sensitivity of rectal and perirectal cultures suggest that both are comparable.Reference Weinstein, Tallapragada, Farrel and Dembry 74
MULTIDRUG-RESISTANT ENTEROBACTERIACEAE (MDR-E)
Current Recommendations Regarding the Discontinuation of CP
The CDC HICPAC MDRO guidelines state that “In general, it seems reasonable to discontinue CP when 3 or more surveillance cultures for the target MDRO are repeatedly negative over the course of 1 or 2 weeks in a patient who has not received antimicrobial therapy for several weeks, especially in the absence of a draining wound, profuse respiratory secretions, or evidence implicating the specific patient in ongoing transmission of the MDRO within the facility.” The CDC CRE Tool Kit 2015 update states that “Currently, there is not enough evidence to make a firm recommendation about when to discontinue use of CP for infected or colonized patients; however, CRE colonization can be prolonged (>6 months). If surveillance cultures are used to decide if a patient remains colonized, >1 culture should be collected to improve sensitivity. Regardless of whether surveillance cultures are performed, the presence of risk factors for ongoing carriage or ongoing CRE exposure should be considered in the decision about discontinuing CP.” 75
In a survey among SHEA Research Network members, among 49 respondents, CP for patients infected with ESBL-E were reportedly maintained for the duration of active illness by 8.2% of respondents, for the duration of hospitalization by 26.5%, until negative surveillance cultures were obtained by 32.7%, and indefinitely by 34.7%.Reference Drees, Pineles, Harris and Morgan 76 Overall, 55% of respondents reported isolating patients at readmission. For those respondents requiring negative cultures for “clearance” (n=16), cultures were obtained after completion of antibiotics by 37.5% of respopndents, after hospital discharge by 25%, and within 3 months by 12.5%. Among 62 respondents regarding CRE, CP were reportedly maintained for the duration of active illness by 6.5% of respondents, for the duration of hospitalization by 12.9%, until negative surveillance cultures by 29%, and indefinitely by 43.5%. Furthermore, 72% of respondents instituted isolation at readmission for CRE. Among respondents reporting that they require surveillance cultures for CRE clearance (n=18), cultures could be obtained after completion of antibiotics by 44.4% respondents, after hospital discharge by 22.2%, within 3 months by 27.8%, and at or after 1 year by 5.6%.
In another survey of infection control practices related to MDR-E among 15 acute-care hospitals in Toronto, Canada, CP were more likely to be instituted universally for CRE patients than for ESBL-E patients for whom risk factors for transmission (eg, diarrhea, draining wounds, etc) were considered when instituting CP. For ESBL-E, CP was maintained until discharge by 5 of 15 hospitals, discontinued after 1 negative specimen by 2 of 15 hospitals, and after 3 negative specimens 1 week apart by 8 of 15 hospitals.Reference Lowe, Katz, McGeer, Muller and Group 77 For CRE, CP was maintained until discharge by 8 of 15 hospitals, discontinued after 1 negative specimen from original positive site by 1 of 15 hospitals, after 3 negative specimens 1 week apart by 4 of 15 hospitals, and was not yet determined by 2 of 15 hospitals.
Duration of Colonization
Several studies have evaluated colonization with MDR-E longitudinally in a variety of patient populations in different countries and healthcare settings.Reference Bart, Paul, Eluk, Geffen, Rabino and Hussein 21 – Reference Vehreschild, Hamprecht and Peterson 23 , Reference Titelman, Hasan and Iversen 78 – Reference Lewis, Enfield, Mathers, Giannetta and Sifri 90 Most studies were conducted in the acute-care settingReference Bart, Paul, Eluk, Geffen, Rabino and Hussein 21 – Reference Vehreschild, Hamprecht and Peterson 23 , Reference Birgand, Armand-Lefevre, Lolom, Ruppe, Andremont and Lucet 79 – Reference Feldman, Adler and Molshatzki 81 , Reference Weintrob, Roediger and Barber 83 , Reference Kola, Holst, Chaberny, Ziesing, Suerbaum and Gastmeier 85 , Reference Zimmerman, Assous, Bdolah-Abram, Lachish, Yinnon and Wiener-Well 87 , Reference Schechner, Kotlovsky and Tarabeia 89 – Reference Kim, Han and Song 91 and identified a population of patients infected and/or colonized with MDR-E, either ESBL-E or CRE, or MDR-Enterobacteriaceae defined by resistance to multiple classes of drugs. Patients were assessed for persistence of colonization at study-specific intervals and variable durations of follow-up. The definition of “clearance” varied between a single negative screening culture,Reference Birgand, Armand-Lefevre, Lolom, Ruppe, Andremont and Lucet 79 , Reference Zimmerman, Assous, Bdolah-Abram, Lachish, Yinnon and Wiener-Well 87 , Reference Lewis, Enfield, Mathers, Giannetta and Sifri 90 at least 2 negative screening cultures,Reference Bart, Paul, Eluk, Geffen, Rabino and Hussein 21 , Reference Vehreschild, Hamprecht and Peterson 23 , Reference Feldman, Adler and Molshatzki 81 , Reference O’Fallon, Gautam and D’Agata 84 or at least 3 negative screening cultures swabs.Reference Löhr, Rettedal, Natås, Naseer, Oymar and Sundsfjord 80 , Reference Kola, Holst, Chaberny, Ziesing, Suerbaum and Gastmeier 85 , Reference Kim, Han and Song 91
ESBL-E
Among hematology-oncology patients in 5 hospitals in Germany undergoing rectal swab sampling every 10 ± 2 days (mean duration of hospitalization, 36.6 days), 22 of 55 patients (40.0%) colonized with ESBL-E converted to a negative ESBL-E carrier status, and 18 of those 22 (81.8%) maintained this status.Reference Vehreschild, Hamprecht and Peterson 23 In another university hospital cohort in Germany, with clearance defined by 3 negative cultures 1 week or more apart obtained without ESBL-directed antibiotic therapy, only 10 cases (6.8%) were cleared from colonization.Reference Kola, Holst, Chaberny, Ziesing, Suerbaum and Gastmeier 85 In a Swedish cohort in which subjects provided self-collected fecal samples, the prevalence of ESBL-E carriage was as follows: 51 of 61 (84%) after 1 month, 36 of 61 (66%) after 3 months, 31 of 61 (55%) after 6 months, and 26 of 61 (43%) after 12 months from a positive clinical culture.Reference Titelman, Hasan and Iversen 78 In a French university hospital cohort (n=448) undergoing routine active surveillance on admission for previous positive culture, 40% were still colonized at first readmission; 25.6% were still colonized after 1 year, and 8.9% were still colonized after 2 years, with a median time to ESBL-E clearance (single negative rectal swab) of 6.6 months (range, 3.4–13.4 months).Reference Birgand, Armand-Lefevre, Lolom, Ruppe, Andremont and Lucet 79 , Reference Kim, Han and Song 91
CRE
In a postdischarge surveillance study of tertiary-care hospital and long-term care facility patients infected or colonized with KPC-Klebsiella pneumoniae in Israel, resolution of carriage (defined as 2 consecutive negative cultures and blaKPC-PCR tests with no subsequent positive test) occurred in 65 of 125 (52%) patients followed for 5 months.Reference Feldman, Adler and Molshatzki 81 In a tertiary-hospital–based prospective cohort in Hong Kong with a median follow-up of 54 days (range, 15–421 days), the median duration of stool CRE carriage was 43 days (range, 13–119 days).Reference Cheng, Chan and Wong 22 In another tertiary-care hospital–based study in Israel, known CRE (predominantly K. pneumoniae) patients with a positive rectal screen at a subsequent hospital encounter (cases) were compared with those with a negative screen (controls). The time interval between the first positive CRE test and the next CRE surveillance test was significantly shorter among cases (median, 51 days; interquartile range [IQR], 15–131) than controls (median, 145 days; IQR, 63–287; P=.003).Reference Schechner, Kotlovsky and Tarabeia 89 In another case-control study among tertiary-care hospital patients in Israel, eradication was defined on the basis of 2 negative rectal cultures plus a negative culture from the original source of CRE isolation (eg, urine, sputum, or wounds). This screening procedure to declare presumed eradication was performed at a median of 11.9 months (IQR, 7.7–19.3) after the last positive CRE culture. The median time to CRE recurrence was 49 days (IQR, 16–130), with recurrence of the same CRE in 30 of 36 case patients. Recurrent CRE was isolated only in screening samples in 21 patients (58%), from urine in 11 patients (31%), and from other clinical samples in 4 patients (11%).Reference Bart, Paul, Eluk, Geffen, Rabino and Hussein 21
Variably Defined MDR-E
In a study involving patients with a history of military deployment hospitalized at Walter Reed National Military Medical Center, those colonized with MDR-E (defined as ESBL-producing organism or resistant to 3 of 5 antibiotic classes [ie, penicillins, cephalosporins, carbapenems, aminoglycosides, or fluoroquinolones]) underwent sequential culturing for 8 weeks or until hospital discharge. Only 1 deployed subject became decolonized over the course of the study, with a median duration of colonization of 26 days (IQR, 9–33).Reference Weintrob, Roediger and Barber 83 This was the only study to culture multiple anatomic sites over time; the results showed that the groin was the most sensitive anatomic site for detecting MDR-E overall but sensitivity differed by organism. In a community-based cohort in France, stool samples from adults returning after travel to tropical regions were screened for Enterobacteriaceae producing an ESBL, AmpC, and/or carbapenemase. Detection rates were 60% at 1 month, 57% at both 2 and 3 months, and 63% at both 6 and 12 months.Reference Ruppé, Armand-Lefèvre and Estellat 86
Patient Groups at Risk for Prolonged Colonization
For ESBL-E, Phylogroup B2 and CTX-M-gr-9 were associated with ESBL-E carriage at 12 months in a Swedish cohort.Reference Titelman, Hasan and Iversen 78 In a French university hospital, having the first positive culture on screening samples only (compared to clinical±screening samples) was associated with ESBL-E clearance at readmission.Reference Birgand, Armand-Lefevre, Lolom, Ruppe, Andremont and Lucet 79 Similarly, among KPC-producing CRE carriers in a university hospital in Israel, the index culture being a clinical culture (vs surveillance culture) was associated with longer mean time to negativity.Reference Zimmerman, Assous, Bdolah-Abram, Lachish, Yinnon and Wiener-Well 87 Recurrent hospitalization between the first and last culture was also associated with a longer time to clearance of CRE colonization. This finding was also reported in a case-control study of risk factors for CRE recurrence in Israel, where a shorter time between the last positive CRE culture and presumed eradication, recurrent hospitalization, and the presence of a foreign body at the time of presumed eradication were associated with CRE recurrence.Reference Bart, Paul, Eluk, Geffen, Rabino and Hussein 21 In another hospital-based study in Israel, persistent CRE-positive patients (n=23) were more often admitted from another hospital (59.1% vs 26.3%; P=.012) and were exposed to antimicrobials during the preceding 30 days, although the latter did not reach statistical significance (57% vs 33%; P=.059).Reference Schechner, Kotlovsky and Tarabeia 89 In a study of CRE carriers in a university hospital in Hong Kong that employed serial quantitative detection via fecal specimens, a higher bacterial load on initial detection of CRE, and the use of cephalosporins, carbapenems, and fluoroquinolones after CRE detection were associated with persistent fecal carriage.Reference Cheng, Chan and Wong 22 In a postdischarge surveillance study in Israel, resolution of CRE carriage was more likely to occur in recent acquisitions (first positive <4 months prior to enrollment) with 29 of 75 (39%) remaining positive compared to remote positives (first positive >4 months before enrollment) with 36 of 50 (72%) remaining positive (P<.001). Furthermore, in remotely colonized patients, the presence of an invasive device and high Charlson score were associated with persistent carriage.Reference Feldman, Adler and Molshatzki 81 Among adults returning to France after traveling to tropical regions and found to carry ESBL-E, CRE, or AmpC-producing Enterobacteriaceae, travelers returning from Asia had more prolonged colonization than travelers returning from sub-Saharan Africa or Latin America, and carriers of E. coli had a lower risk of prolonged carriage than carriers of other Enterobacteriaceae species.Reference Ruppé, Armand-Lefèvre and Estellat 86
Sites of Colonization
Generally, the lower gastrointestinal tract is considered the primary site of colonization with MDR-E, though there is no universally accepted surveillance sampling strategy. The gastrointestinal tract sampling was included in nearly all of these studies. Most studies evaluating duration of colonization with MDR-E utilized stool samples,Reference Titelman, Hasan and Iversen 78 , Reference Löhr, Rettedal, Natås, Naseer, Oymar and Sundsfjord 80 , Reference Tham, Walder, Melander and Odenholt 82 , Reference Ruppé, Armand-Lefèvre and Estellat 86 , Reference Tandé, Boisramé-Gastrin and Münck 88 perirectal swab samples,Reference Bart, Paul, Eluk, Geffen, Rabino and Hussein 21 , Reference Birgand, Armand-Lefevre, Lolom, Ruppe, Andremont and Lucet 79 , Reference Feldman, Adler and Molshatzki 81 , Reference O’Fallon, Gautam and D’Agata 84 or either.Reference Vehreschild, Hamprecht and Peterson 23 , Reference Kola, Holst, Chaberny, Ziesing, Suerbaum and Gastmeier 85 Original sites of infection were tested (if still relevant) in one study,Reference Bart, Paul, Eluk, Geffen, Rabino and Hussein 21 and another study included cultures of samples obtained from multiple anatomic sites (ie, forehead, axillae, finger webs, groin, toe webs, and perirectal area).Reference Weintrob, Roediger and Barber 83
CLOSTRIDIUM DIFFICILE
Shedding Time
Sethi et alReference Sethi, Al-Nassir, Nerandzic, Bobulsky and Donskey 25 demonstrated that 1-4 weeks after conclusion of CDI treatment with vancomycin the frequencies of skin and environmental shedding increased to 58% and 50%, respectively (Figure 1). These percentages were greater than they were at the end of CDI treatment (32% and 14%, respectively). This study suggests that skin contamination and environmental surface shedding of C. difficile often persist at the time of resolution of diarrhea, and recurrent shedding is common 1–4 weeks after therapy. These results provide support for the recommendation that CP be continued until hospital discharge if rates of CDI remain high despite implementation of standard infection control measures.
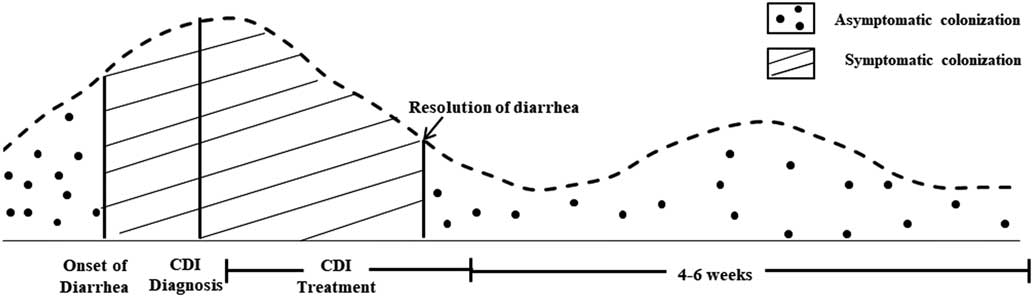
FIGURE 1 Frequency of Skin Contamination and Environmental SheddingReference Donskey, Kundrapu and Deshpande 116
Jinno et alReference Jinno, Kundrapu, Guerrero, Jury, Nerandzic and Donskey 26 compared shedding of spores in patients with resolved CDI within the past month (the time from the end of therapy to 1 month after completion of therapy) and those with active CDI (time from diagnosis until completion of CDI treatment or until completion of 14 days of treatment in patients receiving prolonged tapering courses of vancomycin). Patients with active CDI were found to have high frequencies of positive stool, skin, and environmental cultures (100%, 63%, and 51%, respectively).Reference Jinno, Kundrapu, Guerrero, Jury, Nerandzic and Donskey 26 Among the patients with resolved CDI, the frequency of positive stool, skin, and environmental cultures was significantly higher among patients cultured during the month after completion of treatment versus those cultured >1 month after treatment (50%, 46%, and 29% vs 18%, 5%, and 5%, respectively; P<.01 for each comparison). None of the patients whose CDI had resolved 6–24 months after completion of treatment had positive skin or environmental cultures. These results indicate that patients with resolved CDI may demonstrate significantly more shedding during the month following completion of treatment than those cultured >1 month following treatment.
Donskey et alReference Donskey, Sunkesula and Jencson 92 showed that the likelihood of carriers having a positive skin or environmental culture or positive perirectal PCR result increased as the number of colonies per swab increased. In comparison to carriers with 1–25 colonies per swab, those with ≥26 colonies per swab had significantly higher frequencies of skin shedding (1 of 10 [10%] versus 10 of 15 [67%]; P=.012) and environmental shedding (0 of 10 versus 9 [60%] of 15; P=.003) and were more likely to have positive perirectal PCR results (3 of 10 [30%] versus 14 of 15 [93%]; P=.002). The number of colonies recovered from perirectal swabs varied widely, and a low burden of colonization was associated with a low risk of skin or environmental shedding or a positive perirectal PCR result.
Shrestha et alReference Shrestha, Sunkesula, Kundrapu, Tomas, Nerandzic and Donskey 93 and colleagues described the contamination of healthcare personnel (HCP) hands with C. difficile after caring for patients with resolved CDI who were no longer under CP. Patient CDI was either recent (2 days to 6 weeks after the end of CDI treatment without diarrhea) or remote (6–24 weeks after CDI treatment). Not surprisingly, the frequencies of hand contamination of HCP after contact with patients with active and recent CDI were similar. Although less common, hand contamination occurred during care of patients with remote CDI, which may support placing patients on CP for the duration of admission as well as re-admissions. Continuing CP after resolution of diarrhea may also reduce transmission.Reference Shrestha, Sunkesula, Kundrapu, Tomas, Nerandzic and Donskey 93
The clinical impact of CP based on shedding might be extrapolated from studies that investigated the effect of CP on asymptomatic C. difficile carriers. Lanzas and DubberkeReference Lanzas and Dubberke 94 used agent-based modeling to evaluate the potential effectiveness of methods targeting asymptomatic carriers to control C. difficile colonization and infection rates by screening patients at admission to detect asymptomatic C. difficile carriers and placing positive patients on CP. Scenarios simulating screening test characteristics, colonization prevalence, and type of strain present at admission were used. The investigators found on average that testing asymptomatic carriers reduced the number of new colonization cases by 40%–50% and hospital-onset CDI cases by 10%–25%, compared with the baseline scenario.
A 2016 crossover experimental study showed that placing asymptomatic carriers on CP based on universal screening on admission significantly decreased the incidence of CDI (3 hospital-associated [HA] CDI cases per 10,000 patient days) compared to a control unit (6.9 HA-CDI per 10,000 patient days; P<.001). The researchers estimate the intervention prevented approximately 63 cases.Reference Longtin, Paquet-Bolduc and Gilca 27 , Reference Longtin, Gilca and Loo 95
Patients with C. difficile Infection
A 2009 survey of 33 acute-care hospitals in Canada identified different approaches related to the discontinuation of CP; 13 hospitals (39%) reported that CP were discontinued as soon as the patients were asymptomatic, and 19 hospitals (58%) required a period of 24–72 hours with no symptoms prior to discontinuation of CP. One hospital reported continuing CP until the end of the treatment for CDI; this hospital had the greatest percentage of positive test results (32% vs 12% for all other hospitals combined; P<.001). No respondent continued additional CP until discharge.Reference Gravel, Gardam and Taylor 96
Johnson et alReference Johnson, Gerding and Olson 97 performed a prospective, controlled study of the use of universal gloves on the incidence of CDI and C. difficile colonization. They found that the units in which the glove intervention was performed demonstrated a decrease of C. difficile incidence from 7.7 to 1.5 cases per 1,000 patient discharges (P=.015). Contrary to this finding, the control units without the intervention had unchanged incidence in the intervention and control phases. Colonization rates also decreased in the units undergoing the interventions (17% vs 9%; P=.029). This study suggests that the use of vinyl gloves may significantly reduce the transmission of C. difficile diarrhea and therefore should be used for the duration of hospitalization.Reference Johnson, Gerding and Olson 97
USE OF MOLECULAR TESTING TO DETERMINE DURATION OF CP
Various methods and procedures have been used to screen patients for MRSA, VRE, ESBL, and CRE colonization. The simplest approach is to culture swab specimens directly on solid agar media. Alternatively, sensitivity can be enhanced by first placing swabs in enrichment broth. Following incubation, broth is inoculated on to agar plates. Broth incubation enhances recovery of MRSA,Reference Wolk, Picton and Johnson 98 but lengthens laboratory turnaround time of results.
Comparison of Molecular and Standard Methods on Sensitivity, Specificity, and Duration of Positivity
The diagnostic advantage of molecular methods (eg, PCR) is enhanced sensitivity compared to culture. Unfortunately, scientific documentation of this phenomenon has been challenged using weak study methodology. The most common methodology involves performing PCR in parallel with culture, with the latter designated as the gold standard. PCR-positive/culture-negative specimens are then retested with an “arbiter” PCR. If positive, the specimen is assigned positive screen status.Reference Yam, Siu and Ho 99 – Reference Babady, Gilhuley, Cianciminio-Bordelon and Tang 114 This methodology (called “partial verification”) introduces bias in favor of PCR. 115 For MRSA, Yam et alReference Yam, Siu and Ho 99 tested 1,246 nares swab specimens with the Lightcycler MRSA Advanced (Roche Molecular Diagnostics, Basel Switzerland), a laboratory developed test (LDT) PCR and, in parallel, cultured all specimens. The gold standard was defined as positive culture or positivity by both molecular assays. A preferable method may be to perform multiple PCR assays with similar sensitivity on all specimens using a “consensus” gold standard definition. The sensitivities of culture, Lightcycler MRSA Advanced, and the LDT were 84%, 83%, and 76%, respectively, with no apparent statistical significance.Reference Yam, Siu and Ho 99 No similar studies were available for VRE or CRE assays.
Recently, FDA-cleared molecular methods (eg, PCR) for detecting MRSA, VRE, and CRE have become commercially available in the United States. For MRSA, commonly used assays include the Xpert MRSA XT and Xpert SA Nasal Complete (Cepheid, Sunnyvale, CA); BD MAX MRSA and BD MAX StaphSR (Becton Dickinson, Franklin Lakes, NJ); Lightcycler MRSA Advanced (Roche Molecular Diagnostics, Basel Switzerland); and the Cobas MRSA/SA Test (Roche Molecular Diagnostics). The Xpert VanA assay (Cepheid) has been cleared by the FDA to detect VRE mediated by vanA, and the Xpert CarbaR (Cepheid) detects various determinants of CRE including blaKPC, blaNDM, blaVIM, blaIMP-1 and blaOXA-48 (including OXA-48-like variants OXA-181 and OXA-232). At this time, no molecular assays that detect ESBL determinants have been cleared by the FDA.
DEVELOPING POLICIES FOR DISCONTINUATION OF CONTACT PRECAUTIONS
Policies dictating the duration of contact precautions can provide guidance to clinicians and infection prevention and control programs in acute-care hospitals. Given the limited data on the duration of organism colonization and the impact of CP in reducing spread of organisms in the healthcare environment, no universally recommended approach exists for making decisions regarding CP duration or discontinuation for any epidemiologically significant organism. However, hospital policies addressing duration of CP should include several components (see Table 2). In adopting a policy to guide decisions regarding discontinuation of CP, hospitals should carefully assess their institutional risks, priorities, and resources. Infection prevention and control leadership should revisit and revise policies if the epidemiology of specific organisms of concern change, particularly in an outbreak or hyperendemic situation. Additional factors, such as the cost and feasibility of implementation, should be considered in adopting a policy for CP discontinuation.
TABLE 2 Potential Components for Inclusion in a Policy for Duration of Contact Precautions With Examples
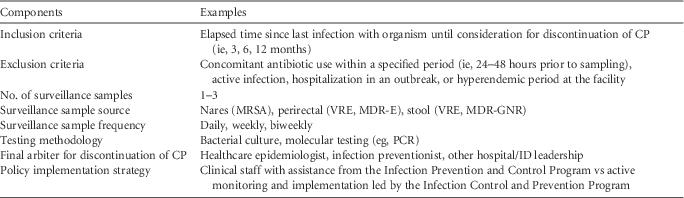
NOTE. CP, contact precautions; MRSA, methicillin-resistant Staphylococcus aureus; VRE, vancomycin-resistant enterococci (VRE); PCR, polymerase chain reaction; ID, infectious diseases; MDR-GNR, multidrug-resistant Gram-negative rods; CRE, carbapenem-resistant Enterobacteriaceae; ESBL, extended-spectrum β-lactamase
CONCLUSIONS AND AREAS FOR FUTURE RESEARCH
Data guiding decisions regarding the duration of CP are generally limited to studies that often describe the duration of low-level organism colonization rather than interventional studies evaluating the clinical impact of discontinuing CP in different settings. Extrapolation of published data is limited based on variability in definitions of colonization and an inadequate understanding of the impact of CP on preventing organism transmission. Further research is needed to improve our understanding of the role of molecular testing in evaluating the transmissibility of organisms in healthcare settings, and how these tests perform in comparison to culture-based methodologies in guiding decisions regarding the duration of CP. Research studies should be performed in “real-world” settings to establish a stronger evidence base on which to optimize the use of CP in reducing the spread of MDROs and C. difficile. Large, multicenter, prospective cluster-randomized trials using appropriate outcomes and time spans to evaluate these outcomes would be valuable in the development of evidence-based guidelines for the implementation of CP, including decisions regarding the duration of CP in acute-care hospitals.
ACKNOWLEDGMENTS
This study was supported in part by the SHEA Research Network (SRN). We express immense gratitude to Valerie M. Deloney, MBA for her editorial assistance and invaluable organizational expertise in coordinating this project. We also wish to thank Erica S. Shenoy, MD, PhD, for her extensive and thoughtful review of the manuscript.
The following disclosures are a reflection of what has been reported to SHEA. To provide thorough transparency, SHEA requires full disclosure of all relationships, regardless of relevancy to the guidance topic. Evaluation of such relationships as potential conflicts of interest is determined by a review process which includes assessment by the SHEA Conflict of Interest Committee. The assessment of disclosed relationships for possible conflicts of interest is based on the relative weight of the financial relationship (ie, monetary amount) and the relevance of the relationship (ie, the degree to which an association might reasonably be interpreted by an independent observer as related to the topic or recommendation of consideration). Readers of this guidance should be mindful of this when reviewing the list of disclosures.
D.B., G.B., K.P., M.B., and S.L. report that they have nothing to disclose. D.M. Springer Nature for book and journal editing and research grants from NIH, AHRQ, VA HSRD and CDC. R.M. reports a research grant/contract with Medimmune and Johns Hopkins (lead in multicenter trial: HAIs Among Surgical and ICU Patients: Implications for Interventions and Treatment). L.S.M.P. reports an advisory/consultant role with Xenex/Clorox, and speaker honorarium from Ecolab. K.V.S. reports research grants/contracts with Nanosphere, Inc. (Performance of the Verigene Gram-Negative Blood Culture Test [BC-GN] in Pediatric Patients), Techlab, The Children’s Hospital of Philadelphia (Evaluation of the Performance of the Shiga Toxin Quik Chek), and Premier EHEC and CHROMagar 0157 (The Detection of EHEC Infections in Children). J.H. reports an advisory/consultant role with Gilead. T.W. reports an advisory/consultant role and research grant with Clorox Healthcare (What is the Role of Improved Hydrogen Peroxide in the Operating Room?) and research grants/contracts with the CDC (Enhancing Surveillance Among Refugees), Pfizer (Understanding Why Patients Accept Vaccines: Socio-Behavioral Approach), and University of Louisville research grants >$25,000 with Pfizer and Clorox Healthcare.







