Background
The major events sparking life on Earth on our 4.6-billion-year-old planet remain enigmatic, although there is general agreement that first life likely arose about 3.7–4.1 billion years ago, during the early Archean or late Hadean eons (Abramov and Mojzsis, Reference Abramov and Mojzsis2009; Deamer, Reference Deamer2011; Bell et al., Reference Bell, Boehnke, Harrison and Mao2015; Knoll, Reference Knoll2015). Evidence for the presence of isoprenoid compounds has been reported in ancient sediments not long after, suggesting the early rise of Archaea (Hahn and Haug, Reference Hahn and Haug1986; Ventura et al., Reference Ventura, Kenig, Reddy, Schieber, Frysinger, Nelson, Dinel, Gaines and Schaeffer2007). The early rise of Archaea is also suggested by phylogenic studies, although lateral gene transfers have complicated their interpretation (Lange et al., Reference Lange, Rujan, Martin and Croteau2000; Kennedy et al., Reference Kennedy, Ng, Salzberg, Hood and DasSarma2001; Brochier-Armanet et al., Reference Brochier-Armanet, Forterre and Gribaldo2011; Hoshino and Gaucher, Reference Hoshino and Gaucher2018). Stromatolites representing fossilized microbial mats have been estimated to be up to 3.7 billion-years-old (Walter et al., Reference Walter, Buick and Dunlop1980; Vankranendonk et al., Reference Vankranendonk, Philipott, Lepot, Bodorkos and Piranjno2008; Nutman et al., Reference Nutman, Bennett, Friend, Van Kranendonk and Chivas2016) and radiocarbon dating has shown 12C enrichment from this early period, consistent with the development of photosynthetic microorganisms (Ohtomo et al., Reference Ohtomo, Kakegawa, Ishida, Nagase and Rosing2014). There is wide agreement that anoxygenic photosynthesis preceded oxygenic photosynthesis, though the length of the interval for this transition is uncertain (Olson, Reference Olson2006; Buick, Reference Buick2008; Rothschild, Reference Rothschild2008). Some geochemical proxy records suggest that the earliest oxygenic photosynthesizers may have appeared by ~2.9–3 Ga with geochemical sinks arresting oxygen's accumulation for a time (Nisbet et al., Reference Nisbet, Grassineau, Howe, Abell, Regelous and Nisbet2007; Planavsky et al., Reference Planavsky, Asael, Hofmann, Reinhard, Lalonde, Knudsen, Wang, Ossa Ossa, Pecoits, Smith, Beukes, Bekker, Johnson, Konhauser, Lyons and Rouxel2014). Ultimately, because of oxygenic photosynthesis and additional, poorly understood factors, the Earth experienced a Great Oxidation Event about 2.3 billion years ago, which indelibly altered the prevailing chemical conditions of our planet's atmosphere (Kump, Reference Kump2008; Lyons et al., Reference Lyons, Reinhard and Planavsky2014; Luo et al., Reference Luo, Ono, Beukes, Wang, Xie and Summons2016).
What were the important evolutionary events predating the rise of photosynthesis during the early history of life on Earth? Although the events during this very early time are not clear, in this paper, we discuss a speculative hypothesis for early evolution, called the ‘Purple Earth,’ which posits the rise of retinal pigment-based phototrophic life forms on Earth's surface prior to anoxygenic and oxygenic photosynthesis. In this view, retinal pigments may have competed with and affected the evolution of photosynthetic pigments and indeed still complements them today in Earth's oceans and other environments. Early microorganisms employing retinal pigments for generating metabolic energy may have dominated, as halophilic Archaea do today in hypersaline environments, providing a scenario which may serve to guide our search for detectable biosignatures on other worlds.
Early evolution on Earth
During the first half of Earth's history, stretching over 2 billion years, dramatic and long-lasting evolutionary inventions occurred through processes that we are only beginning to understand (Fig. 1; Deamer, Reference Deamer2011; Knoll, Reference Knoll2015). They include prebiotic evolution and the development of cellularity, the foundation of the last universal common ancestor (LUCA) and evolution of the universal genetic code (Fenchel, Reference Fenchel2002). Other factors critical for the success of early life were the evolution of transmembrane potential and chemiosmotic coupling for creating and storing bioenergy, pigments for the capture of light energy for phototrophy and photosynthesis and respiratory chains for anaerobic and aerobic respiration (Zannoni, Reference Zannoni2004). In addition, a ‘frozen accident’ has been proposed to establish the genetic code as a universal feature in all extant life on Earth (Crick, Reference Crick1968; Söll and RajBhandary, Reference Söll and RajBhandary2006). During the earliest period in evolutionary history, well-defined phylogenetic lineages may not yet have been established; instead extensive lateral gene transfers allowed for ready sharing of new innovations until such time when the last common ancestor experienced competitive selective forces and diverged into the three primary ‘Domains of Life’ (Woese, Reference Woese2002).
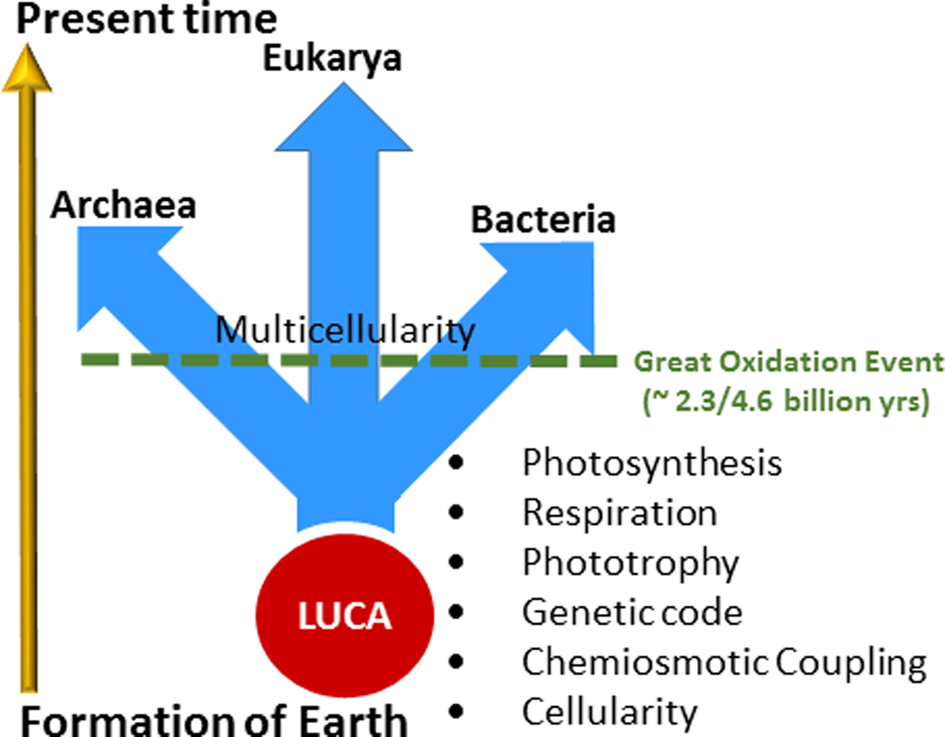
Fig. 1. Evolutionary timeline and events. The arrow at the left roughly indicates time from the formation of the Earth to the present, about 4.6 billion years. Geochemical and fossil evidence indicate that life arose soon after the Earth formed, with many key evolutionary inventions following: cellularity, chemiosmotic coupling, genetic code, phototrophy, respiration and photosynthesis. Light-driven proton pumping by retinal proteins are hypothesized to have evolved during this early stage in evolution. The last universal common ancestor (LUCA) predated the divergence of life into three Domains: Archaea, Bacteria and Eukarya. The rise of anoxygenic and then oxygenic photosynthesis allowed the productivity of Earth's microbial biosphere to increase immensely (Des Marais, Reference Des Marais2000). The Great Oxidation Event followed, about 2.3 billion years ago and led to the development of multicellularity and evolution of higher life forms.
Even prior to the evolution of the three Domains, the development of a protocell must have been facilitated by the evolution of a water-tight cell membrane as a permeability barrier, preventing the free diffusion of chemicals into and out of cells, critical for generating and storing cellular energy (Gunner et al., Reference Gunner, Amin, Zhu and Lu2013). The intracellular milieu provided a microenvironment in which biomolecular functions, such as the biosynthesis of macromolecules and the genetic code could be established. Transmembrane ion pumps acting as energy transduction and storage systems must have been among the earliest inventions. In one scenario proposed here, a simple light-harvesting system incorporating a retinal pigment allowed light-driven proton pumping and led to a proton-motive gradient. Based on its ubiquity, the transmembrane electrochemical potential (i.e. proton-motive gradient) as well as phosphoric anhydride bonds, such as in adenosine triphosphate (ATP), became established and universal due to their kinetic stability and bioenergetic capabilities in the aqueous environment. Subsequently, retinal as well as a variety of more complex anaerobic and oxygenic light-harvesting systems were invented and resulted in the evolution of diverse phototrophic and photosynthetic microorganisms.
Appearance of purple retinal pigments
The earliest life-forms probably arose in the early Archean or possibly late Hadean Eons, with some molecular clock estimates putting life's origin as early as 4 Ga (Hedges, Reference Hedges2002). While the exact timing of appearance of retinal pigments is not clear, it may have been a very early metabolic invention coincident with or occurring soon after the development of cellular life. A retinal chromophore bound to a single polypeptide allows a system for phototrophy by forming a chromoprotein, like bacteriorhodopsin in halophilic Archaea dominant in hypersaline environments and proteorhodopsin in pelagic bacteria distributed throughout the oceans (Béjà et al., Reference Béjà, Spudich, Spudich, Leclerc and DeLong2001; Stoeckenius et al., Reference Stoeckenius, Lozier and Bogomolni1979). The absorption of light by this chromoprotein in the 490–600 nm region, a highly energy-rich region of the solar spectrum (Fig. 2), is directly coupled to pumping of protons and the resulting electrochemical gradient chemiosmotically drives ATP synthesis. This type of retinal-dependent phototrophy is considerably simpler albeit less efficient than photosynthesis and it neither results in fixation of carbon nor production of oxygen (Pinhassi et al., Reference Pinhassi, DeLong, Béjà, González and Pedrós-Alió2016). Nevertheless, the widespread distribution of retinal chromoproteins in nature and their unique utilization of the energy-rich, yellow-green region of the spectrum for production of cellular energy suggest their early appearance on Earth.
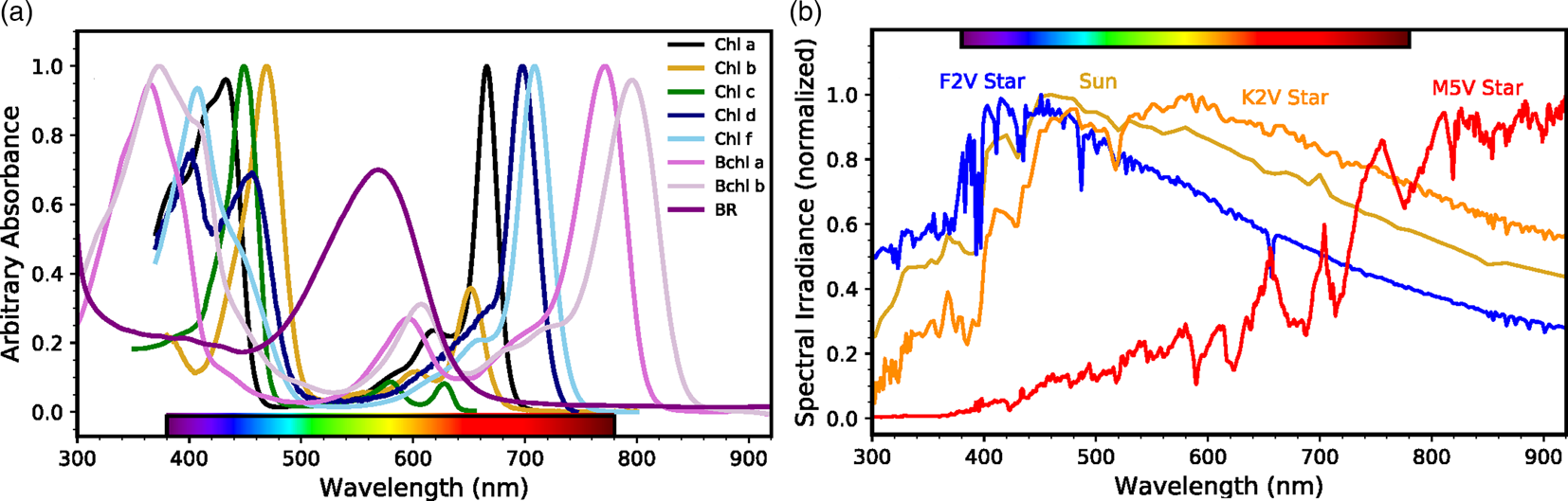
Fig. 2. Phototrophic pigment absorption and stellar radiation as a function of wavelength. (a) Absorbance spectra of phototrophic pigments including chlorophyll a, b, c, d and f (Chen et al., Reference Chen, Schliep, Willows, Cai, Neilan and Scheer2010; Chen and Blankenship, Reference Chen and Blankenship2011; Jeffrey, Reference Jeffrey1963); bacteriochlorophyll a and b (Frigaard et al., Reference Frigaard, Larsen and Cox1996); and bacteriorhodopsin (BR; credit: Victoria Laye and Priya DasSarma). Note the strong BR absorption where (bacterio)chlorophylls are least absorptive. (b) Normalized spectral energy distributions at the top of the atmosphere for FGKM-type stars, including the Sun (G-type), from the Virtual Planetary Laboratory (Meadows et al., Reference Meadows, Arney, Schwieterman, Lustig-Yaeger, Lincowski, Robinson, Domagal-Goldman, Deitrick, Barnes, Fleming, Luger, Driscoll, Quinn and Crisp2018; Segura et al., Reference Segura, Krelove, Kasting, Sommerlatt, Meadows, Crisp, Cohen and Mlawer2003).
Evidence for the existence of isoprenoid compounds that are part of the biosynthetic pathway to retinal as well as archaeal lipids in the early history of the Earth has also been provided (Hahn and Haug, Reference Hahn and Haug1986; Ventura et al., Reference Ventura, Kenig, Reddy, Schieber, Frysinger, Nelson, Dinel, Gaines and Schaeffer2007). It is likely that the evolutionary invention of retinal pigments was coincident with other membrane lipids, which together established the molecular basis for chemiosmotic coupling and phototrophic capabilities (Boucher and Doolittle, Reference Boucher and Doolittle2000). Retinal is produced by a branch of the isoprenoid metabolic pathway leading to carotenoids and branched-chain lipids, which are found in cell membranes (Fig. 3). Retinal pigments occur in both major prokaryotic phylogenetic groups, Archaea and Bacteria, as well as in eukaryotes, where they are essential components of the visual system (Ernst et al., Reference Ernst, Lodowski, Elstner, Hegemann, Brown and Kandori2014). Among the pigments prevalent in nature, retinal has a simple structure compared with many others that are used for photosynthesis and respiration, e.g. chlorophyll and other porphyrins, which may be produced by a branch of the tricarboxylic acid (TCA) cycle, a pathway used by all aerobic organisms (Mailloux et al., Reference Mailloux, Bériault, Lemire, Singh, Chénier, Hamel and Appanna2007). These findings, together with the central position of retinal at the intersection of lipid metabolism and bioenergetics, as well as its widespread distribution suggest that retinal played an important role in the early evolution of life on Earth.
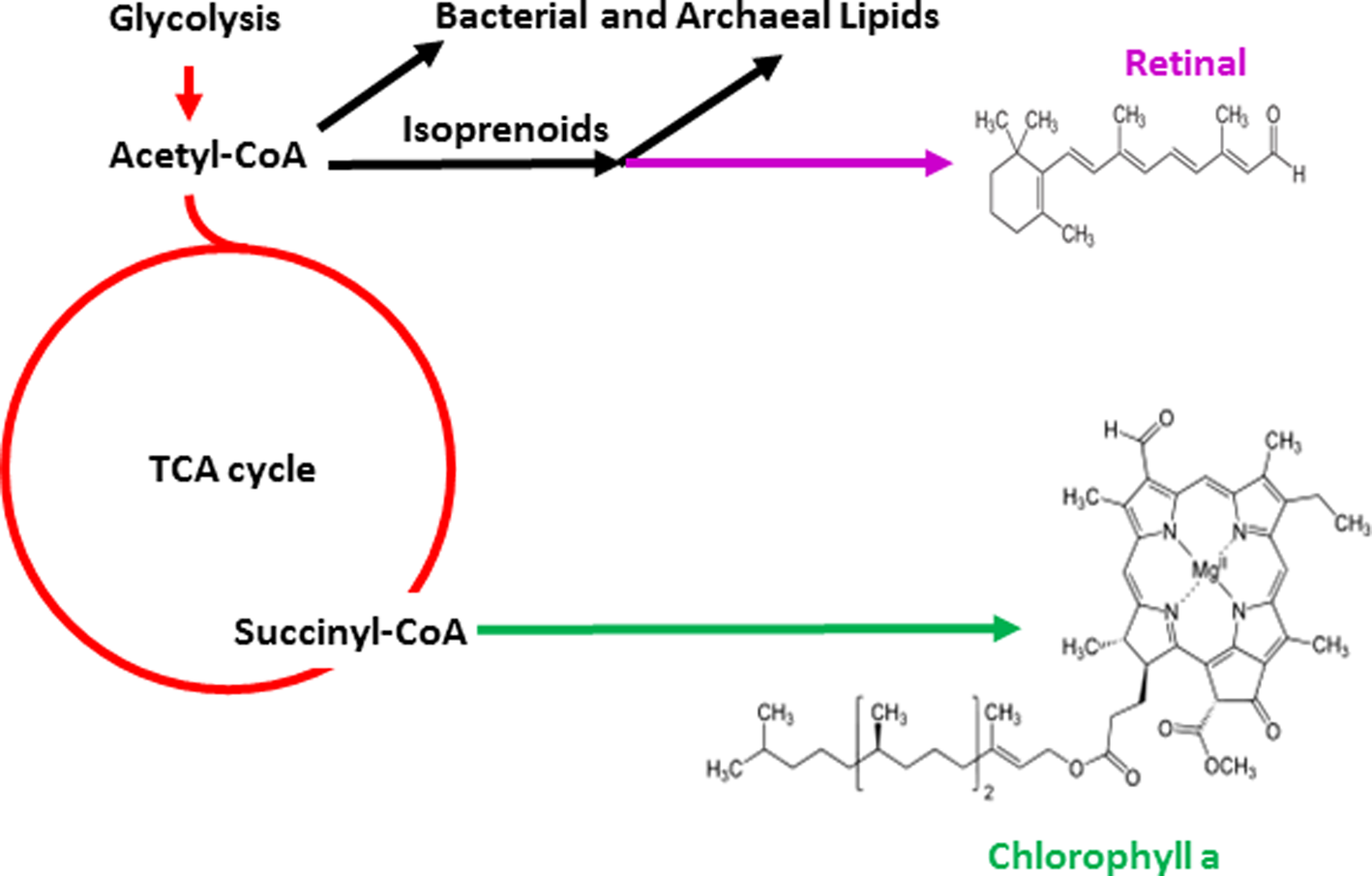
Fig. 3. Biosynthetic pathways for photopigments. Pathways leading to retinal (purple) and chlorophyll (green) branching from central metabolism (red) are shown. Glycolysis and the TCA cycle are depicted as are structures of the simpler retinal chromophore and the more complex chlorophyll a.
The light-driven proton pumping activity of retinal pigments such as the chromoprotein bacteriorhodopsin in the membrane of an early cell would have allowed the development of chemiosmotic coupling, linking of membrane potential to other transmembrane transport processes and ATP synthesis (Stoeckenius et al., Reference Stoeckenius, Lozier and Bogomolni1979). A retinal-based phototrophic system clearly represents one of the simplest bioenergetic mechanisms conceivable, requiring only a single opsin inserted in a membrane vesicle and membrane-potential coupled ATP synthase (Fig. 4). Indeed, such a model phototrophic system, inside out, was established in vitro in the 1970s using haloarchaeal bacteriorhodopsin and mitochondrial ATP synthase in artificial lipid vesicles (Racker and Stoeckenius, Reference Racker and Stoeckenius1974). This seminal work was credited with helping to establish the validity of Mitchell's chemiosmotic coupling hypothesis (Mitchell, Reference Mitchell1961) and also forms the foundation of one of the simplest and as proposed, earliest metabolic capabilities in evolution, retinal-based phototrophy.
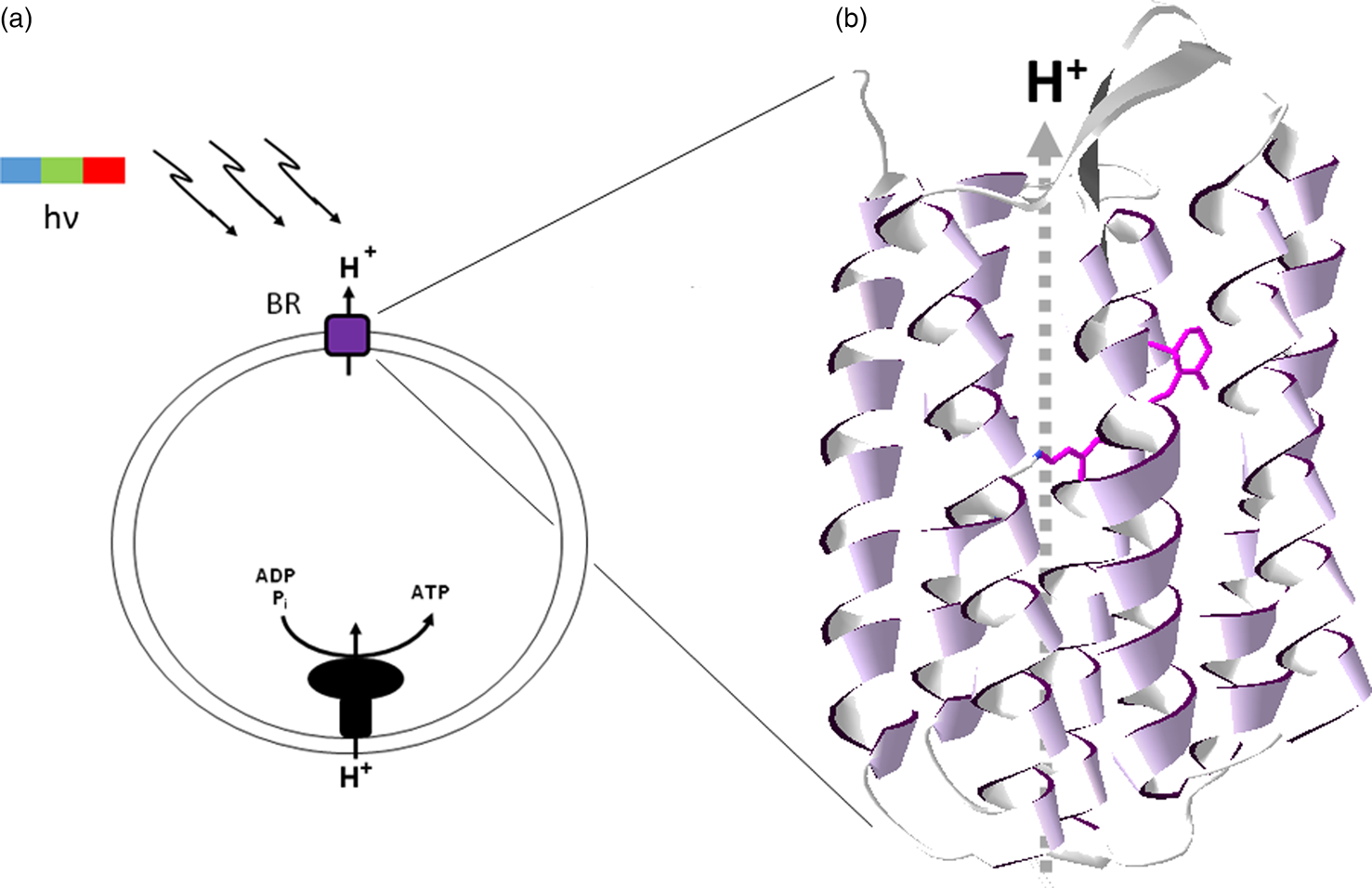
Fig. 4. Bacteriorhodopsin and chemiosmotic coupling. (a) Light-driven (hν) proton pumping by bacteriorhodopsin (BR) results in ATP synthesis by chemiosmotically coupling to the proton-motive force. (b) Bacteriorhodopsin structure showing seven-transmembrane α-helical segments (ribbons) and bound retinal chromophore (purple wire structure), with proton pumping (dashed arrow, H+).
Early Earth environments would have lacked abundant free O2 in contrast to highly oxic modern environments and required the production of retinal using a terminal oxidative step in a likely strictly anaerobic environment. A number of potential mechanisms have been proposed for generating such an oxidative potential, such as pyrite-induced aqueous hydrogen peroxide and hydroxide radical formation (Borda et al., Reference Borda, Elsetinow, Schoonen and Strongin2001; Cohn et al., Reference Cohn, Mueller, Wimmer, Leifer, Greenbaum, Strongin and Schoonen2006). Other anaerobic oxidation reactions are also known, such as anaerobic oxidation of methane and ammonium and transformation of isoprenoids by anaerobic microorganisms (Hallam et al., Reference Hallam, Putnam, Preston, Detter, Rokhsar, Richardson and DeLong2004; Hylemon and Harder, Reference Hylemon and Harder1998; Strous and Jetten, Reference Strous and Jetten2004).
Also notable is that modern halophilic Archaea are facultative, rather than obligate aerobes and can respire nitrate and TMAO/DMSO (Mancinelli and Hochstein, Reference Mancinelli and Hochstein1986; Müller and DasSarma, Reference Müller and DasSarma2005). Indeed, Haloarchaea have been shown to engage in phototrophy in microaerobic or anoxic laboratory conditions (Sumper et al., Reference Sumper, Reitmeier and Oesterhelt1976; DasSarma et al., Reference DasSarma, Zamora, Müller and DasSarma2012; Laye et al., Reference Laye, Karan, Kim, Pecher, DasSarma and DasSarma2017). Additionally, a considerable amount of evidence suggests that the genes for aerobic respiration were laterally transferred to halophilic Archaea (Kennedy et al., Reference Kennedy, Ng, Salzberg, Hood and DasSarma2001) and their ultimate origin may have been as an anaerobic chemolithoautotrophic methanogen (Nelson-Sathi et al., Reference Nelson-Sathi, Dagan, Landan, Janssen, Steel, McInerney, Deppenmeier and Martin2012; Aouad et al., Reference Aouad, Taib, Oudart, Lecocq, Gouy and Brochier-Armanet2018). Hence, haloarchaeal phototrophic metabolism was probably developed well before genes for aerobic respiration were acquired, possibly in Archaea inhabiting hypersaline environments (Stevenson et al., Reference Stevenson, Burkhardt, Cockell, Cray, Dijksterhuis, Fox-Powell, Kee, Kminek, McGenity, Timmis, Timson, Voytek, Westall, Yakimov and Hallsworth2015). While these modern haloarchaeal organisms have certainly changed over the eons from the original retinal-based phototrophs, the available evidence illustrates the potential capacity of Haloarchaea to have survived the anaerobic conditions that prevailed on ancient Earth.
In modern halophilic Archaea, the retinal protein bacteriorhodopsin trimers form a hexagonal lattice which can cover a large fraction of the cell surface (Stoeckenius et al., Reference Stoeckenius, Lozier and Bogomolni1979), imparting a bright purple colour to some salt ponds where they dominate (Fig. 5). The resulting purple membrane can be easily isolated using sucrose density gradients and has been the subject of extensive structural and functional analysis of transmembrane ion translocation (Henderson and Unwin, Reference Henderson and Unwin1975; Stoeckenius et al., Reference Stoeckenius, Lozier and Bogomolni1979; Krebs and Khorana, Reference Krebs and Khorana1993; Hirai et al., Reference Hirai, Subramaniam and Lanyi2009). Bacteriorhodopsin is a prototype of integral membrane proteins with seven-transmembrane α-helical segments where the retinal chromophore is bound by a Schiff's base linkage to the ε-amino group of a lysine residue (Bayley et al., Reference Bayley, Huang, Radhakrishnan, Ross, Takagaki and Khorana1981). The photobiology of bacteriorhodopsin has been intensively studied, including characterization of the molecular dynamics and role of retinal during photocycling (Hirai et al., Reference Hirai, Subramaniam and Lanyi2009). The bacteriorhodopsin resting state is notable for the characteristic colour purple resulting from the strong absorption peak maximum at 568 nm in the yellow-green region of the spectrum.
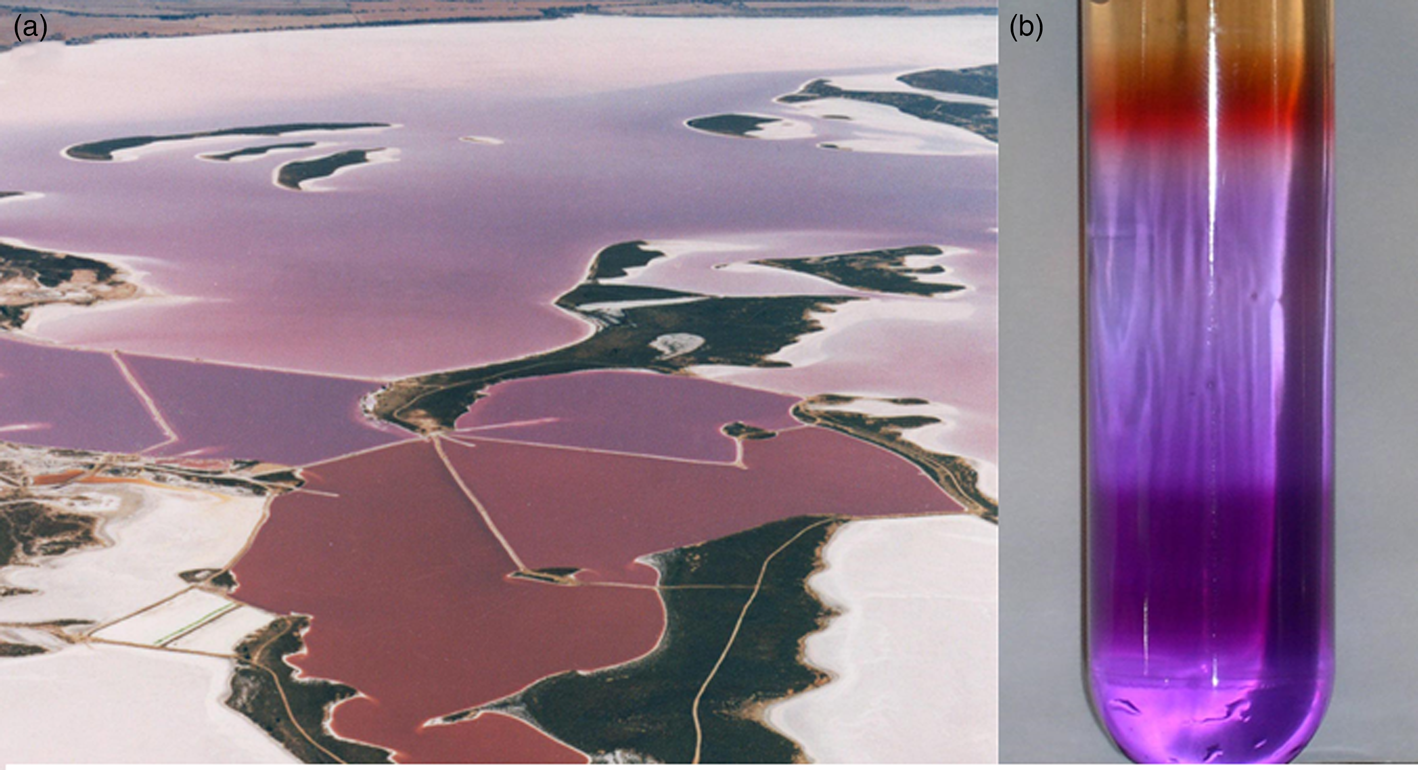
Fig. 5. Purple microorganisms and purple membrane. (a) Australian salt pond with a bloom of purple microorganisms (Courtesy Cheetham Salt Co.). (b) Sucrose gradient separating Halobacterium sp. cell lysate, including both red (upper) and purple (lower) pigments (Credit: Victoria Laye and Priya DasSarma).
Spectral complementarity of photopigments
Comparison of the spectrum of bacteriorhodopsin with the major photosynthetic pigments containing chlorophyll and bacteriochlorophylls shows them to be complementary, i.e. the purple pigment absorption peaks in the region with a trough for the green pigments (Fig. 2a). If the evolution of the simpler retinal pigments predated chlorophyll pigments in the evolutionary history, as proposed, it is conceivable that they may have affected the development of the spectral characteristics of evolving chlorophyll pigments (Goldsworthy, Reference Goldsworthy1987; DasSarma, Reference DasSarma2006). This may have been the consequence of filtering of light by retinal chromoproteins, resulting in a deficit of wavelengths of light centred around the peak of bacteriorhodopsin absorption in the yellow-green region of the spectrum. The resulting deficit, particularly in a stratified community of microorganisms such as those observed in stromatolites, may explain why chlorophylls and bacteriochlorophylls evolved to absorb relatively little light in the yellow-green energy-rich portion of the electromagnetic spectrum, instead absorbing light primarily in the flanking blue and red regions of the solar spectrum.
Modern stromatolites represent microbial communities with on-going spectral competition and spectral tuning of chromoproteins (Croce and van Amerongen, Reference Croce and van Amerongen2014). Phototrophic and photosynthetic microorganisms in microbial mats are commonly stratified based on predictable photosystem characteristics as well as oxygen requirements. In such communities, oxygenic cyanobacteria are found near the surface oxic zone while anaerobic phototrophic and photosynthetic microbes are buried at lower anoxic regions. If these modern stratified microbial communities are like those present in ancient stromatolites, filtering of wavelengths of light would have been an important and pervasive characteristic of microbial communities. Modern microbial communities all over the world support planktonic retinal-containing halophilic Archaea and Bacteria inhabiting brines above photosynthetic mats (Cohen and Rosenberg, Reference Cohen and Rosenberg1989). If co-evolution of retinal and chlorophyll photopigments occurred in deep evolutionary history, stratification within such niches may have played an important role in the evolution of spectral properties.
Importantly, modern rhodopsin-based phototrophy is present throughout the oceanic and terrestrial biosphere, including non-hypersaline conditions and environments that may have been common in the distant geologic past. While originally discovered in halophilic Archaea, microbial rhodopsins are common in oceanic planktonic Bacteria (Kandori, Reference Kandori2015). For example, Pelagibacter ubique is a widely distributed marine bacterium that produces the retinal chromoprotein, proteorhodopsin, with the ability to use its light-driven proton pumping activity for energy generation. Moreover, the absorption characteristics of proteorhodopsins show spectral variations in oceanic planktonic bacteria isolated from different depths, consistent with spectral tuning (Rangarajan et al., Reference Rangarajan, Galan, Whited and Birge2007). Rhodopsin-based phototrophy in the ocean may be so widespread as to rival the total light capture of photosynthesizers (Brown, Reference Brown and Hohmann-Marriott2014; Gómez-Consarnau et al., Reference Gómez-Consarnau, Levine, Cutter, Wang, Seegers, Arístegui, Fuhrman, Gasol and Sañudo-Wilhelmy2017). Furthermore, metagenomic analyses have recently uncovered evidence for the widespread presence of rhodopsins in the terrestrial biosphere including in the phyllosphere (leaf surfaces) and even edaphic systems and hypolithic communities in the Antarctic Dry Valley (Atamna-Ismaeel et al., Reference Atamna-Ismaeel, Finkel, Glaser, Sharon, Schneider, Post, Spudich, von Mering, Vorholt, Iluz, Béjà and Belkin2012; Guerrero et al., Reference Guerrero, Vikram, Makhalanyane and Cowan2017). These findings are consistent with the notion that microorganisms evolve specialized photosystems that make use of any available spectral region with sufficient energy. The widespread presence of microbial rhodopsins in modern environments, along with inefficient chlorophyll absorption in the middle of the visible spectrum where rhodopsin light capture is most efficient, suggests a co-evolution that is consistent with the earlier appearance of retinal-based phototrophy (PBS Eons 2018).
Rise of photosynthesis
The rise of anaerobic and oxygenic photosynthesis and retreat of retinal-based life must have occurred in discrete stages, which are not fully understood. For example, the time of appearance of a wide diversity of bacteria with anoxygenic photosynthetic systems is not precisely known (Jeffrey, Reference Jeffrey1963; Frigaard et al., Reference Frigaard, Larsen and Cox1996; Rothschild, Reference Rothschild2008; Chen et al., Reference Chen, Schliep, Willows, Cai, Neilan and Scheer2010; Chen and Blankenship, Reference Chen and Blankenship2011; Croce and van Amerongen, Reference Croce and van Amerongen2014). The purple and green bacteria possessing bacteriochlorophyll may have been an early evolutionary development with photosynthetic reaction centres evolving from electron transport chain components such as cytochromes (Williamson et al., Reference Williamson, Conlan, Hillier and Wydrzynski2011; Mazor et al., Reference Mazor, Greenberg, Toporik, Béjà and Nelson2012). Alternatively, a simplified photosystem may have evolved previously, like those in some heliobacteria (Xiong et al., Reference Xiong, Inoue and Bauer1998). In either case, evolution likely first led to the evolution of anoxygenic photosynthesis with the development of more complex oxygenic photosynthetic membrane systems like those in modern cyanobacteria, including two photosynthetic reaction centres and a host of membrane components, developing later.
With multiple evolutionary steps leading to progressively higher efficiency chlorophyll pigments along with the invention of accessory pigments, photosynthetic microorganisms out-competed retinal-based phototrophic microorganisms in most environments. Evolution of anoxygenic photosynthesizers was followed by oxygenic photosynthesizing cyanobacteria and ultimately eukaryotic algae and plants. Interestingly, a distinct hypothesis that purple sulphur bacteria may have dominated euxinic oceans during the mid-Proterozoic eon has also been proposed, resulting in a second Purple Earth (Brocks et al., Reference Brocks, Love, Summons, Knoll, Logan and Bowden2005; Sanromá et al., Reference Sanromá, Pallé, Parenteau, Kiang, Gutiérrez-Navarro, López and Montañés-Rodríguez2014), which would have been long after the retreat of retinal-based life dominating the first Purple Earth to ecological niches resembling those of today. The development of eukaryotic algae and complex plants and their spread throughout the terrestrial environment allowed the evolution of land animals and ultimately intelligent life (Catling et al., Reference Catling, Glein, Zahnle and McKay2005; Reinhard et al., Reference Reinhard, Planavsky, Olson, Lyons and Erwin2016). At every step of this progression, it is not clear to what degree evolutionary contingency has played a role and which developments would be inevitable given sufficient time and the appropriate environmental conditions. As a result, the capacity for evolution to generate diverse phototrophic and photosynthetic systems on Earth, even those that do not dominate today, may have considerable implications for the development of novel pigments on other habitable worlds (Johnson et al., Reference Johnson, Zhao, Caycedo, Manrique, Qi, Rodriguez and Quiroga2013).
Retinal-based phototrophy as an astronomical biosignature
Regardless of the evolutionary sequence of events leading to retinal phototrophy on Earth, analog photopigments may have arisen independently in other habitable environments in the universe. For example, exoplanets within the habitable zones of most stars would receive ample photon fluxes to power significant levels of (bacterio)chlorophyll or rhodopsin analog phototrophy with some differences in total capacity based on the photospheric temperature and consequent spectral energy distribution of those stars (Kiang et al., Reference Kiang, Siefert, Govindjee and Blankenship2007b; Reference Kiang, Segura, Tinetti, Govindjee, Blankenship, Cohen, Siefert, Crisp and Meadows2007a; Komatsu et al., Reference Komatsu, Umemura, Shoji, Kayanuma, Yabana and Shiraishi2015; Ritchie et al., Reference Ritchie, Larkum and Ribas2018). One exception may be the dimmest and reddest M-stars, which produce the least flux in the 400–700 nm wavelength range (Fig. 2b). For these stellar systems, total global productivity may be photon-limited rather than reductant or nutrient limited as it is on Earth (Lehmer et al., Reference Lehmer, Catling, Parenteau and Hoehler2018). However, FGK stellar systems are the more likely targets for future space-based direct-imaging missions capable of detecting astronomical biosignatures. This is due to the wider angular separation of star and planet in the habitable zone (Stark et al., Reference Stark, Roberge, Mandell, Clampin, Domagal-goldman, Mcelwain and Stapelfeldt2015; Reference Stark, Roberge, Mandell and Robinson2014) and the productivity of biospheres on planets orbiting these stars would not be photon-limited. It is, therefore, worthwhile to examine what the remote signatures of rhodopsin-like phototrophy would be on exoplanets and how they would compare to those produced by analogs to chlorophyll-based photosynthesis.
The most commonly referenced surface signature of life is the vegetation red edge (VRE), the steep increase in reflectivity of vegetation (primarily green vascular plants) at ~700 nm (Gates et al., Reference Gates, Keegan, Schleter and Weidner1965; Knipling, Reference Knipling1970). This increase is due to the contrast between the absorption of chlorophyll at red wavelengths and its high albedo at infrared wavelengths due to intracellular scattering. The VRE effect is commonly used to map vegetation by employing broadband observations from Earth-observing satellites (Huete et al., Reference Huete, Justice and Liu1994; Tucker et al., Reference Tucker, Pinzon, Brown, Slayback, Pak, Mahoney, Vermote and El Saleous2005). While the VRE has been extensively examined as a possible exoplanet biosignature (Sagan et al., Reference Sagan, Thompson, Carlson, Gurnett and Hord1993; Arnold et al., Reference Arnold, Gillet, Lardiere, Riaud and Schneider2002; Des Marais et al., Reference Des Marais, Harwit, Jucks, Kasting, Lin, Lunine, Schneider, Seager, Traub and Woolf2002; Seager et al., Reference Seager, Turner, Schafer and Ford2005; Brandt and Spiegel, Reference Brandt and Spiegel2014), its applicability is limited to those planets that have, like Earth, evolved chlorophyll-analog (i.e., red-absorbing, infrared reflecting) powered vegetation with significant continental surface covering fractions.
Even on Earth, green vascular planets have only existed for just the last ~10% of the planet's history, about 470 million years out of 4.6-billion years (Kenrick and Crane, Reference Kenrick and Crane1997). In order to address the limited time of presence of the VRE, astrobiologists interested in remote biosignatures have begun to consider and catalog surface reflectance signatures from a diverse array of known pigmented organisms including oxygenic and anoxygenic photosynthesizers, rhodopsin-based phototrophs and non-photosynthetic microbes that use pigments as a UV screen or antioxidant, or for other purposes (DasSarma, Reference DasSarma2006; Kiang et al., Reference Kiang, Siefert, Govindjee and Blankenship2007b, Reference Kiang, Segura, Tinetti, Govindjee, Blankenship, Cohen, Siefert, Crisp and Meadows2007a; Cockell, Reference Cockell2014; Hegde et al., Reference Hegde, Paulino-Lima, Kent, Kaltenegger and Rothschild2015; Poch et al., Reference Poch, Frey, Roditi, Pommerol, Jost and Thomas2017; Schwieterman, Reference Schwieterman, Deeg and Belmont2018; Schwieterman et al., Reference Schwieterman, Kiang, Parenteau, Harman, DasSarma, Fisher, Arney, Hartnett, Reinhard, Olson, Meadows, Cockell, Walker, Grenfell, Hegde, Rugheimer, Hu and Lyons2018; Reference Schwieterman, Cockell and Meadows2015). The possible existence of a Purple Earth extends and expands the possible biological history of a planet when alternate biosignatures may be detectable, and it also enhances the number of possible evolutionary trajectories for which surface biosignatures may be found.
The photochemical properties of known prokaryotic rhodopsins on Earth are particularly worthy of study as a potential remote biosignature because of their capacity to generate chemical energy using an energy-rich portion of the electromagnetic spectrum. The most consequential difference between rhodopsin and chlorophyll-based phototrophy is the wavelength of maximum absorption. While the absorption peak of chlorophyll a is near 700 nm, bacteriorhodopsin absorption peaks near 570 nm. However, the expression of this signal would differ depending on whether the phototrophic organisms were on dry land or suspended in aquatic environments.
A true bacteriorhodopsin-based analog to terrestrial vegetation would possess a ‘green-edge’ comparable with the vegetation ‘red-edge.’ Fig. 6a illustrates the differences between the reflective spectra of a red-edge producing conifer forest (Baldridge et al., Reference Baldridge, Hook, Grove and Rivera2009) and the green-edge producing Haloarchaea (Schwieterman et al., Reference Schwieterman, Cockell and Meadows2015). While the green colour of the conifer forest results from inefficient absorption in a broad wavelength region centred near 550 nm, the pink colour of halophiles results from their high reflectivity at orange and red wavelengths due both to bacteriorhodopsin and carotenoid pigments such as bacterioruberins (Kushwaha and Kates, Reference Kushwaha and Kates1979; Oren et al., Reference Oren, Stambler and Dubinsky1992; Oren and Dubinsky, Reference Oren and Dubinsky1994). Green plants are similarly bright in the infrared due to their high reflectivity on the non-visible, long-wavelength side of the VRE. Notably, a wide variety of other organisms have spectral ‘edges’ at various visible wavelengths. Hegde et al. (Reference Hegde, Paulino-Lima, Kent, Kaltenegger and Rothschild2015) conducted an extensive series of reflectance measurements of plated cultures of extremophiles from 350 to 2500 nm showing a distribution of ‘edge’ features for various phototrophic and non-phototrophic species throughout the UV-visible spectrum. The radiotolerant species Deinococcus radiodurans also possess a ‘green-edge’ in plated cultures due to its primary pigment deinoxanthin (Cockell, Reference Cockell2014; Schwieterman et al., Reference Schwieterman, Cockell and Meadows2015), which likely functions as an antioxidant. Depending on the environmental context, some of these pigments may also serve as alternative biosignatures. Rhodopsin-based (and other) phototrophs may have both biological and detectability advantage, however, in that they can harvest light energy for growth and accumulate at the surface of aquatic environments. Consequently, it is also important to consider the spectral differences between plated and suspended cultures, which would map to different planetary environments (e.g., land versus ocean).
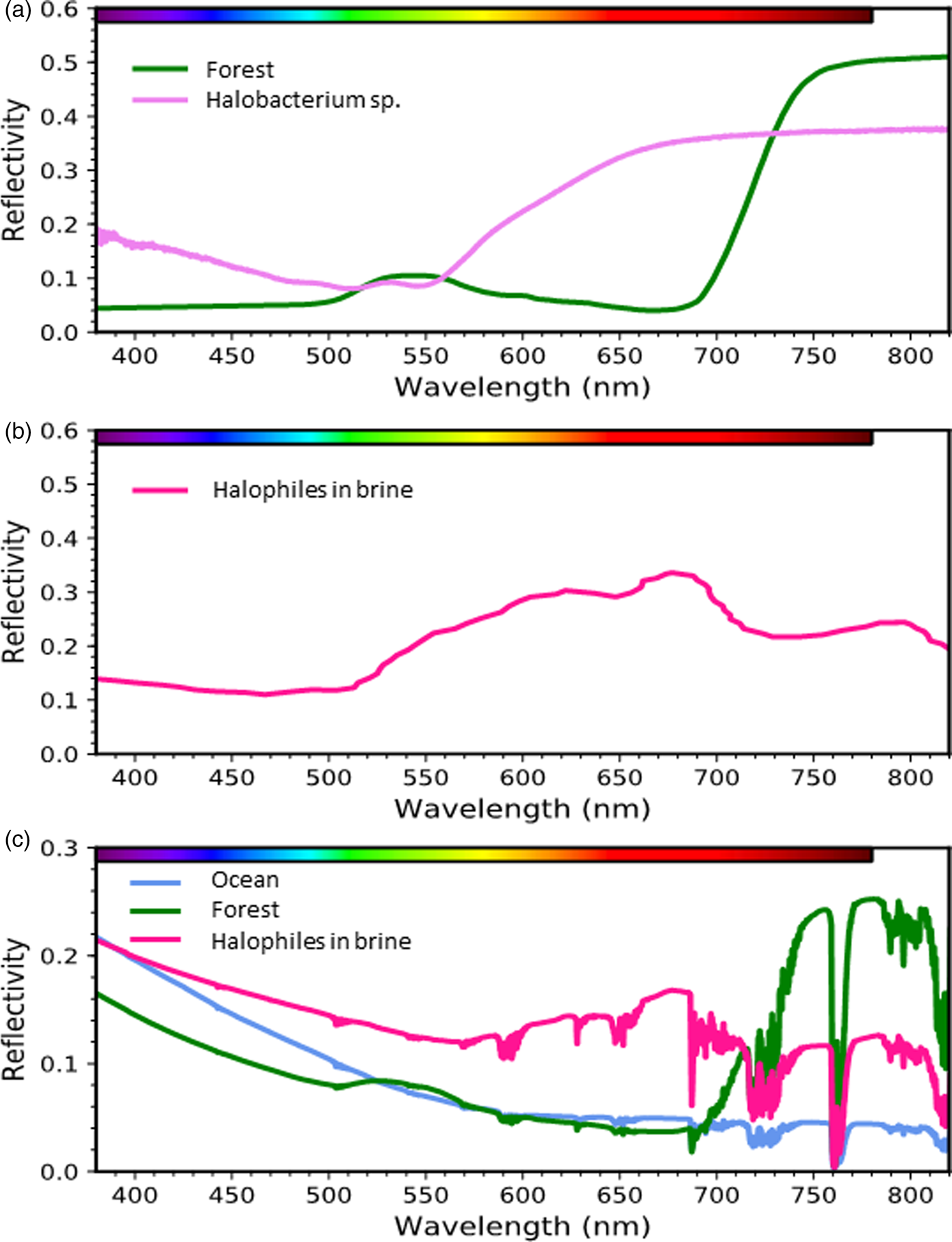
Fig. 6. Surface signatures of retinal and chlorophyll-based phototrophy. (a) Reflectance spectrum of a conifer forest (Baldridge et al., Reference Baldridge, Hook, Grove and Rivera2009) and a culture of the phototrophic archaeon Halobacterium sp. (Schwieterman et al., Reference Schwieterman, Cockell and Meadows2015). (b) Environmental spectrum of a halophile-dominated saltern pond in San Francisco Bay (Dalton et al., Reference Dalton, Palmer-Moloney, Rogoff, Hlavka and Duncan2009). (c) Simulated spectra of planets consisting of 100% sterile ocean, conifer forest, or a halophile-dominated saltern pond under an Earth-like atmosphere generated with a radiative transfer model (Schwieterman et al., Reference Schwieterman, Cockell and Meadows2015).
The spectral signature of pigmented organisms like Haloarchea suspended in a lake or ocean would also be affected by the low reflectivity and strong absorption properties of aquatic environments. For example, halophilic communities present in saltern ponds possess a peak in brightness near ~680 nm due to increasing reflectivity of bacteriorhodopsin and carotenoid pigments from the green to the red combined with strong water absorption at the reddest wavelengths (Fig. 6b; also see Dalton et al., (Reference Dalton, Palmer-Moloney, Rogoff, Hlavka and Duncan2009)). Importantly, the brightness of halophilic pigments at orange and red wavelengths confers a detectability advantage over chlorophyll-containing cyanobacteria and algae suspended in water, because chlorophyll's high infrared reflectivity is counteracted by water vapour absorption, while chlorophyll is most absorptive at wavelengths where water is relatively transparent.
The remote signatures of these phototrophic organisms would further change from the spectral impact (i.e., absorption and scattering) of the overlying atmosphere (Fig. 6c). The spectral signatures of halophilic organisms are again somewhat favoured in this case because of the impact of overlying water vapour absorption nearly coincident with the VRE. Of course, the detectability of these signatures on an exoplanet will also be strongly sensitive to the land covering fraction, cell densities if suspended in water or brine and cloud cover effects (Sanromá et al., Reference Sanromá, Pallé, Parenteau, Kiang, Gutiérrez-Navarro, López and Montañés-Rodríguez2014; Schwieterman et al., Reference Schwieterman, Cockell and Meadows2015). The detectability potential of retinal photopigments and other halophilic pigment-analogs should be considered when anticipating the variety of potential surface biosignatures of exoplanets.
The capacity to detect surface biosignatures is an ongoing consideration in the design mandate of large, space-based telescopes (Fujii et al., Reference Fujii, Angerhausen, Deitrick, Domagal-Goldman, Grenfell, Hori, Kane, Pallé, Rauer, Siegler, Stapelfeldt and Stevenson2018; Schwieterman et al., Reference Schwieterman, Kiang, Parenteau, Harman, DasSarma, Fisher, Arney, Hartnett, Reinhard, Olson, Meadows, Cockell, Walker, Grenfell, Hegde, Rugheimer, Hu and Lyons2018) such as the conceived HabEx and LUVOIR/HDST missions (Dalcanton et al., Reference Dalcanton, Seager, Aigrain, Hirata, Battel, Mather, Brandt, Postman, Conroy, Redding, Feinberg, Schiminiovich, Gezari, Stahl, Guyon, Tumilinson and Harris2015; Mennesson et al., Reference Mennesson, Gaudi, Seager, Cahoy, Domagal-Goldman, Feinberg, Guyon, Kasdin, Marois, Mawet, Tamura, Mouillet, Prusti, Quirrenbach, Robinson, Rogers, Scowen, Somerville, Stapelfeldt, Stern, Still, Turnbull, Booth, Kiessling, Kuan, Warfield, MacEwen, Fazio, Lystrup, Batalha, Siegler and Tong2016; Rauscher et al., Reference Rauscher, Canavan, Moseley, Sadleir and Stevenson2016; Bolcar et al., Reference Bolcar, Aloezos, Crooke, Dressing, Fantano, Hylan, Tompkins, Bolcar, Bly, Collins, Feinberg, France, Gochar, Gong, Jones, Linares, Postman, Pueyo, Roberge, Sacks, West, MacEwen and Breckinridge2017). Direct imaging spectra and spectrophotometry will allow characterization of the surfaces of terrestrial planets in the habitable zone, producing constraints on surface types, including surface biosignatures, providing the cloud covering fraction is sufficiently low (Sanromá et al., Reference Sanromá, Pallé, Parenteau, Kiang, Gutiérrez-Navarro, López and Montañés-Rodríguez2014, Reference Sanromá, Pallé and García Munõz2013). The detectability potential of retinal photopigments and halophile-analogs suggests wavelengths shortward of the traditional VRE (λ< 700 nm) will be important to observe and analyse for ‘edge’ features suggestive of diverse phototrophic pigments and should be considered in the search for life outside the solar system.
Concluding remarks
Although of enormous scientific interest, our understanding of the early evolutionary history of phototrophic life on Earth has remained limited. We propose here that the biochemical simplicity of retinal-based phototrophy, the spectral complementarity of bacteriorhodopsin pigments with chlorophylls and the newly uncovered widespread diversity of microbial rhodopsins throughout aquatic and terrestrial ecosystems are suggestive of the fundamental role retinal may have played in the early history of life on Earth. We posit here that domination by retinal-based phototrophs in the early history of life may have created the first ‘Purple Earth’ that at some point gave way to modern photosynthesizers before the rise of atmospheric oxygen. If correct, this early phototrophic metabolism would have greatly shaped the evolution of photosynthesis and indeed much of life on Earth. In fact, we know it continues to play a significant role in many environments today.
To test this Purple Earth hypothesis, future work should further explore natural communities of retinal-based phototrophs in diverse environments (e.g. arid, high altitude and polar locations). Additional studies are needed to explore the diversity and light capture capacity of retinal-based phototrophy in modern environments as they may continue to reveal unexpected roles and niches for this metabolism and inform its evolutionary origin. Additionally, future genomic analyses should be designed to consider the importance of the timing of the introduction of aerobic respiration in Haloarchaea in relation to the development of phototrophy during their metamorphosis from anaerobic chemolithoautotrophic methanogens to aerobic photoheterotrophs.
Considering an even broader view, the quest to understand the origin of life and early evolutionary events on our planet has gained increasing urgency with the discovery of thousands of new extrasolar planets, many of which are within the habitable zones of their host stars. Consequently, we may soon have the ability to characterize other potentially living worlds and finally answer the age-old question ‘Are we alone in the universe?’ To realize this goal, however, we need to improve our understanding of major events sparking life on Earth and determine what biosignatures early life produced, especially those which may be detectable by remote sensing.
Simple retinal-based light-harvesting systems like that of the purple chromoprotein bacteriorhodopsin, may potentially serve as remote biosignatures for exoplanet research through the search for brightness peaks about 680 nm like that seen in hypersaline environments on Earth or by spectral ‘edges’ at green-yellow wavelengths (~550 nm) analogous to the traditional vegetation ‘red-edge’ seen at 700 nm. These features are within the wavelength sensitivity window of planned next-generation space-based telescopes capable of directly imaging exoplanets and should be considered in the search for life in the universe.
Acknowledgements
Exobiology research in the S.D. laboratory is supported by NASA grant NNX15AM07G. E.S. is supported by a NASA Postdoctoral Fellowship, administered by the Universities Space Research Association and by the NASA Astrobiology Institute's Alternative Earths and Virtual Planetary Laboratory teams under Cooperative Agreement Nos. NNA15BB03A and NNA13AA93A, respectively. We thank Priya DasSarma for critical reading of the manuscript.











