Over the last three decades, there has been an increase in the number of dairy products available to consumers along with significant growth in fermented dairy products containing probiotic bacteria (Karimi et al., Reference Karimi, Mortazavian and Amiri-Rigi2012; Song et al., Reference Song, Ibrahim, Hayek and Rigobelo2012). At present, dairy products such as yogurt, fermented sour milk, and cheese remain the major probiotic delivery systems. The use of standard media such as MRS (as a laboratory medium) and skim milk and whey-based media (as bulk media) for the cultivation of lactic acid bacteria (LAB) has limitations such as cost effectiveness, preparation, low cell density, and quality control requirements. For example, MRS is considered to be an expensive medium due to its nitrogen source (meat and yeast extract) and does not support the growth of all LAB and probiotic cultures. With regard to bulk media, additional steps are required for preparation and handling, and trained individuals must be involved in the preparation of the starter culture. There are also issues related to these media such as achieving high cell density and additional quality control steps to ensure that LAB reach maximum growth (Ibrahim and Daguri, Reference Ibrahim and Daguri1995, Reference Ibrahim and Daguri1996; Yamani and Ibrahim, Reference Yamani and Ibrahim1996; Burns et al., Reference Burns, Vinderola, Molinari and Reinheimer2008; Djeghri-Hocine et al., Reference Djeghri-Hocine, Boukhemis and Amrane2010; Hayek and Ibrahim, Reference Hayek and Ibrahim2013). The literature reveals significant research aimed at developing new media or finding alternative low cost ingredients to lower costs or to obtain higher cell density (Kurbanoglu, Reference Kurbanoglu2004; Vázquez et al., Reference Vázquez, González and Murado2004a, Reference Vázquez, González and Murado2004b; Djeghri- Hocine et al., 2007a; Aguirre et al., Reference Aguirre, Garro and De Giori2008; Burns et al., Reference Burns, Vinderola, Molinari and Reinheimer2008; Zhang et al., Reference Zhang, Mills and Block2009; Djeghri-Hocine et al., Reference Djeghri-Hocine, Boukhemis and Amrane2010). In addition to increased cell mass production, the cultivation media have a critical role in dairy quality control applications. For quality control, most dairies still rely on conventional cultivation techniques for the enumeration of starter cultures and probiotics (Tharmaraj and Shah, Reference Tharmaraj and Shah2003; Van De Casteele et al., Reference Van De Casteele, Vanheuverzwijn, Ruyssen, Van Assche, Swings and Huys2006; Saccaro et al., Reference Saccaro, Hirota, Tamime and De Oliveira2012; Menon et al., Reference Menon, Shields, Duong and Sturino2013; Davis, Reference Davis2014). These quality control processes include various microbial tests such as differentiation, selective enumeration, isolation, and identification which cannot be achieved using standard media. Consequently, an increasing number of studies have been aimed at modifying standard media in order to cultivate LAB for dairy quality control applications (Ashraf and Shah, Reference Ashraf and Shah2011; Karimi et al., Reference Karimi, Mortazavian and Amiri-Rigi2012; Davis, Reference Davis2014). It is imperative that researchers look to develop a new growth medium with alternative low-cost ingredients that are likely to perform well for such applications.
LAB are the most widely used starter culture in fermented foods, especially dairy products (Peighambardoust et al., Reference Peighambardoust, Tafti and Hesari2011). LAB have the ability to adapt to different environments, which could explain their wide use in the fermentation of diverse food products. During the twentieth century, a significant amount of research related to LAB cultivation media was conducted. In the 1920s, Walter L. Kulp demonstrated that Lactobacillus (Lb.) acidophilus and Lb. bulgaricus grow poorly in a peptone sugar agar medium, so he developed a new medium using tomato juice as the basic ingredient. Since whey and tomato juice agar failed to give consistent results, a medium from dehydrated ingredients, trypticase sugar agar, was created (McLaughlin, Reference McLaughlin1946). Due to the discovery of different essential vitamins and the development of methods for quantitative determination of vitamins and amino acids in the 1940s, it became possible to form a medium with chemically defined components. Following these discoveries, a medium for lactobacilli based on the modification of the original growth medium, BRIGGS (Briggs, Reference Briggs1953), and a medium for streptococci and lactobacilli, LAE (Lactic-Agar-Elliker), were developed (Elliker et al., Reference Elliker, Anderson and Hannesson1956). A number of lactobacilli strains did not grow well in any of these media, so a nonselective medium known as lactobacilli MRS that was able to support the growth of lactobacilli was developed (De Man et al., Reference De Man, Rogosa and Sharpe1960). Many strains of LAB grow well at 42–43 °C and give higher recoveries when enumerated using modified MRS media (Mullan, Reference Mullan, Batt and Tortorello2014). In the 1970s, Lowrie and Pearce observed that not all LAB strains, and especially streptococci, were able to grow well in MRS media, so an alternative medium, M16, was developed (Lowrie and Pearce, Reference Lowrie and Pearce1971). However, due to the rapid decline of pH due to the growth of streptococci in M16, led to the development of a new medium called M17 with improved buffering capacity (Terzaghi and Sandine, Reference Terzaghi and Sandine1975). Since then, MRS and M17 have remained the most commonly used standard media, exhibiting consistent growth for LAB.
The purpose of the present review is thus to summarize research efforts that have focused on the search for alternative low-cost ingredients for the development of cultivation media that are suitable for dairy quality control applications. Topics related to LAB cultivation media including ingredients that are used to form LAB media, standard cultivation media of LAB, and chemically defined media are also briefly reviewed.
Composition of cultivation media
Bacteria in general require an appropriate biochemical and biophysical environment in order to grow. The biochemical environment is made available as a culture medium that contains appropriate amounts of nutrients. LAB is a group of fastidious bacteria that require rich, complex cultivation media for normal growth and cannot grow on simple mineral media supplemented with only a carbon source (Hébert et al., Reference Hébert, Raya, De Giori, Walker, Spencer and Ragout de Spencer2004a, Reference Hébert, Raya and De Giori2004b; Vera Pingitore et al., Reference Vera Pingitore, Hebert, Sesma and Nader-Macías2009). In addition to carbohydrates (simple sugars such as dextrose, sucrose, maltose, or lactose), LAB cultivation media normally contain various nitrogen sources (such as peptone, yeast extract, beef extract, or whey protein), minerals (mainly Mn2+ and Mg2+), and buffering agents (such as sodium acetate and di-sodium-glycerophosphate) (Hébert et al., Reference Hébert, Raya, De Giori, Walker, Spencer and Ragout de Spencer2004a, Reference Hébert, Raya and De Giori2004b; John et al., Reference John, Nampoothiri and Pandey2007; Djeghri-Hocine et al., Reference Djeghri-Hocine, Boukhemis, Zidoune and Amrane2007a; Adebayo-Tayo and Onilude, Reference Adebayo-Tayo and Onilude2009; Dicks and Endo, Reference Dicks and Endo2009; Hayek and Ibrahim, Reference Hayek and Ibrahim2013). Simple carbohydrates or sugars are the primary sources of carbon and energy for bacteria growth (Deutscher, Reference Deutscher2008; O'donnell et al., Reference O'donnell, Forde, Neville, Ross and O'toole2011). Carbon and energy can also be obtained from other organic components such as nitrogen sources. Glycerol was also suggested as a good carbon and energy source for some LAB species such as Lactobacillus reuteri (Lb. reuteri) (Da Silva et al., Reference Da Silva, Mack and Contiero2009). Dextrose (glucose) is the most commonly used sugar for bacterial growth, while lactose, the principal sugar in dairy product, is hydrolyzed relatively slowly and is one of the least utilized forms of sugars by lactobacilli strains (Srinivas et al., Reference Srinivas, Mital and Garg1990).
Most LAB require a wide range of growth factors. Nitrogen sources such as peptones, beef extract and yeast extract are sources of amino acids, peptides, nucleic acid derivatives, minerals and vitamins (Aasen et al., Reference Aasen, Møretrø, Katla, Axelsson and Storrø2000; Lechiancole et al., Reference Lechiancole, Ricciardi and Parente2002; Aspmo et al., Reference Aspmo, Horn and Eijsink2005; Horn et al., Reference Horn, Aspmo and Eijsink2005). Peptones are most often obtained by enzymatic digestion or the acid hydrolysis of natural products such as animal tissues, milk, plants, or microbial cultures. In the dairy industry, milk, skim milk powder, whey protein, and reconstituted whey are more commonly used as peptides and amino acids sources. More than one type of nitrogen source is usually included in LAB media. Manganese sulfate (MnSO4·5H2O) and magnesium sulfate (MgSO4·7H2O) are also typically included in LAB cultivation media in trace amounts. Mn2+ is essential for the growth and metabolic activity of most microorganisms, including LAB. In addition, Mn2+ is an important element that acts against endogenous oxygen radicals for some LAB and is responsible for the catalytic scavenging of O2 that is necessary for anaerobic growth. Similarly, Mg2+ can stimulate the growth and improve survival of LAB. It was shown that Mg2+ is the only essential oligo-element necessary for the growth of Lb. delbrueckii ssp. lactis (Hébert et al., Reference Hébert, Raya, De Giori, Walker, Spencer and Ragout de Spencer2004a, Reference Hébert, Raya and De Giori2004b), and it is also an essential metal ion for the growth of Streptococcus thermophilus (St. thermophilus) (Letort and Juillard, Reference Letort and Juillard2001).
Buffering agents in the cultivation media of LAB are required in order to maintain the pH at optimum levels due to the production of acid during bacterial growth. Sodium acetate (CH3COONa), trisodium citrate (Na3C6H5O7), or Di-sodium-glycerophosphate (C3H7Na2O6P) are commonly used buffers in LAB media. Sodium acetate was found to support the growth of Lb. strains (De Man et al., Reference De Man, Rogosa and Sharpe1960; Snell, Reference Snell1989), and omitting sodium acetate lowered the growth of Lb. plantarum due to the rapid decline in pH (Sawatari et al., Reference Sawatari, Hirano and Yokota2006). Di-sodium-glycerophosphate was included in M17 due to its buffering capacity that can maintain the pH above 5.7 during 24 h of St. growth (Terzaghi and Sandine, Reference Terzaghi and Sandine1975). Other components that have been used in LAB media with buffering activity include disodium phosphate (Na2HPO4), ammonium citrate (NH4C6H5O7), trisodium phosphate (Na3PO4), potassium biphosphate (KH2PO4), magnesium phosphate tribasic Mg3(PO4)2, calcium carbonate (CaCO3), and dipotassium phosphate (K2HPO4) (Hayek et al., Reference Hayek, Shahbazi, Awaisheh, Shah and Ibrahim2013).
Surfactants such as lecithin or Tweens can also be used in LAB media. Surfactants protect cells against harsh conditions, improve nutrient uptake and enhance the growth of LAB. Tweens such as Tween 80 and 85 contain oleic acid, and Tween 20 contains lauric acid. Fatty acids such as these enhance the growth of LAB (Jenkins and Courtney, Reference Jenkins and Courtney2003). Not all surfactants are important for LAB growth, although Tween 80 is well known for its ability to enhance the growth of most LAB genera (Kaneko et al., Reference Kaneko, Suzuki and Takahashi1987; Jenkins and Courtney, Reference Jenkins and Courtney2003; Ibrahim et al., Reference Ibrahim, Ahmed and Song2009). It has been reported that Tween 80 has a significant effect on recovery ability (Li et al., Reference Li, Zhang, Du, Han, Yi, Guo, Zhang, Luo, Zhang, Shan and Hou2011), bile tolerance (Kimoto et al., Reference Kimoto, Ohmomo and Okamoto2002; Ibrahim et al., Reference Ibrahim, Ahmed and Song2009), and metabolic activity (Lechiancole et al., Reference Lechiancole, Ricciardi and Parente2002) of LAB.
Standard media
During the first half of the twentieth century, there was extensive research on the nutritional requirements and growth media of LAB. After the discovery of required nutrients that can support the growth of LAB, it became possible to form a medium with defined components. As a result, the lactobacillus MRS was developed in the 1960s to serve the selective cultivation of Lb. spp. (De Man et al., Reference De Man, Rogosa and Sharpe1960). In the 1970s, it was observed that streptococci cannot grow well in MRS, so M16 was developed and later improved to form the present day M17 in order to serve the selective cultivation of St. spp. (Terzaghi and Sandine, Reference Terzaghi and Sandine1975). Compositions of original and commercially available MRS and M17 are shown in Tables 1 and 2. Some commercial suppliers have made slight modifications to the original compositions of MRS and M17 in order to better comply with industrial requirements. For example, commercial MRS broth from Difco contains proteose peptone No. 3, which is an enzymatic digest of animal tissue (bovine and porcine protein). This ingredient was developed as an alternative nitrogen source in dehydrated culture media to accommodate various nutritional requirements of LAB. Other non-animal protein sources such as phytone peptone have also been shown to support the growth of LAB (Atilola et al., Reference Atilola, Gyawali, Aljaloud and Ibrahim2015). Phytone peptone is an ultra-filtered enzymatic digest of soybean meal and has a high buffering capacity that helps to maintain the pH level during the fermentation process. This protein has been shown to enhance growth and increase the cell mass of Lb. reuteri (Atilola et al., Reference Atilola, Gyawali, Aljaloud and Ibrahim2015). The primary ingredient in M17 is tryptone peptone which is a pancreatic digest of casein that is recommended for the rapid growth and detection of microorganisms present in low concentrations. The original M17 medium contains polypeptone which is a mixture of peptones having a high amino acid content and small polypeptides characteristic of pancreatic digest of casein plus larger polypeptides characteristic of the peptic digest of animal tissue. As listed in Tables 1 and 2, both media contain other ingredients as sources of carbon, nitrogen, vitamins, and minerals. Tween 80, acetate, magnesium and manganese provide growth factors for culturing a variety of lactobacilli. Yeast extract supplies B-complex vitamins which stimulate bacterial growth. Disodium- β-glycerophosphate buffers the medium as acid is produced from the fermentation of lactose. Ascorbic acid stimulates the growth of lactic streptococci, and magnesium sulfate provides essential ions for growth. It is also possible that these ingredients may inhibit the growth of some organisms other than lactobacilli in MRS as well as M17.
Table 1. Chemical compositions of original and commercial lactobacilli MRS broth

a Composition of original MRS was obtained from De Man et al. (Reference De Man, Rogosa and Sharpe1960) and compositions of commercial MRS were obtained from the corresponding supplier.
Table 2. Chemical compositions of original and commercial M17 broth

a Composition of original M17 was obtained from Terzaghi and Sandine (Reference Terzaghi and Sandine1975) and compositions of commercial M17 were obtained from the corresponding supplier.
Other media such as APT (All Purpose Tween 80) and St. thermophilus agar (ST) can also be used to isolate Lb. and St., respectively. MRS is not completely selective; consequently, much research effort has been dedicated to finding alternative selective media (Menon et al., Reference Menon, Shields, Duong and Sturino2013; Davis, Reference Davis2014). In addition, other genera of LAB such as Leuconostoc (Ln.), Pediococcus, Enterococcus and Weissella can grow in MRS. Members of Lb., Ln., Pediococcus, and Weissella share similar physiological properties and generally respond to conditions or inhibitory compounds in similar ways (Schillinger and Holzapfel, Reference Schillinger, Holzapfel, Corry, Curtis and Baird2011). Ln. can grow well on MRS, but other media such as Rogosa, yeast extract glucose citrate, and tetrazolium-sucrose (TS) medium are commonly used to grow these genera. Bille et al. (Reference Bille, Espie and Mullan1993) evaluated the effectiveness of MRS with vancomycin for isolating Ln. spp. from fermented dairy products containing lactococci such as Lactococcus lactis (Lc. lactis), Lc. diacetylactis and non-starter LAB such as lactobacilli and pediocci. These authors reported the poor selectivity of the medium toward the recovery of leuconostocs. This could be attributed to bacterial resistance to vancomycin. M17 has been widely used for the selective enumeration of Lc. spp. with or without lactose supplementation (Karimi et al., Reference Karimi, Mortazavian and Amiri-Rigi2012). Consequently, MRS and M17 are not selective media, but are standard, commonly used media that have been widely used to serve the growth of most genera of LAB including: Lb., St., Ln., Lc., Pediococcus, Enterococcus, Weissella, Aerococcus, and Oenococcus (Carr et al., Reference Carr, Chill and Maida2002; Schillinger and Holzapfel, Reference Schillinger, Holzapfel, Corry, Curtis and Baird2011). Due to differences in the nutritional requirements among LAB species, such standard media cannot provide optimum growth conditions for all genera of LAB; consequently, a large number of media was proposed to serve the cultivation of LAB. Table 3 shows some examples of commonly used standard cultivation media for LAB. MRS has been used widely as a general culture medium as well as a basal medium to perform different tests such as temperature, pH, alcohol, salt, and Teepol concentration (Carr et al., Reference Carr, Chill and Maida2002). Modification of MRS to obtain selectivity for some groups of LAB, particularly for microbial analysis in dairy applications, has also been a topic of research interest among food scientists (Ashraf and Shah, Reference Ashraf and Shah2011; Karimi et al., Reference Karimi, Mortazavian and Amiri-Rigi2012; Davis, Reference Davis2014). In a recent paper, Nwamaioha and Ibrahim (Reference Nwamaioha and Ibrahim2018) found that reinforced clostridial media supplemented with 0.025% CaCl2, 0.01% uracil, 0.2% Tween 80 and 0.01% aniline blue dye was superior to the standard MRS medium with regard to enumeration and differentiation of Lb. bulgaricus in mixed LAB cultures. More details on the applications of MRS as a basal medium are provided in the dairy section below.
Table 3. Common culture media used for detection, isolation, and cultivation of LAB

Alternative low-cost ingredients
Lab have a high demand for diversified nitrogen sources. To meet these demands, peptone, tryptone, beef extract and yeast extract are included in the standard media of LAB. Nitrogen sources are relatively expensive and are thus the main contributors to the high cost of LAB cultivation media (Macedo et al., Reference Macedo, Lacroix, Gardner and Champagne2002; Kurbanoglu, Reference Kurbanoglu2004; Djeghri-Hocine et al., Reference Djeghri-Hocine, Boukhemis, Zidoune and Amrane2007a, Reference Djeghri-Hocine, Boukhemis, Zidoune and Amrane2007b; Burns et al., Reference Burns, Vinderola, Molinari and Reinheimer2008; Yu et al., Reference Yu, Lei, Ren, Pei and Feng2008; Hayek et al., Reference Hayek, Shahbazi, Awaisheh, Shah and Ibrahim2013; Ayad et al., Reference Ayad, El-Rab, Shahbazi, Worku, Schimmel, Ejimakor, Zimmerman and Ibrahim2016). The use of standard cultivation media is primarily limited to quality control, laboratory analysis, research studies, and academic purposes. As a result, scientists and industries (especially in dairy and probiotics) are actively searching for low cost products that can replace expensive ingredients and support the growth and cell mass production of LAB. A lot of widely available agricultural waste such as crop residues, woody materials, and food by-products have potential economic and environmental benefits and are being considered for the cultivation of LAB (Wang et al., Reference Wang, Tashiro and Sonomoto2015). These products can also be used for lactic acid production, the primary commercial product of LAB (Kurbanoglu, Reference Kurbanoglu2004; Yu et al., Reference Yu, Lei, Ren, Pei and Feng2008), production of beneficial compounds (Macedo et al., Reference Macedo, Lacroix, Gardner and Champagne2002), cell mass production (Champagne et al., Reference Champagne, Savard and Barrette2009; Zhen-Hua, Reference Zhen-Hua2009; Ziadi et al., Reference Ziadi, Rezouga, Bouallagui, Baâti, Othman, Thonart and Hamdi2010; Krzywonos and Eberhard, Reference Krzywonos and Eberhard2011), or as alternatives to develop low cost media (Macedo et al., Reference Macedo, Lacroix, Gardner and Champagne2002; Djeghri-Hocine et al., Reference Djeghri-Hocine, Boukhemis, Zidoune and Amrane2007a; Burns et al., Reference Burns, Vinderola, Molinari and Reinheimer2008; Hayek et al., Reference Hayek, Shahbazi, Awaisheh, Shah and Ibrahim2013). With these aims, several studies were carried out. A group of these studies is listed in Table 4. Since MRS is the most commonly used standard medium for LAB, most of the proposed media were compared with MRS as the benchmark. Many of the tested products have shown significant improvement in LAB growth and functionality compared to MRS when supplemented with small amounts of external nitrogen sources (Burns et al., Reference Burns, Vinderola, Molinari and Reinheimer2008; Yu et al., Reference Yu, Lei, Ren, Pei and Feng2008; Ziadi et al., Reference Ziadi, Rezouga, Bouallagui, Baâti, Othman, Thonart and Hamdi2010; Hayek et al., Reference Hayek, Shahbazi, Awaisheh, Shah and Ibrahim2013).
Table 4. Studies on evaluation of agricultural waste and food byproducts as alternatives that can be used to serve the growth of LAB compared to MRS
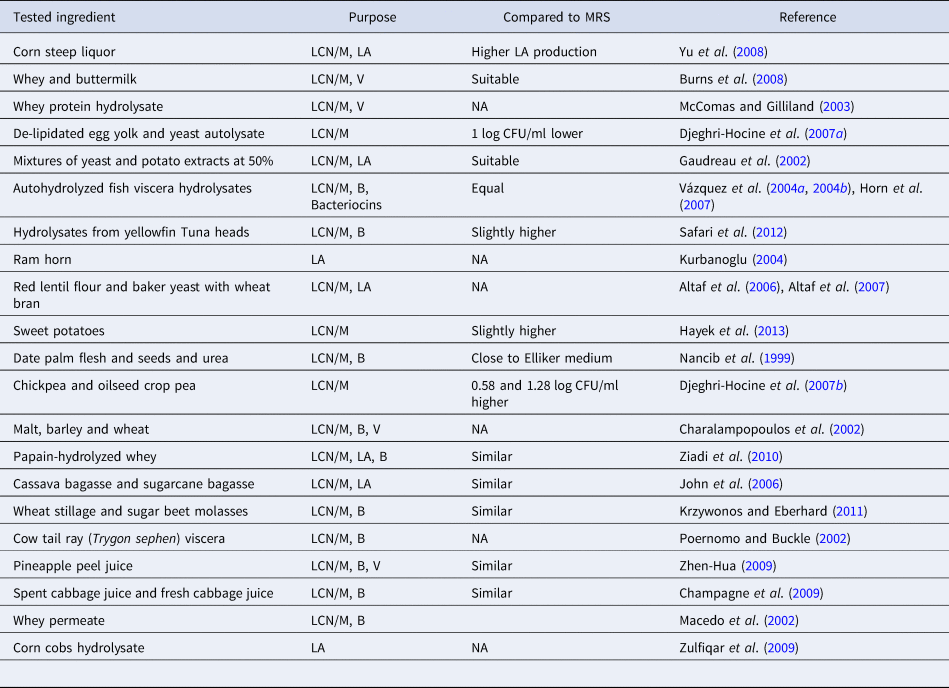
LCN/M, low cost nitrogen source/medium; LA, lactic acid production; B, cell biomass production; V, enhancing cells viability.
Low cost nitrogen sources can be obtained from fish processing byproducts, slaughtering byproducts, plant products, agricultural waste, and dairy industry byproducts. For example, the byproducts of fish processing (heads, viscera, chitinous material, wastewater, etc.) are excellent nutrient sources for microbial growth (Vázquez et al., Reference Vázquez, González and Murado2004a, Reference Vázquez, González and Murado2004b; Safari et al., Reference Safari, Motamedzadegan, Ovissipour, Regenstein, Gildberg and Rasco2012; Rebah and Miled, Reference Rebah and Miled2013). These byproducts are also useful in the production of enzymes such as protease, lipase, chitinolytic and ligninolytic enzymes (Rebah and Miled, Reference Rebah and Miled2013). For instance, the enzymatic hydrolysis of yellowfin tuna (Thunnus albacares) head is an excellent source of nitrogen and is effective in promoting the growth of LAB (Safari et al., Reference Safari, Motamedzadegan, Ovissipour, Regenstein, Gildberg and Rasco2012). Peptones obtained from the enzymatic hydrolysis of yellowfin tuna were found to be better at promoting LAB growth than MRS. Autohydrolyzed fish viscera promote the production of biomass and bacteriocin that equals or surpasses that obtained with MRS (Vázquez et al., Reference Vázquez, González and Murado2004a, Reference Vázquez, González and Murado2004b). The highest cell biomass production of LAB was obtained at low hydrolysis times with the hydrolysis pH having a limited influence on the final levels (Vázquez et al., Reference Vázquez, González and Murado2004a, Reference Vázquez, González and Murado2004b). Cowtail ray (Trygon sephen), a waste product in the fish industry, was converted into low cost microbiological peptones (Poernomo and Buckle, Reference Poernomo and Buckle2002). This process included ensilation using a 3% (v/w) mixture of propionic and formic acids (1 : 1, v/v), followed by vacuum evaporation to prepare crude liquid peptones from cowtail ray viscera. The microbial growth in crude peptones from cowtail ray viscera was similar to, or even surpassed that of commercial peptones (Poernomo and Buckle, Reference Poernomo and Buckle2002). It was also reported that the proteolytic enzyme used to hydrolyze the fish waste had a significant impact on the performance of the resultant hydrolysates (Poernomo and Buckle, Reference Poernomo and Buckle2002; Vázquez et al., Reference Vázquez, González and Murado2004a, Reference Vázquez, González and Murado2004b; Safari et al., Reference Safari, Motamedzadegan, Ovissipour, Regenstein, Gildberg and Rasco2012). Corn steep liquor was used to completely replace yeast extract, and the optimized medium resulted in a 30.4% higher lactic acid production than yeast extract medium (Yu et al., Reference Yu, Lei, Ren, Pei and Feng2008). Wheat bran was used for the production of lactic acid by Lb. amylophilus GV6 where peptone and yeast extract were completely replaced by inexpensive red lentil flour and baker's yeast in a modified MRS medium (Altaf et al., Reference Altaf, Naveena and Reddy2007). This fermentation resulted in a 92% lactic acid yield efficiency, indicating that such growth media could be used as an economic source for the production of lactic acid using LAB. In another comparison study with malt, barley, and wheat on the growth and metabolic activity of LAB, the malt medium supported the growth of all LAB strains more than the other two cereals (Charalampopoulos et al., Reference Charalampopoulos, Pandiella and Webb2002).
Cassava bagasse and sugarcane bagasse in low cost production media were used to enhance the fermentative production of L-lactic acid (John et al., Reference John, Nampoothiri and Pandey2006), offering a cost-effective technology to scale up lactic acid production. The authors demonstrated that Lb. delbrueckii NCIM 2025 was able to grow in a sugarcane bagasse and effectively utilize the medium's available sugar. The results of this study showed that the normal growth of LAB can be supported with minimum supplementation of ammonium salt, yeast extract, and cassava hydrolysate prepared from bagasse as the only carbon source. Similarly, the feasibility of using a vegetal substrate as a protein source to replace nitrogen sources such as yeast extract, beef extract, and peptones for the growth of LAB has been studied. For example, horse bean extract was used to replace peptone and beef extract in the developed medium for the cultivation of LAB (Djeghri-Hocine et al., Reference Djeghri-Hocine, Boukhemis, Zidoune and Amrane2006). The tested Lb. strains showed higher bacterial populations in the horse bean extract medium than in MRS and M17. The authors indicated that horse bean extract was a cost-effective medium for the growth of LAB species isolated from plants. Oilseed crop pea and chickpea were likewise used to replace expensive nitrogen sources in a LAB cultivation medium (Djeghri-Hocine et al., Reference Djeghri-Hocine, Boukhemis, Zidoune and Amrane2007b). The developed medium was supplemented with small amounts of yeast extract, meat extract or tryptic casein peptone, 5 g/l. The authors indicated that a vegetal substrate (pea) is efficient for the growth of LAB species isolated from plants such as Lb. plantarum. For example, oilseed crop pea and chickpea-based media showed higher bacterial populations than MRS. These plants are rich in protein and thus can be used to partially replace the expensive nitrogen sources that are typically used in LAB media (Djeghri-Hocine et al., Reference Djeghri-Hocine, Boukhemis, Zidoune and Amrane2007b). A combination of sieved wheat stillage and a sugar production byproduct, molasses, was suggested for the low cost, industrial production of Lb. plantarum (Krzywonos and Eberhard, Reference Krzywonos and Eberhard2011). In order to allow a high Lb. plantarum cell biomass, sieved wheat stillage containing 10% molasses (v/v) was enriched with 1.77 g/l yeast extract and NH4OH for pH adjustment. Other plant products such as pineapple peel juice (Zhen-Hua, Reference Zhen-Hua2009), spent cabbage juice and fresh cabbage juice (Champagne et al., Reference Champagne, Savard and Barrette2009), and corn cob hydrolysates (Zulfiqar et al., Reference Zulfiqar, Anjum and Zahoor2009) were also suggested as novel low-cost alternatives for the cultivation and biomass production of LAB. However, supplementation of plant products with external nitrogen sources at low concentrations (5 g/l) was required in most studies in order to achieve results similar to those with MRS. Recently, Campanella et al. (Reference Campanella, Rizzello and Fasciano2017) used grape marc as a substrate for the growth LAB (Lb. plantarum 12A and PU1, Lb. paracasei 14A). Grape marc media was prepared with 0.4% yeast extract in a 0.1% peptone solution (pH 6.0). The authors found the highest bacterial cell density (>9.0 log CFU/g) in the presence of 1% glucose. This study demonstrated that grape marc fermented by LAB strains could be used as a functional food dietary supplement or for other dairy applications.
Exopolysaccharides (EPSs)-producing LAB are industrially important microorganisms that are used as starter cultures in the manufacture of fermented milk. In particular, EPS producing bacteria are essential for the proper consistency and texture of yogurt (Gyawali and Ibrahim, Reference Gyawali and Ibrahim2016). As a result, these polysaccharides are known to improve the rheological properties of LAB-fermented products by directly influencing viscosity, syneresis, firmness and sensory attributes. Less expensive substrates such as waste or by-products of food processing or agro-industry can also be utilized for EPS production in LAB leading to a significant decrease in production costs. These lower priced substrates are generally either in liquid form such as syrups, molasses, juices, cheese whey, and olive mill wastewater or solid-like lignocellulosic biomass and pomaces (Özcan and Öner, Reference Özcan, Öner, Ramawat and Merillon2015). For example, the common fermentation process known as submerged fermentation is carried out in liquid media. However, substrates used in solid-state fermentation processes utilize by-products, or the waste of the agro-industrial, forestry, or food processing industry. These solid-state substrates have been recently explored for use in the production of biopolymers such as EPSs whose yields are comparable to those obtained from conventional submerged cultivation (Özcan and Öner, Reference Özcan, Öner, Ramawat and Merillon2015). These substrates can also be utilized for the production of EPSs from LAB. Huang et al. (Reference Huang, Huang, Kao and Fang2017) evaluated the effects of orange peel fiber powder on the EPS production of Lb. plantarum SLC 13. Maximum SLC 13 EPS production was observed when 2% of peel fiber pretreated with 3 N HCl was added to the MRS medium. The results showed that orange peel fiber powder promotes the growth, EPS production and antibacterial property of Lb. plantarum SLC 13, suggesting that such fruit peel fibers have potential as a more affordable growth substrate in industrial dairy applications. In our earlier study, we developed a sweet potato based medium (SPM) supplemented with different nitrogen sources. Our results showed that Lb. spp. grown in SPM media was found to be similar to that of standard MRS (Hayek et al., Reference Hayek, Shahbazi, Awaisheh, Shah and Ibrahim2013). In addition, the enzymatic activity of Lb. growing in the SPM was higher than that in MRS (Hayek et al., Reference Hayek, Shahbazi, Awaisheh, Shah and Ibrahim2013). These results indicate that SPM can be a suitable medium for the growth of Lb. and is also a lower cost alternative to MRS.
In the dairy industry, several dairy byproducts that are rich sources of nitrogen and nutrients were suggested to support the growth of LAB at low cost. Acid whey, a dairy by-product, is often disposed of as a waste and causes significant environmental contamination (Gyawali and Ibrahim, Reference Gyawali and Ibrahim2016). Whey is rich in protein, organic acids, lactose, vitamins, and minerals. Thus, whey can be utilized as a growth medium for several LAB species in dairy applications. Whey and a lupin-hydrolysates medium supplemented with 1.56 ml/l Tween 80 provided good growth of Lb. fermentum DSM 20049. Based on a comparison of Lb. fermentum DSM 20049 growth on this formulated medium with that observed on a standard MRS medium, the growth of the strain in the formulated whey medium was found to be higher than that of MRS (Hanoune et al., Reference Hanoune, Djeghri-Hocine, Kassas, Derradji, Boudour and Boukhemis2015). These results thus demonstrated that whey lupin could also be used as a lower cost alternative source of nitrogen for the cultivation of LAB in industrial applications.
Whey hydrolysis can improve the availability of amino acids and other nutrients and thus better serve the growth of LAB. A whey permeated medium supported the biomass production of LAB without external supplementation with nitrogen sources (Macedo et al., Reference Macedo, Lacroix, Gardner and Champagne2002). The effect of whey hydrolyzed by papain on the growth of LAB was also studied (Ziadi et al., Reference Ziadi, Rezouga, Bouallagui, Baâti, Othman, Thonart and Hamdi2010). The highest biomass production was observed after 30 min of hydrolysis at pH 5.0 and 60 °C. Whey and buttermilk were also used to serve the growth of LAB (Burns et al., Reference Burns, Vinderola, Molinari and Reinheimer2008). However, supplementation with external nitrogen sources at a low concentration of 0.3% yeast extract was necessary in order to achieve satisfactory growth of LAB when compared to MRS. Milk can also provide a suitable medium for LAB. The growth of probiotic LAB in milk can be improved by supplementing milk with whey protein hydrolysate (McComas and Gilliland, Reference McComas and Gilliland2003). A partially deproteinized whey medium containing 1% peptone that is prepared by adjusting the pH of skim milk to 4.6 and removing the precipitate by centrifugation can provide a higher cell biomass compared to that of MRS (Adebayo-Tayo and Onilude, Reference Adebayo-Tayo and Onilude2009). Thus, hydrolysis of dairy byproducts is recommended for suitable growth and biomass production of LAB.
Cultivation media in the dairy industry
Fermented dairy products have become increasingly popular among consumers because these products are easily accessible, nutritious, stable, natural, and healthy. Strains of LAB that are used in dairy starter culture applications are primarily homofermentative and basically produce lactic acid. In some fermented dairy products, additional bacteria that are referred to as secondary microflora are added to produce carbon dioxide in order to enhance the flavor and texture of the final product (Rose, Reference Rose1981). The dairy industry obtains starter cultures from commercial sources that are typically in liquid, frozen, spray dried, or lyophilized form (Carvalho et al., Reference Carvalho, Silva, Ho, Teixeira, Malcata and Gibbs2004). In general, starter cultures are available in the form of freeze-dried granules and frozen pellets. Drying is the traditional method for dairy starter culture delivery and preservation and includes spray and freeze drying. Earlier, commercial starter cultures in liquid form were employed. However, the use of dried starter culture eliminates the plant subculturing step, reduces the costs associated with bulk culture preparation and lowers the risk of bacteriophage infection (Santivarangkna et al., Reference Santivarangkna, Kulozik and Foerst2007; Peighambardoust et al., Reference Peighambardoust, Tafti and Hesari2011). Microbial cell survival throughout the drying and storage phases depends on the initial concentration of microorganisms, growth conditions, growth medium, drying medium, and rehydration conditions (Carvalho et al., Reference Carvalho, Silva, Ho, Teixeira, Malcata and Gibbs2004). Protective substrates added to the cultivation media can enhance the viability of dried starter cultures which are the most widely used in the dairy industry (Peighambardoust et al., Reference Peighambardoust, Tafti and Hesari2011). Before drying, a high cell density is required. This density is usually obtained using bulky culture media such as a semi-synthetic liquid medium, skim milk medium, or cheese whey-based medium (Sawatari et al., Reference Sawatari, Hirano and Yokota2006). However, many dairies still prepare their own bulk starters using mother cultures.
The skim milk medium is the most frequently used medium for starter culture production in the dairy industry. Reconstituted skim milk medium or fresh milk are also used as alternatives. Mn2+ is usually included in the media to promote the growth of certain LAB genera such as Lb. and Ln. Phage inhibiting media, which contain phosphates, citrates, or other chelating agents can also be used for the production of single or multiple strain cultures. A skim milk medium with phage inhibiting capacity is also available for starter production (Bylund, Reference Bylund2003). Whey permeate supplemented with salts and a nitrogen source has also been used for the biomass production of LAB in the dairy industry (Macedo et al., Reference Macedo, Lacroix, Gardner and Champagne2002). Due to the fastidious nutritional requirements of LAB, the dairy industry must pay more attention to the media composition. Media composition can influence functionality, metabolic activity, biomass growth, cell viability, and lactic acid production (Macedo et al., Reference Macedo, Lacroix, Gardner and Champagne2002; Adebayo-Tayo and Onilude, Reference Adebayo-Tayo and Onilude2009; Peighambardoust et al., Reference Peighambardoust, Tafti and Hesari2011; Hayek and Ibrahim, Reference Hayek and Ibrahim2013; Bulatović et al., Reference Bulatović, Rakin, Vukašinović-Sekulić, Mojović and Krunić2014). For example, the viable cell count and biomass production of Lb. johnsonii growing in a reconstituted whey medium can vary significantly according to the various combinations of yeast extract, inulin, sucrose, and pyridoxal (Bulatović et al., Reference Bulatović, Rakin, Vukašinović-Sekulić, Mojović and Krunić2014).
Fermented dairy products are considered to be the most suitable vehicles for probiotic bacteria. This is due to the fact that dairy products provide a suitable environment for probiotic bacteria, support the growth and viability of probiotics, and most milk and milk products are stored at refrigerated temperatures (Song et al., Reference Song, Ibrahim, Hayek and Rigobelo2012). Due to the growing interest in probiotic strains, a significant number of dairy products contain both starter cultures and probiotics. In order to ensure that a minimal number of probiotic bacteria is present in the end product, reliable methods for routine enumeration are warranted. Often, dairies that rely on conventional methods for the enumeration of starter cultures and probiotics may require rapid and other reliable techniques for routine enumeration in order to ensure a high quality end product (Tharmaraj and Shah, Reference Tharmaraj and Shah2003; Van De Casteele et al., Reference Van De Casteele, Vanheuverzwijn, Ruyssen, Van Assche, Swings and Huys2006; Saccaro et al., Reference Saccaro, Hirota, Tamime and De Oliveira2012; Menon et al., Reference Menon, Shields, Duong and Sturino2013). Such routine enumeration is also essential in order to monitor changes in the probiotic bacterial population during storage. Table 5 lists a group of studies aimed at developing suitable media for dairy quality control. Ingredients such as fructose, sucrose, maltose, bile, sorbitol, raffinose, HCl, or antibiotics have been used for these purposes. For example, L-cysteine hydrochloride can be added to MRS at a low concentration (0.05–0.1%, w/v) to support microaerophilic conditions and to favor the isolation of anaerobic species of LAB (Hartemink et al., Reference Hartemink, Domenech and Rombouts1997; Simpson et al., Reference Simpson, Fitzgerald, Stanton and Ross2006; Dicks and Endo, Reference Dicks and Endo2009). More details on the selective enumeration of probiotic bacteria in dairy products were previously reviewed (Ashraf and Shah, Reference Ashraf and Shah2011; Karimi et al., Reference Karimi, Mortazavian and Amiri-Rigi2012).
Table 5. A list of developed media for enumeration, differentiation, selective enumeration, isolation, and identification of starter cultures and probiotic bacteria in dairy products

Microbial quality, probiotic viability, and starter culture functionality tests require selective or differential media that can provide reliable, consistent results. While several media are considered to be selective or differential, it is also necessary to confirm that bacterial counts from the same yogurt sample do not vary with different media. In a comparison among different selective media including MRS-Sorbitol, LC, MRS-Bile, and various Bifidobacterium (Bb.) selective media for their suitability to provide reliable counts for Lb. acidophilus, Bb. spp. and L. casei in yogurt, no selective or differential medium was able to provide reliable counts of probiotic bacteria in yogurt (Talwalkar and Kailasapathy, Reference Talwalkar and Kailasapathy2004). A comparison of Lb. Anaerobic MRS with vancomycin and bromocresol green (LAMVAB) and Rogosa agar for the detection and enumeration of Lb. spp. revealed that neither medium is fully accurate in its representation of Lb. (Jackson et al., Reference Jackson, Bird and Mcorist2002). In a study that compared the growth of probiotic LAB and starter culture LAB on twenty-one nonselective and selective culture media, Lb. acidophilus, Lb. casei, and Bb. animalis grew in most tested media (de Carvalho et al., Reference de Carvalho Lima, Kruger, Behrens, Destro, Landgraf and De Melo Franco2009). Four of these media were selected for quantitative enumeration of Lb. acidophilus, Lb. casei, and Bb. animalis. LC agar, which is recommended for the differential enumeration of Lb. casei, proved to be less selective than expected since Lb. acidophilus also grew in this medium (de Carvalho et al., Reference de Carvalho Lima, Kruger, Behrens, Destro, Landgraf and De Melo Franco2009). The LC medium was also recommended for the enumeration of Lb. rhamnosus and Lb. paracasei in yogurt products (Van De Casteele et al., Reference Van De Casteele, Vanheuverzwijn, Ruyssen, Van Assche, Swings and Huys2006). In addition, a semi -chemically defined medium called MS for the semi- selective cultivation of Lb. as an alternative to MRS was developed (Menon et al., Reference Menon, Shields, Duong and Sturino2013). MS, a carbohydrate-supplemented medium with low background coloration, was found to exhibit greater semi -selectivity against non -LAB than MRS. Thus, there is still a need to develop a reliable technique to accurately enumerate probiotic bacteria in dairy products. In addition, culturing methods can only measure viable culturable or replicating cells. During processing, some live probiotic cells may enter a viable but non-culturable (VBNC) in which the probiotic cells are dormant but metabolically active (Davis, Reference Davis2014). As a result, the research community has turned toward developing culture-independent methods for microbiological analysis of dairy products such as flow cytometry (Díaz et al., Reference Díaz, Herrero, García and Quirós2010; Geng et al., Reference Geng, Chiron and Combrisson2014) and polymerase chain reaction (Boyer and Combrisson, Reference Boyer and Combrisson2013). These methods offer the potential to enumerate both culturable and VBNC bacteria.
Research studies related to LAB cultivation media have also been done using laboratory media and laboratory strains of LAB that were sometimes endowed with plasmid(s) that enabled growth in a milk-based medium (Steele et al., Reference Steele, Broadbent and Kok2013). The use of a milk-based medium or food grade medium in such research could help to bridge the gap between laboratory research and the dairy industry. As a result, it might be appropriate to develop a medium based on milk or dairy byproducts for dairy research applications.
Chemically defined media
Cultivation media can be either undefined (or contain complex natural ingredients with a largely undefined composition) or chemically defined (synthetic) and composed of pure chemicals in precisely known proportions (Zhang and Greasham, Reference Zhang and Greasham1999; Menon et al., Reference Menon, Shields, Duong and Sturino2013). A semi-defined medium is also available and is composed of defined components with one or two complex nutrients. A minimal medium contains only nutrients that satisfy the minimal nutritional requirements for bacteria growth (Zhang and Greasham, Reference Zhang and Greasham1999; Wegkamp et al., Reference Wegkamp, Teusink, DeVos and Smid2010). Standard media are largely undefined and are not optimum for high cell density production. Determining the appropriate nutrients at appropriate concentrations can result in maximum levels of biomass production and could eliminate unnecessary elements (Hébert et al., Reference Hébert, Raya, De Giori, Walker, Spencer and Ragout de Spencer2004a, Reference Hébert, Raya and De Giori2004b). Consequently, a chemically defined medium (CDM) that can eliminate unrequired nutrients and are capable of supporting the growth of nutritionally-fastidious LAB for high cell density production has been targeted in several studies (Foucaud et al., Reference Foucaud, Francois and Richard1997; Letort and Juillard, Reference Letort and Juillard2001; Hébert et al., Reference Hébert, Raya, De Giori, Walker, Spencer and Ragout de Spencer2004a, Reference Hébert, Raya and De Giori2004b; Zhang et al., Reference Zhang, Mills and Block2009; Wegkamp et al., Reference Wegkamp, Teusink, DeVos and Smid2010). CDM has also been gaining more interest for use in commercial fermentation, particularly for biomass production and for the preparation of biological products (Zhang et al., Reference Zhang, Mills and Block2009).
Traditionally, biochemical studies or a single factor approach were used to optimize media by the examination of one nutritional factor at a time (Letort and Juillard, Reference Letort and Juillard2001; Lechiancole et al., Reference Lechiancole, Ricciardi and Parente2002; Hébert et al., Reference Hébert, Raya, De Giori, Walker, Spencer and Ragout de Spencer2004a, Reference Hébert, Raya and De Giori2004b; Wegkamp et al., Reference Wegkamp, Teusink, DeVos and Smid2010). This single factor technique is based on the assumption that components can be omitted without affecting bacterial growth. Using CDM, complicated interactions among complex components in the media can be minimized, and the target metabolite can be easily detected (Hébert et al., Reference Hébert, Raya, De Giori, Walker, Spencer and Ragout de Spencer2004a, Reference Hébert, Raya and De Giori2004b; Teusink et al., Reference Teusink, Van Enckevort, Francke, Wiersma, Wegkamp, Smid and Siezen2005; Zhang et al., Reference Zhang, Mills and Block2009; Wegkamp et al., Reference Wegkamp, Teusink, DeVos and Smid2010). For example, the minimum growth requirements of St. thermophilus for exponential growth were established using CDM (Letort and Juillard, Reference Letort and Juillard2001). This medium included lactose, six amino acids, two metallic ions, six vitamins and urea. Final St. thermophilus populations were approximately 8 log CFU/ml. The authors indicated that St. thermophilus was less demanding on nutrients than other LAB. Consequently, a CDM that supports the high cell density growth of Lc., Enterococcus, and St., was developed (Zhang et al., Reference Zhang, Mills and Block2009). The authors used an experimental leave-one-out technique to determine essential nutrients among 57 components including amino acids, vitamins, minerals, and various other chemicals. The developed CDM thus supported the high-cell-density of Lc. lactis, Enterococcus faecalis, and St. thermophilus. The maximum biomass production of Lc. lactis in the developed medium was more than 50% higher than that of M17. A minimal CDM that can support the sustained growth of Lb. delbrueckii subsp. lactis was developed using the single omission technique (Hébert et al., Reference Hébert, Raya, De Giori, Walker, Spencer and Ragout de Spencer2004a, Reference Hébert, Raya and De Giori2004b). The authors indicated that the developed medium was simple and well defined and should be preferable to complex media for conducting future biochemical, physiological, and genetic studies on Lb. delbrueckii subsp. lactis. The specific growth rate of Lb. delbrueckii subsp. lactis in the medium developed by Hebert and colleagues was 0.31/h. A CDM for the growth of Ln. mesenteroides containing lactose, Mn2+, Mg2+, 12 amino acids, 8 vitamins, adenine, uracil, Tween 80, and potassium phosphate was also developed (Foucaud et al., Reference Foucaud, Francois and Richard1997). This developed medium supported the high growth rate of Ln. mesenteroides and reached 0.85 ± 0.10/h.
The CDM and semi-defined media that were developed to serve the growth of LAB usually provide a greater biomass yield compared to standard media and thus allow an increased focus on metabolism and regulation for research purposes (Lechiancole et al., Reference Lechiancole, Ricciardi and Parente2002; Zhang et al., Reference Zhang, Mills and Block2009). CDMs that were developed in order to study specific characteristics of specific LAB genera or strains typically provide less growth than those obtained with complex media (Zhang et al., Reference Zhang, Mills and Block2009). A minimal growth medium for Lb. plantarum was developed using repetitive single omission experiments but showed a 63% lower specific growth rate compared to MRS (Wegkamp et al., Reference Wegkamp, Teusink, DeVos and Smid2010). The authors eliminated all nutrients that could be omitted without the complete abolishment of growth, so nutrients were minimized and growth was also reduced. A minimal medium such as this can help to determine essential growth factors and to understand growth behaviors yet may not support high cell biomass production. In order to determine the required nutrients and the concentrations of these nutrients, the CDM can be optimized by biochemical studies or by using a single factor approach that examines one nutritional factor at a time (Letort and Juillard, Reference Letort and Juillard2001; Wegkamp et al., Reference Wegkamp, Teusink, DeVos and Smid2010). However, this type of experiment would be inefficient for testing multiple components or for testing important interactions between components in complex media (Zhang et al., Reference Zhang, Mills and Block2009).
In conclusion, LAB are a group of fastidious bacteria that require rich complex media for normal growth. Consequently, the cultivation media of LAB have been extensively studied in order to support growing LAB applications, particularly in the dairy industry. Along with fermented carbohydrates (carbon and energy sources), LAB cultivation media are being supplemented with nitrogen sources, minerals, and buffering agents in addition to Tween 80. Several cultivation media have been developed to serve LAB, but MRS and M17 remain the most commonly used standard media. However, such standard cultivation media have a number of negative attributes that limit their uses for academic purposes including cost, specific preparation steps, and extensive incubation time (Hayek and Ibrahim, Reference Hayek and Ibrahim2013). Thus, the recent literature contains a significant amount of research aimed at developing new media at low cost or to improving the existing media. For example, food byproducts, agriculture products, agriculture wastes, and inexpensive, rich sources of nutrients are receiving more attention in scientific communities as part of an effort to develop low cost cultivation media. These low-cost ingredients could replace, at least partially, the complex and expensive nitrogen supplements ordinarily used for the growth of LAB strains. Even though many low-cost products have been proposed as nitrogen replacement sources in LAB media, the development of appropriate cultivation media for LAB continues to be a timely issue. Thus, more attention from the scientific community is warranted in order to develop a new cultivation medium that can better support the growth and functionality of LAB and meet industry requirements. Although most media were developed using laboratory strains, an alternative medium based on milk or dairy byproducts could help to bridge the gap between laboratory research and the dairy industry.
Acknowledgements
This publication was made possible by grant number NC.X-267-5-12-170-1 from the National Institute of Food and Agriculture (NIFA), by NIZO Food Research BV, The Netherlands and by Jarrow Formulas, USA. The content herein is solely the responsibility of the authors and does not necessarily represent the official view of NIFA.









