Introduction
Sufficiency in vitamin B9 (folate) and vitamin B12 (cobalamin) status in pregnancy is important for normal fetal development. Reference Molloy, Kirke, Brody, Scott and Mills1 Deficiencies of folate and vitamin B12 are associated with adverse outcomes including maternal anaemia and infant neural tube and birth defects. Reference Hanson, Bardsley and De-Regil2 These vitamins play a role in one-carbon metabolism and the remethylation of homocysteine to methionine and associated pathways which generate substrates for DNA synthesis and methyl groups for numerous methylation reactions including those with DNA. Reference Finer, Saravanan, Hitman and Yajnik3,Reference Godfrey, Lillycrop, Burdge, Gluckman and Hanson4
Nutrient deficits or excesses which occur at ‘critical’ periods of development influence gene expression and thus determine future disease risk. Reference Godfrey, Lillycrop, Burdge, Gluckman and Hanson4 Maternal folate status is reported to play a key role in infant DNA methylation in genes associated with developmental pathways, metabolism and regulation of DNA methylation, among others, Reference Joubert, den Dekker and Felix5–Reference Pauwels, Ghosh and Duca7 although the responsible mechanisms are not fully established.
Excess or deficiency of these B vitamins during pregnancy has been linked to a higher risk of developing gestational diabetes mellitus (GDM) in some reports Reference Kouroglou, Anagnostis, Daponte and Bargiota8–Reference Sukumar, Venkataraman and Wilson12 but not others. Reference Barzilay, Moon and Plumptre13–Reference Tarim, Bagis and Kilicdag17 Two found that a combination of folate sufficiency and vitamin B12 deficiency was associated with a higher risk of GDM, Reference Lai, Pang and Cai9,Reference Li, Hou and Yan10 and others showed an association between lower vitamin B12 status and GDM. Reference Kouroglou, Anagnostis, Daponte and Bargiota8,Reference Krishnaveni, Hill and Veena11,Reference Sukumar, Venkataraman and Wilson12 In addition, an inverse correlation was observed, in one small prospective study, between vitamin B12 status and fasting glucose. Reference Radzicka, Ziolkowska, Zaborowski, Brazert and Pietryga18 To our knowledge, there has been no investigation of relationships between maternal folate and vitamin B12 status and GDM in pregnant women with obesity, who have a higher risk of developing GDM than those of recommended weight. Reference Di Cianni, Miccoli, Volpe, Lencioni and Del Prato19
While there are several reports of relationships between folate and/or vitamin B12 status and GDM/insulin resistance, the mechanism remains unclear. In obese subjects, folate or vitamin B12 deficiency has been linked to reduced mitochondrial DNA methylation in association with insulin resistance. Reference Zheng, Linarelli and Liu20 A causative relationship could explain why glucose homoeostasis is impaired in a folate and B12-deficient state.
Large cohort studies have observed relationships between lower folate and vitamin B12 concentrations and a higher body mass index (BMI) in pregnant women Reference Knight, Shields and Brook21–Reference Scholing, Olthof, Jonker and Vrijkotte23 which may reflect the suboptimal micronutrient status among obese individuals in general, often attributed to multiple factors including poor quality diet, socio-economic status and altered metabolism. Reference García, Long and Rosado24
A study from the UK Pregnancies Better Eating and Activity Trial (UPBEAT), an antenatal randomised controlled trial of a behavioural intervention in pregnant women with obesity (BMI ≥ 30 kg/m2), recently reported that a diagnosis of GDM, fasting and 1-h and 2-h plasma glucose concentrations following an oral glucose tolerance test (OGTT) were associated with in total 732 differentially methylated cytosine-phosphate-guanine (CpG) sites in neonatal cord blood DNA. These differentially methylated sites occurred amongst genes involved in transcriptional regulation and cell signalling. Reference Antoun, Kitaba and Titcombe25 This study has addressed the hypothesis that the proposed relationship in obese women between methylation status and glucose homoeostasis is a result of altered maternal folate and/or vitamin B12 status. Specific aims were 1) to determine folate and vitamin B12 status in a large cohort (n = 951) of pregnant women with obesity, 2) to investigate the relationship between maternal folate or vitamin B12 status and cord blood DNA methylation at CpG sites previously observed in association with dysglycaemia Reference Antoun, Kitaba and Titcombe25 and 3) to determine associations between folate, vitamin B12 and glucose homoeostasis.
Method
Subjects and study design
This is a secondary analysis of data and samples from pregnant women who participated in the UPBEAT study. UPBEAT was a multicentre, randomised controlled trial in the UK examining the effects of a behavioural intervention of diet and physical activity versus standard antenatal care in pregnant women with obesity. The primary outcomes were GDM and delivery of a large for gestational age infant. The study protocol has been published elsewhere. Reference Briley, Barr and Badger26 Briefly, women were recruited in eight UK centres between March 2009 and June 2014. Women were eligible for inclusion if they were >16 years of age, had a BMI ≥ 30 kg/m2 and a singleton pregnancy and were between 15+0 and 18+6 weeks’ of gestation. Women were excluded if they were unwilling or unable to give informed consent, if they had underlying health disorders or were prescribed metformin. The study was approved by the NHS Research Ethics Committee (UK integrated research application system, reference 09/H0802/5). Participants allocated to the intervention group attended eight weekly sessions with a health trainer prior to the OGTT. The control group received standard antenatal care according to local healthcare provision.
There was no reduction in the incidence of GDM or large-for-gestational-age infants in the intervention group compared to the control group; however, there were improvements in maternal dietary intake, physical activity and adiposity, metabolic parameters and a reduction in gestational weight gain. Reference Poston, Bell and Croker27,Reference Mills, Patel and White28
Data and sample collection
Participants attended three study visits. Women who provided blood samples at the OGTT visit (23–30 weeks’ gestation) were included in this study. Social and demographic data were collected at the baseline visit at 15+0–18+6 weeks’ gestation, including maternal age (years), BMI (kg/m2), medical history (e.g. previous history of GDM), family history (e.g. type 2 diabetes), ethnicity (Black, White, Asian, other), parity (nulliparous, multiparous), smoking (smoking during pregnancy, ex-smoker, non-smoker), educational attainment (none, general certificate of secondary education (GCSE), vocational qualification, A-level, first- or higher degree) and index of multiple deprivation Reference Noble, Wright, Smith and Dibben29 (score from 1, least deprived, to 5, most deprived).
Assessment of folate and vitamin B12
Blood samples were processed and serum stored at −80 °C until analysis. Samples for plasma glucose analyses were transferred to the clinical laboratory for analysis. Chemiluminescent microparticle immunoassay technology (Architect 2000 Series analyser, Abbott Diagnostics) was used to assess the serum concentrations of folate and vitamin B12. The linearity of the assays was 1.5–20.0 µg/L and 125–2000 ng/L, and inter assay coefficients of variation were <7.1% and <8.0% for folate and vitamin B12, respectively. Haemolysed or insufficient samples were excluded from the analysis (n = 4). A priori decision was made to access folate status regardless of folic acid supplementation.
Vitamin B12 deficiency was defined as serum concentration <200 ng/L (148 pmol/L), and folate deficiency was defined as serum concentration <3 µg/L (6.8 nmol/L). Reference Sobczynska-Malefora and Harrington30
Assessment of GDM
All participants attended an OGTT between 23 and 30 weeks’ gestation (75 g, mean 27 weeks’ gestation) following fasting for at least 10 h. Diagnosis of GDM was according to the criteria from the International Association of Diabetes and Pregnancy Study Groups (IADPSG): fasting venous glucose of ≥5.1 mmol/L, 1-h venous glucose ≥ 10.0 mmol/L or 2-h venous glucose of ≥8.5 mmol/L. Reference Metzger, Gabbe and Persson31 Women diagnosed with GDM were referred to the local antenatal diabetes service and managed according to local practice, with dietary management, metformin and/or insulin treatment as appropriate.
Cord blood DNA methylation
Umbilical cord blood was collected shortly after delivery, processed and the buffy coat stored at −80°C until further analysis. The evaluation of the neonatal epigenome was carried out as detailed previously. Reference Antoun, Kitaba and Titcombe25 DNA was extracted from the buffy coat using the QIAamp Blood DNA mini kit (Qiagen). Agarose gel electrophoresis was used to check the DNA quality, and the NanoDrop ND-1000 (NanoDrop Technologies) was used to determine the DNA quantity. DNA was converted with sodium bisulphite (Zymo EZ DNA Methylation-Gold kit, ZymoResearch, Irvine, California, USA, D5007) and analysed by the Centre for Molecular Medicine and Therapeutics for DNA methylation using the Infinium Methylation EPIC BeadChip kit (Infinium, California, USA). Infinium 850K data processing was carried out by Antoun et al. Reference Antoun, Kitaba and Titcombe25 Antoun et al. calculated the beta values for DNA methylation which were used in the current study to examine the association between maternal serum folate or vitamin B12 and the epigenome. Only the 732 significant differentially methylated CpGs (false discovery rate corrected (FDR) p < 0.05) found to be associated with GDM, fasting and 1-h and 2-h plasma glucose concentrations post OGTT were analysed for the purpose of this study. Reference Antoun, Kitaba and Titcombe25
Confounders
Confounders were identified from previous relevant literature Reference Lai, Pang and Cai9–Reference Sukumar, Venkataraman and Wilson12,Reference Idzior-Walus, Cyganek and Sztefko15,Reference Seghieri, Breschi and Anichini16,Reference Knight, Shields and Brook21,Reference Scholing, Olthof, Jonker and Vrijkotte23,Reference Antoun, Kitaba and Titcombe25,Reference Tomedi, Chang and Newby32 in the assessment of the relationship between maternal folate or vitamin B12 status and maternal GDM/glucose concentrations and included: maternal age, ethnicity, parity, cigarette smoking, maternal BMI, education, level of deprivation and intervention allocation. Only significant associations (p < 0.05) were adjusted for confounders.
Confounders were entered into three models: Model 1: adjusted for maternal age, ethnicity, parity, cigarette smoking, maternal BMI, education, level of deprivation and treatment allocation; Model 2 included all confounders in model 1 plus previous history of GDM and family history of type 2 diabetes mellitus; Model 3 included all confounders in model 2 plus mutual adjustment for maternal folate and vitamin B12.
To assess the association between maternal folate or vitamin B12 and cord blood DNA methylation, the following confounders were included Reference Antoun, Kitaba and Titcombe25 maternal age, ethnicity, parity, cigarette smoking, maternal BMI, GDM, neonate sex, macrosomia (defined as a birth weight >4 kg) and to adjust for the contribution of different cells subtypes to the neonatal DNA epigenome, the predicted values for B-cells, CD4 T-cells, CD8 T-cells, granulocytes, monocytes, natural killer cells and nucleated red blood cell composition. Reference Bakulski, Feinberg and Andrews33
Statistical analysis
All statistical analyses were performed in Stata (version 15.0; StataCorp LP, College Station, Texas). The normality of variables was assessed visually using histograms and Q–Q plots. Descriptive statistics summarised the characteristics of the study population, stratified by GDM diagnosis. Characteristics of both groups, women with GDM and women without GDM were compared using logistic regressions. Unpaired t-test for continuous data and Chi-square test for categorical data were used for the sensitivity analyses to compare all women in the current study with women who participated in UPBEAT who did not provide blood samples.
Vitamin B12 was not normally distributed and was log transformed. Associations between maternal folate or vitamin B12 concentrations and maternal characteristics were examined using linear regression. Folate and vitamin B12 were included as continuous variables. All significant associations were adjusted for confounders. Linear- or logistic regression with appropriate adjustment for confounders was used to examine the association between maternal folate or vitamin B12 concentrations and fasting, 1-h glucose and 2-h glucose post OGTT (as continuous variables) or GDM diagnosis (IADPSG criteria), respectively. Unadjusted and adjusted (Models 1, 2 and 3) regression coefficients or odds ratio (OR) and 95% confidence intervals (95% CI) are presented. A receiver operating characteristic (ROC) curve was created to assess if folate:B12 ratio was a predictor for GDM. Statistical significance was taken at p < 0.05.
To determine the association between maternal folate or vitamin B12 and cord blood DNA methylation, regression models were used. The Benjamini–Hochberg adjustment for FDR was used for this analysis. As not all women provided both blood samples at the OGTT visit and cord blood samples of their neonate, a subgroup of the current cohort was used to determine the association between maternal folate or vitamin B12 and cord blood DNA methylation. A comparison of this subgroup with the complete cohort was undertaken using unpaired t-test for continuous data and Chi-square test for categorical data.
Results
Characteristics of the study population
Of the 1555 pregnant women who took part in the UPBEAT study, 959 provided blood samples at the OGTT visit which were assessed for folate and vitamin B12 status (Fig. 1). Eight blood samples were excluded. Data were available for the epigenome of 504 cord blood samples, in which previous analysis had determined 732 CpGs to be associated with maternal dysglycaemia.
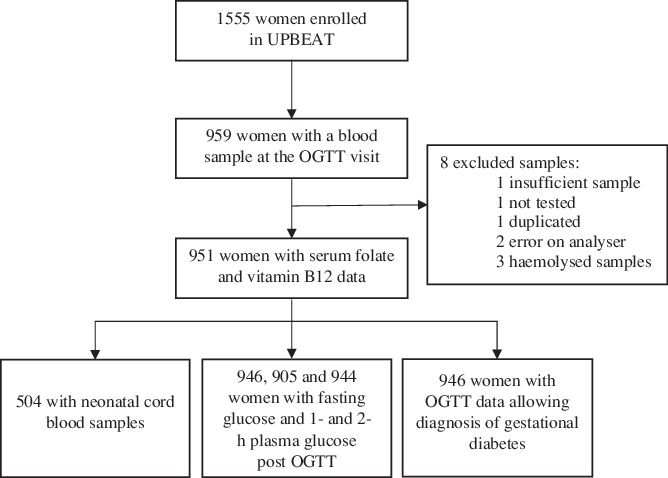
Fig. 1. Flow chart. UPBEAT, UK Pregnancies Better Eating and Activity Trial; OGTT, oral glucose tolerance test. (a) Serum folate. (b) Serum vitamin B12.
The characteristics of the participants are included in Table 1. The mean (± standard deviation (SD)) age of the women was 30.7 ± 5.5 years, the median (interquartile range (IQR)) BMI was 35.2 (32.8–38.8) kg/m2. Forty-four point eight percentage of women were nulliparous and 6.8% smoked during pregnancy. The majority of women were White (66.7%), followed by Black (21.4%), Asian (6.9%) and other (5.1%) ethnic groups. Forty-two point one percentage women had no education or obtained a GCSE or vocational qualification, whereas 57.9% had an A-level, first- or higher degree. Two-hundred and seventy-one women (28%) developed GDM. Women with GDM were older and had a higher BMI than women without GDM.
Table 1. Characteristics of the study participants, stratified by GDM diagnosis
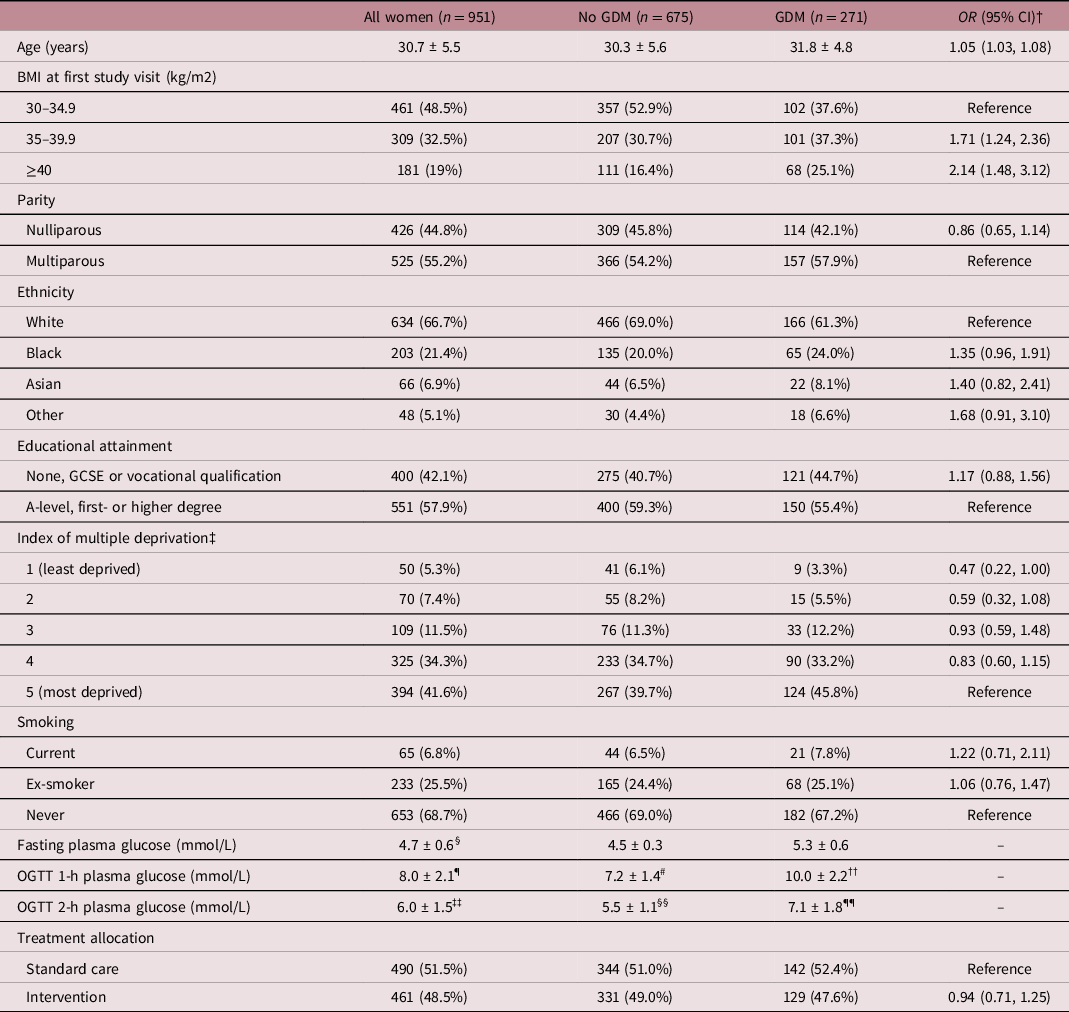
Data presented as mean ± standard deviation (SD) for continuous variables or number (%) for categorical variables. GDM, gestational diabetes mellitus; OR, odds ratio; CI, confidence interval; BMI, body mass index; GCSE, general certificate of secondary education; OGTT, oral glucose tolerance test.
† Logistic regression was used for the comparison of GDM to no GDM.
‡ n = 948 for all women and n = 672 for no GDM group.
§ n = 946;
¶ n = 905.
# n = 639;
†† n = 266.
‡‡ n = 944.
§§ n = 674.
¶¶ n = 270.
In comparison to women from the UBPEAT study who did not provide a blood sample at the OGTT visit (n = 603), women included in this study lived in less deprived areas, had a higher educational attainment and were more likely to be White (Supplementary Table S1).
Maternal folate and vitamin B12 status
The mean (SD) folate and median (IQR) vitamin B12 concentrations of the total group were 9.6 ± 4.9 µg/L and 230 (178–304) ng/L, respectively (Table 2). Three-hundred and fifty-six women (37.4%) were classed as vitamin B12 deficient based on a cut-off of <200 ng/L, while 44 women (4.6%) were folate deficient based on a cut-off of <3 µg/L. Three point seven percentage of women with GDM were folate deficient and 34.3% were vitamin B12 deficient, whereas 4.9% of the women without GDM were folate deficient and 38.7% were vitamin B12 deficient.
Table 2. Serum folate and vitamin B12 concentrations at the OGTT visit, stratified by GDM diagnosis
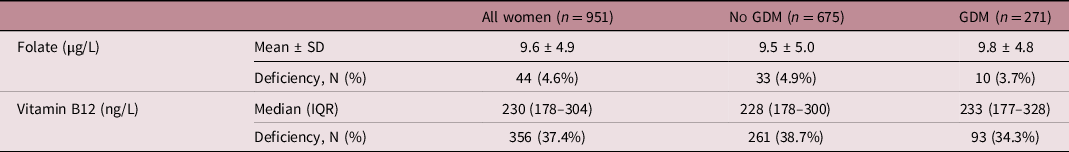
OGTT, oral glucose tolerance test; GDM, gestational diabetes mellitus; SD, standard deviation; IQR, interquartile range.
Association between maternal folate or vitamin B12 status and maternal characteristics
The relationship between maternal folate or vitamin B12 status and maternal characteristics is presented in Supplementary Table S2. Older women had higher folate and vitamin B12 concentrations compared to younger women. Nulliparous women had better folate status compared to multiparous women. Black women had higher vitamin B12 concentration in comparison to White women. In contrast, vitamin B12 and folate concentrations were lower in women with lower educational attainment and in those who smoked. Folate and vitamin B12 concentrations were also lower in women with a higher BMI (35.0–39.9 kg/m2) in comparison to women with a BMI between 30.0 and 34.9 kg/m2. These associations were robust for adjustment for confounders.
Association between maternal folate or vitamin B12 status and plasma glucose concentrations and GDM
There was a positive association between maternal folate and 1-h glucose post OGTT in the unadjusted model (β = 0.046, 95% CI 0.018–0.074, p = 0.001); a 1 µg/L increase in maternal folate being associated with a significant increase of 0.046 mmol/L of plasma glucose. This remained significant following adjustment for confounders in Models 1 and 3 (p < 0.05), and a trend towards significance was observed following adjustment for Model 2 (p = 0.055) (Table 3).
Table 3. Association between serum folate or vitamin B12 and plasma glucose concentrations post OGTT and gestational diabetes
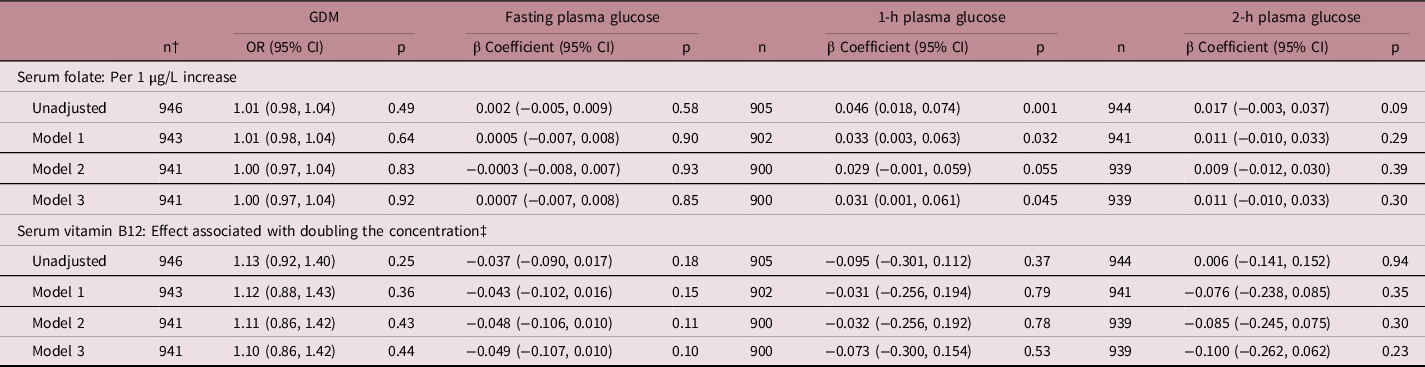
GDM, gestational diabetes mellitus; OR, odds ratio; CI, confidence interval; Model 1: adjusted for maternal age, ethnicity, parity, cigarette smoking, maternal body mass index, education, level of deprivation and treatment allocation; Model 2: adjusted for all confounders in model 1, plus previous history of gestational diabetes and family history of type 2 diabetes mellitus; Model 3: adjusted for all confounders in model 2, plus mutual adjustment for serum folate and vitamin B12.
† Applies for gestational diabetes and fasting plasma glucose.
‡ Logged.
There was no association between maternal folate and fasting or 2-h plasma glucose post OGTT or with GDM diagnosis. Additionally, there was no association between maternal vitamin B12 and fasting glucose and 1-h and 2-h plasma glucose post OGTT or with GDM diagnosis.
A ROC curve was drawn to examine the combined effect of maternal folate and vitamin B12 status on GDM occurrence. The folate:vitamin B12 ratio was not a predictor for GDM (ROC area 0.51, standard error 0.02, CI 0.47, 0.55).
Association between maternal folate or vitamin B12 status and cord blood DNA methylation
Fig. 2 presents volcano plots showing the association between cord blood DNA methylation at CpG sites previously observed in association with dysglycaemia (732 CpGs in total) in 504 neonates and maternal (A) folate or (B) vitamin B12. No significant associations (Benjamini-Hochberg FDR-corrected p < 0.05) were found between maternal folate or vitamin B12 concentrations and DNA methylation of the 732 CpGs associated with glycaemic status examined.
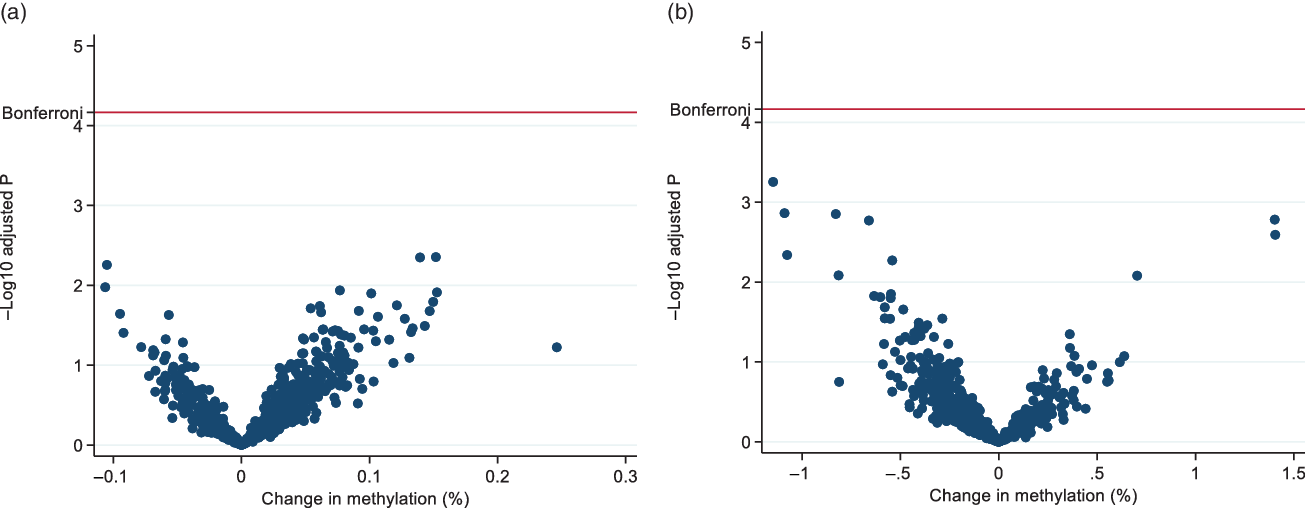
Fig. 2. Volcano plots of the association between cord blood DNA methylation and (a) serum folate or (b) serum vitamin B12. The blue spots represent non-significant associations for individual CpG sites after false discovery rate (FDR) correction. Adjusted for the following confounders: maternal age, ethnicity, parity, cigarette smoking, maternal body mass index, gestational diabetes, neonate sex, macrosomia and the predicted values for B-cells, CD4 T-cells, CD8 T-cells, granulocytes, monocytes, natural killer cells and nucleated red blood cell composition.
The complete list of adjusted regression coefficients for every CpG can be found in Supplementary Table S3.
Discussion
In this large cohort of pregnant women with obesity, over a third were vitamin B12 deficient while only a small proportion were folate deficient. No association was found between maternal folate or vitamin B12 status and cord blood DNA methylation of CpG sites implicated in dysglycaemia. Reference Antoun, Kitaba and Titcombe25 There was a modest but significant association between maternal folate status and maternal 1-h glucose post OGTT, but no association between maternal folate status and GDM occurrence, nor any association between maternal vitamin B12 status and maternal glucose or GDM.
Two previous reports have suggested an association between maternal folate and vitamin B12 concentrations and infant DNA methylation Reference Caramaschi, Sharp and Nohr34,Reference McKay, Groom and Potter35 which contrast to the present results. Those studies differ from the present study since they also included women of recommended weight and did not investigate CpG’s previously observed in association with dysglycaemia. Joubert et al. Reference Joubert, den Dekker and Felix5 found 443 differential methylated CpGs in cord blood to be FDR significantly associated with maternal folate concentrations in pregnancy. Using the Illumina Human Methylation450K BeadChip, the study included 1988 neonates from the Norwegian Mother and Child Cohort Study (MoBa) and the Dutch Generation R Study. Folate and vitamin B12 status was assessed at 18 weeks’ gestation (MoBa study) and 13 weeks’ gestation (Generation R Study). Although the majority of genes identified were not related to any known function of folate, some had an association with neural tube defects and other neurological functions. Folate status was evaluated at an earlier gestation than in the present study, which could provide a rationale for the difference in studies as fetal DNA methylation has been reported to occur predominantly early in pregnancy. Reference Godfrey, Lillycrop, Burdge, Gluckman and Hanson4
A study from Australia which examined the association between maternal folate status >28 weeks’ gestation and infant DNA methylation also used the Illumina Human Methylation450K BeadChip to measure genome-wide DNA methylation in cord blood of 23 neonates. Reference Amarasekera, Martino and Ashley6 They found differentially methylated CpGs at seven regions across the genome and reported a hypomethylated region upstream of the ZFP57 transcriptor, known to be involved in the regulation of DNA methylation in early gestation. A possible explanation for the absence of any association between maternal folate status and differentially methylated CpGs in the present study is that, to address the hypothesis raised, we had focused a priori on differentially methylated CpGs previously associated with maternal glucose concentration and GDM. Reference Antoun, Kitaba and Titcombe25 Our study therefore suggests that the relationship between GDM and differential CpG methylation in the neonate is not mediated by altered maternal folate or vitamin B12 status. This does not preclude a relationship between folate or vitamin B12 status per se and epigenome wide methylation status, the approach taken by others to date.
We found that a higher maternal folate concentration was associated with a modest increase in 1-h plasma glucose post OGTT, concurring with Li et al. Reference Li, Hou and Yan10 who also found an association between higher maternal folate and 1-h plasma glucose post OGTT in 406 Chinese women of heterogenous BMI. To our knowledge, this relationship has not been reported previously in women with obesity. Our finding showing an association between folate concentration and the 1-h plasma glucose measurement could however be due to change. We did not find an association between maternal folate and fasting glucose, 2-h glucose or GDM as observed by others. Reference Krishnaveni, Hill and Veena11–Reference Seghieri, Breschi and Anichini16
The mechanism whereby folate status may influence glucose tolerance in pregnancy has yet to be elucidated. Further research is necessary to understand the mechanism between maternal folate status and glucose homoeostasis.
Over a third of the pregnant women with obesity were vitamin B12 deficient, consistent with a systematic review and meta-analysis of 42 studies which included women from all BMI categories which reported that 29% of women in the third trimester were vitamin B12 deficient. Reference Sukumar, Rafnsson, Kandala, Bhopal, Yajnik and Saravanan36 In a study including 97 obese pregnant women from Ireland, O’Malley et al. Reference O’Malley, Reynolds, Cawley, Woodside, Molloy and Turner22 reported that a quarter of women were vitamin B12 deficient using the same criteria as the present study. Furthermore, in a prospective cohort, the Dutch Amsterdam Born Children and their Development (ABCD) study reported that 22.8% of obese pregnant women were vitamin B12 deficient in early pregnancy (12–15 weeks’ gestation). Reference Scholing, Olthof, Jonker and Vrijkotte23 Together with the findings of the current study, there is increasing evidence that vitamin B12 deficiency is common during pregnancy, including among pregnant women with obesity, and that a higher maternal BMI is associated with lower vitamin B12 status. Reference Krishnaveni, Hill and Veena11,Reference Sukumar, Venkataraman and Wilson12,Reference Radzicka, Ziolkowska, Zaborowski, Brazert and Pietryga18,Reference Knight, Shields and Brook21,Reference O’Malley, Reynolds, Cawley, Woodside, Molloy and Turner22,Reference Rogne, Tielemans and Chong37 There may be associated health consequences as vitamin B12 deficiency is associated with adverse maternal and fetal outcomes including fatigue, anaemia and neurological defects. Reference Hanson, Bardsley and De-Regil2 More data are needed to understand micronutrient deficiency thresholds in pregnancy to determine if there is need for dietary counselling, including advice on vitamin B12-rich food sources, to improve micronutrient intake in this high-risk group.
The observation that a small proportion of women in this study were classed as folate deficient is in contrast to that reported by Scholing et al. Reference Scholing, Olthof, Jonker and Vrijkotte23 in the ABCD study, where 21.8% of women with obesity were folate deficient. However, in that cohort, folate status was assessed in early pregnancy at 13 weeks’ gestation and a higher threshold (folate <4.4 µg/L) was used to define deficiency. Previous reports that pregnant women with a higher BMI have lower folate concentrations, Reference Li, Hou and Yan10,Reference Knight, Shields and Brook21–Reference Scholing, Olthof, Jonker and Vrijkotte23 is in line with the our observation that maternal folate concentration was lower in women with a BMI of 35–39.9 kg/m2 in comparison to women with a BMI between 30 and 34.9 kg/m2.
The mechanism underlying the link between higher maternal BMI and lower vitamin B12 status is still unclear. In non-pregnant populations, some studies suggest that vitamin B12 deficiency is a consequence of obesity, while others indicate deficiency as an exacerbating factor in obesity. Reference García, Long and Rosado24 Women with obesity have been shown to have suboptimal dietary intake, Reference Rush and Yan38 including in pregnancy, Reference Shin, Lee and Song39 which may contribute to vitamin B12 deficiency. Obesity may additionally influence the pharmacokinetics (e.g. absorption, metabolism) of micronutrients. Reference O’Leary and Samman40 Conversely, deficiency in vitamin B12 has been implicated in weight gain through epigenetic modification of genes that contribute to pathways involving lipid metabolism and inflammation. Reference Samavat, Adaikalakoteswari and Boachie41,Reference Milagro, Mansego, De Miguel and Martinez42 Additional studies are required to understand the relationship between vitamin B12 status and obesity in pregnancy.
In accord with most previous studies, including women of heterogenous BMI, maternal folate and vitamin B12 concentrations, were lower in younger, less educated, multiparous and smoking women who had a higher pre-pregnancy BMI. Reference Lai, Pang and Cai9,Reference Li, Hou and Yan10
This study did not find evidence of an association between maternal vitamin B12 status and glucose homoeostasis in obese women, which concurs with some reports in BMI heterogenous groups of women Reference Barzilay, Moon and Plumptre13–Reference Idzior-Walus, Cyganek and Sztefko15,Reference Tarim, Bagis and Kilicdag17 but not all. Reference Kouroglou, Anagnostis, Daponte and Bargiota8–Reference Sukumar, Venkataraman and Wilson12 Reasons can only be speculative; the majority of the larger studies showing an association between vitamin B12 and glucose status have been undertaken in women from Asia, so ethnic differences may contribute, although, one UK study Reference Sukumar, Venkataraman and Wilson12 reported a relationship between vitamin B12 and GDM, albeit with different diagnostic criteria.
This study has several strengths. To our knowledge, there has been no previous investigation of the relationship between maternal folate or vitamin B12 status and glucose, GDM and cord blood epigenome in a large cohort of obese women. Due to the depth of information in the UPBEAT database, we were able to examine the relationship between maternal folate or vitamin B12 status and social and demographic characteristics whilst ensuring appropriate statistical adjustment. Also, we were able to investigate associations between maternal folate or vitamin B12 status and the fasting, 1-h glucose and 2-h glucose concentrations post OGTT as well as GDM diagnosis, whereas all other studies but one Reference Li, Hou and Yan10 did not include these measurements. Finally, this study adds to the literature investigating the relationship between maternal B vitamin status and the infant epigenome.
A limitation of this study was that maternal folate and vitamin B12 status were only assessed at the OGTT visit and not longitudinally throughout gestation. Early pregnancy assessment would be of interest as this is a critical period for embryonic and fetal development. Reference Wu, Imhoff-Kunsch and Girard43 The vitamin B12 and folate status might have been influenced, most likely positively, by the dietary advice given to women in the intervention arm. However, we found no difference in serum folate and vitamin B12 between the two arms in the UPBEAT study (data not shown). It is reported that holo-transcobalamin (holo-TC), the biologically active fraction of vitamin B12, is a better marker than serum vitamin B12 for measuring vitamin B12 status in pregnancy since holo-TC is believed to be less influenced by pregnancy related physiological changes in plasma volume. Reference Sobczynska-Malefora and Harrington30 It would have been preferable to measure red cell folate as this is less affected by recent dietary changes. Reference Sobczynska-Malefora and Harrington30 However, as recommended, serum folate was evaluated after an overnight fast. Cord blood was used for investigating the epigenome of the neonate, and it is not known how representative this is in tissues of interest, e.g. insulin sensitive cell types. Lastly, women in this study participated in a randomised controlled trial addressing a behavioural intervention, which may have introduced selection bias, although the results were adjusted for randomised group allocation. Generalisability to non-trial obese pregnant women may therefore be limited.
Future studies could consider analysing the whole epigenome of the neonate to investigate the association between B vitamins and cord blood DNA methylation more extensively. Also holo-TC may be a useful biomarker to measure in addition to serum vitamin B12.
Conclusion
In a cohort of pregnant women with obesity, maternal folate was associated with 1-h glucose post OGTT, but we found no evidence to support the hypothesis that the relationship between GDM and glycaemic status and differential methylation of neonatal cord blood DNA was mediated by maternal folate or vitamin B12 status. The relationship between maternal folate and glycaemia adds to the evidence that folate status could be involved in maternal glucose homoeostasis; this needs validation in future studies of pregnant women with obesity. Over a third of the women were classed as vitamin B12 deficient. Together with the finding that lower maternal vitamin B12 concentrations were associated with a higher BMI, this study adds to the evidence of sub-optimal micronutrient status in obese pregnant women.
Acknowledgements
The authors thank all staff in the UPBEAT consortium and the participants in the trial for their patience, time, interest and goodwill.
Financial support
This research was funded/supported by the National Institute for Health Research (NIHR) Biomedical Research Centre based at Guy’s and St Thomas’ NHS Foundation Trust and King’s College London and/or the NIHR Clinical Research Facility. The views expressed are those of the author(s) and not necessarily those of the NHS, the NIHR or the Department of Health. The UPBEAT study was funded by UK’s National Institute for Health Research (RP-PG-0407-10452) and the Chief Scientist Office, Scottish Government Health Directorates (Edinburgh) (CZB/A/680). EA, KAL and the epigenome study were funded by Diabetes UK (16/0005454). ACF, SLW and LP are supported by Tommy’s charity. KVD is supported by the British Heart Foundation (FS/17/71/32953). PS is partly funded by King’s Health Partners Institute of Women and Children’s Health, Tommy’s (Registered charity no. 1060508) and by ARC South London (NIHR).
Conflicts of interest
The authors declare no conflicts of interest.
Ethical standards
The authors assert that all procedures contributing to this work comply with the Declaration of Helsinki of 1975, as revised in 2008, and the UPBEAT study and all associated analyses including this current study were approved by the National Health Service Research Ethics Committee (UK integrated research application system, reference 09/H0802/5).
Author contributions
ACF and SLW designed the study. WvW wrote the manuscript. WvW conducted the data and statistical analysis along with PTS. EA, KMG, NTK and KAL provided the epigenome data. All authors have interpreted the data and have read and approved the final manuscript.
Supplementary material
To view supplementary material for this article, please visit https://doi.org/10.1017/S2040174421000246










