Introduction
Dysphonia is a common finding in idiopathic Parkinson's disease, and its treatment remains a challenge.Reference Jankovic1–Reference Herd, Tomlinson, Deane, Brady, Smith and Sackley3 Dysphonia is often accompanied by other disturbances in the upper aerodigestive tract, such as hypokinetic dysarthria and oropharyngeal or oesophageal dysphagia.Reference Herd, Tomlinson, Deane, Brady, Smith and Sackley3 During the course of the disease, up to 90 per cent of idiopathic Parkinson's disease patients will develop voice complaints, including breathiness, hoarseness, reduced loudness, vocal tremor, restricted pitch variability and so on.Reference Skodda, Gronheit, Mancinelli and Schlegel2–Reference Muller, Wenning, Verny, McKee, Chaudhuri and Jellinger4
Voice disorders, among others, may affect speech intelligibility and can have a major impact on health-related quality-of-life in idiopathic Parkinson's disease as they interfere with the communicative abilities of the patient.Reference Silva, Gama, Cardoso, Reis and Bassi5,Reference Wood, Neumiller, Setter and Dobbins6 As with other idiopathic Parkinson's disease-related dysfunctions, dysphonia can be initially treated with levodopa because this medication is usually prescribed to improve the overall motor function of the patient.Reference Ramig, Sapir, Fox and Countryman7–Reference Cushnie-Sparrow, Adams, Abeyesekera, Pieterman, Gilmore and Jog10
If symptoms persist, other therapeutic approaches, such as voice therapy (for instance Lee Silverman Voice Treatment (LOUD)) or in specific cases surgical treatment (i.e. vocal fold augmentation) may be considered.Reference Ramig, Sapir, Fox and Countryman7,Reference Ramig, Fox and Sapir8 These treatments may improve voice-related quality of life and voice functionality, but so far no treatment can completely halt the inevitable decline. The question is whether surface electrical stimulation can be of added value in the rehabilitation of voice complaints.
There are currently no large-scale investigations on voice rehabilitation using surface electrical stimulation of the neck combined with voice therapy (usual care) in idiopathic Parkinson's disease patients. As in sports medicine, where adjunctive electromyostimulation has been used to enhance the effects of muscle training, it is hypothesised that voice therapy with adjunctive surface electrical stimulation may enhance the vocal function.Reference Maffiuletti, Dugnani, Folz, Di Pierno and Mauro11
Guzman et al. carried out a study in this context concluding that surface electrical stimulation in combination with voice therapy might be a useful intervention to improve voice quality in patients with a superior laryngeal nerve injury.Reference Guzman, Rubin, Cox, Landini and Jackson-Menaldi12 Furthermore, seven patients with bilateral vocal fold bowing were enrolled in a study by Lagorio et al.Reference Lagorio, Carnaby-Mann and Crary13 Voice therapy with adjunctive surface electrical stimulation seemed to reduce vocal fold bowing resulting in improved acoustic, laryngeal and patient-reported outcome measures in this study.Reference Lagorio, Carnaby-Mann and Crary13
The rationale of surface electrical stimulation is twofold: first, it achieves stimulation of the nerve and its motor end plate and consequently the muscle fibres, resulting in re-education of functional muscle contraction patterns, which is mainly a peripheral effect.14,Reference Freed, Freed, Chatburn and Christian15 Second, when surface electrical stimulation is applied to the skin at low current levels, it activates the sensory nerve endings in the surface layers of the skin providing sensory feedback to the central nervous system that uses this feedback to make appropriate motor actions. Motor control is fundamentally the integration of this sensory information to generate desired movements or action.Reference Rosenbaum16
It is unknown if surface electrical stimulation can improve voice quality in idiopathic Parkinson's disease patients by improving vocal fold function or if surface electrical stimulation could be used as a cueing tool to improve sensory feedback and internal cueing for voice production.Reference Ramig, Sapir, Fox and Countryman7,Reference Ramig, Fox and Sapir8,Reference Pinnington, Muhiddin, Ellis and Playford17 Based on the aforementioned mechanisms, the aim of this study was to describe the effects of surface electrical stimulation plus voice therapy (usual care) on voice function in dysphonic idiopathic Parkinson's disease patients. The hypothesis is that dysphonic idiopathic Parkinson's disease patients could benefit from suprahyoid surface electrical stimulation using different electrical current intensities as an adjunct to voice therapy.
Materials and methods
Study population
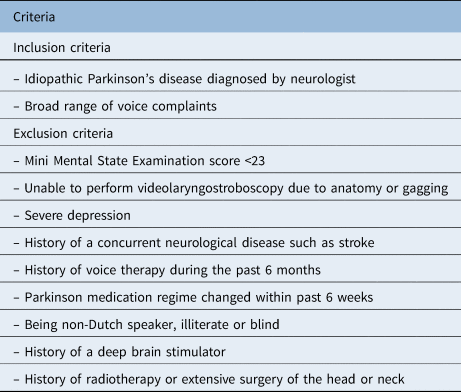
Idiopathic Parkinson's disease patients reporting voice complaints were recruited from hospitals all over the Netherlands. A neurologist clinically diagnosed the idiopathic Parkinson's disease according to the UK Parkinson's Disease Society Brain Bank and the Hoehn and Yahr scale scoring system.Reference Hoehn and Yahr18,Reference Hughes, Daniel, Kilford and Lees19 Inclusion and exclusion criteria are listed in Table 1.
Table 1. Inclusion and exclusion criteria
Study design
Each patient was strictly allocated chronologically to a treatment group (alternation; i.e. the first patient to enter the study was placed in group 1, the second patient in group 2, the third patient in group 3, the fourth patient in group 1 and so on to obtain quasi-randomisation). The idiopathic Parkinson's disease patients were blinded for this allocation during the baseline measurements. All patients received 30 minutes of treatment every day, except for the weekends, during 3 weeks (15 days). Voice therapy (usual care) included the following exercises: airway or breathing exercises to increase respiratory volumes and subglottal air pressure; postural exercises; oral motor exercises; loudness training by active phonation and vocal fold adduction; and exercises to improve sensory awareness.Reference Ramig, Sapir, Fox and Countryman7,Reference Ramig, Fox and Sapir8 The study protocol was approved by the medical ethics committee of the Maastricht University Medical Centre (MEC 05-237).
The aim of the voice therapy was to improve respiratory and laryngeal tract function. The content of this intensive training programme was deemed to be consistent with theories of motor learning and skill acquisition but also with principles of neural plasticity (i.e. the capacity of the nervous system to change in response to signals).Reference Jankovic1,Reference Herd, Tomlinson, Deane, Brady, Smith and Sackley3 Eighty-five speech and language pathologists affiliated with ParkinsonNet® and with experience in voice therapy for idiopathic Parkinson's disease took part in the study.20 ParkinsonNet is a national network of more than 3400 healthcare providers of various disciplines specialised in idiopathic Parkinson's disease.20 This large number of speech and language pathologists minimised the possibility of a therapist effect on group performance or on treatment outcome and enabled patients to receive treatment in their own neighbourhood all over the country. All speech and language pathologists underwent a supervised surface electrical stimulation training that was described in a previous study on dysphagic idiopathic Parkinson's disease patients.Reference Baijens, Speyer, Passos, Pilz, van der Kruis and Haarmans21
All three groups received voice therapy, and group 2 and group 3 also received surface electrical stimulation at the same time, making group 1 the control group. Surface electrical stimulation at motor-level threshold was applied in group 2 and surface electrical stimulation at sensory-level threshold in group 3.
A commercially available electrical stimulator was used (VitalStim® Therapy; frequency 80 Hz, pulse width 700 μs). Two skin electrodes (VitalStim, reference 59035) were placed on the suprahyoid skin slightly medially to the posterior horns of the hyoid bone near the presumed location of the superior laryngeal nerves and connected on each side of the midline of the neck (Figure 1). Adult electrodes are circular, have a 2.1 cm diameter and provide 3.46cm2 surface area of stimulation via a carbon-silver substrate. This electrode position was based on previous surface electrical stimulation studies in non-idiopathic Parkinson's disease patients with dysphonia and on the VitalStim manual.Reference Lagorio, Carnaby-Mann and Crary13–Reference Freed, Freed, Chatburn and Christian15
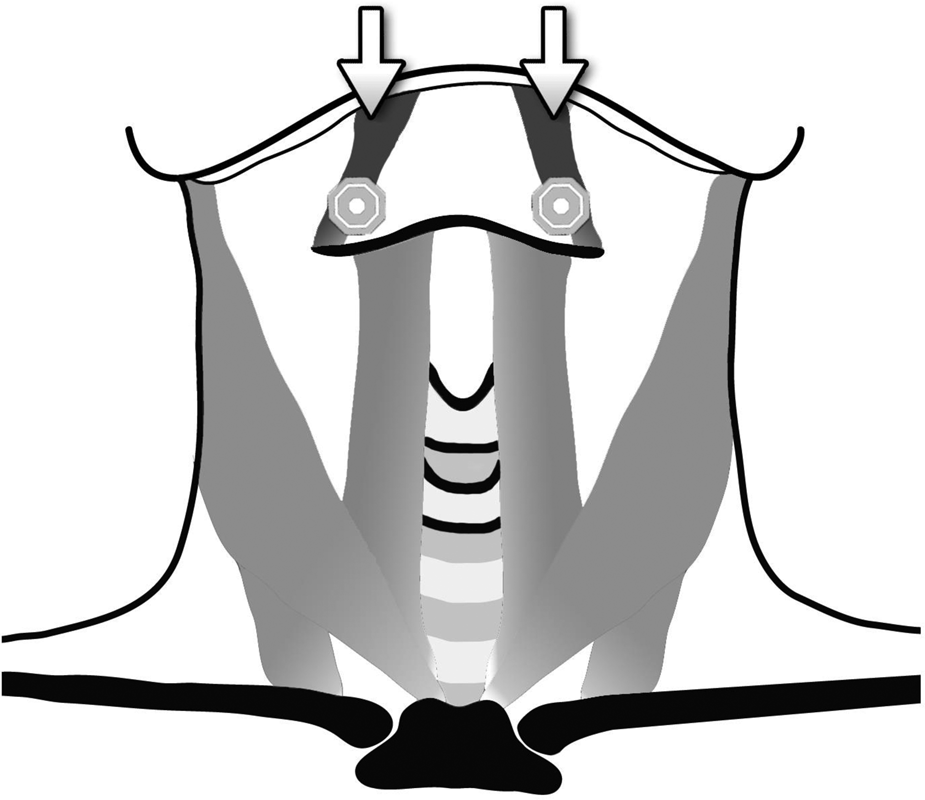
Fig. 1. Electrode position (after cleaning, lifting and shaving the skin): two self-adhesive electrodes (VitalStim®) placed horizontally on the suprahyoid skin slightly medially to the posterior hyoid horns near the location of the superior laryngeal nerves on each side of the midline of the neck (suprahyoid region). The arrows mark the electrodes. The electrodes have a 2.1cm diameter and provide a 3.46 cm2 surface area of stimulation via a carbon-silver substrate.
The suprahyoid triangle has suprahyoid muscles (mylohyoid and digastric muscles) and innervations from cervical and cranial nerves, such as the trigeminal, hypoglossal with ansa cervicalis and superior laryngeal nerves (internal and external branches) that are able to receive and transduce stimuli towards the central nervous system as sensory feedback.Reference Rosenbaum16 The protocol for applying electrical current at a motor-level or sensory-level intensity was based on previous studies.Reference Baijens, Speyer, Passos, Pilz, van der Kruis and Haarmans21,Reference Ludlow, Humbert, Saxon, Poletto, Sonies and Crujido22,Reference Humbert, Poletto, Saxon, Kearney and Ludlow23 The treatment sessions and all examinations were performed within 90–120 minutes after the intake of anti-Parkinsonian medication during the ‘on’ motor phase.Reference Wajsbort24 The ‘on-off’ phenomenon in idiopathic Parkinson's disease refers to a switch between mobility and immobility in levodopa-treated patients, which occurs as an end-of-dose worsening of motor function.Reference Wajsbort24
Outcome measures
All patients underwent a standardised assessment protocol including: a clinical examination (ear, nose, throat anatomical and cranial nerve integrity, and postural behaviour (gait, upper limb movement and so on)), the Voice Handicap IndexReference Leeuw IM, Kuik, De Bodt, Guimaraes, Holmberg and Nawka25–Reference Speyer, Wieneke and Dejonckere27 and a videolaryngostroboscopy. All measurements were performed within one week before the start of treatment and within one week following the end of therapy by the same laryngologist of the research laboratory. During baseline measurements, all patients and the laryngologist were blinded to treatment allocation.
The validated Dutch version of the Voice Handicap Index was used to measure the level of voice handicap and disability experienced by the patients.Reference Leeuw IM, Kuik, De Bodt, Guimaraes, Holmberg and Nawka25 It consists of 30 items divided into three subscales: emotional (Voice Handicap Index-Emotional), functional (Voice Handicap Index-Functional) and physical (Voice Handicap Index-Physical). Each item can be scored from 0 to 4: 0 represents ‘never’ and 4 ‘always’. Adding the scores of the 30 items yields a total Voice Handicap Index score (Voice Handicap Index-Total) ranging from 0 to 120. The higher the total score, the higher the degree of patient-experienced voice handicap.
A videolaryngostroboscopy was performed to assess laryngeal function during phonation and investigate the presence of any laryngeal pathology. The videos were recorded on a digital versatile disc (‘DVD’) at 30 frames per second using a flexible fibre-optic Pentax FNL-10RP3 endoscope (Pentax Canada Mississauga, Canada) together with the Alphatron Stroboview ACLS camera, Alphatron Lightsource, Alphatron contact microphone and IVACX computerised video archiving system (Alphatron Medical Systems, Rotterdam, The Netherlands).
During the examination, patients were seated upright. The field of the image included the laryngeal vestibule, vocal folds, anterior and posterior commissure, and the arytenoids. Video recordings of vocal fold vibration were made during repeated stable phonation of a sustained vowel /a:/ or /i:/ at comfortable pitch and loudness. Each video contained a phonation time long enough to allow the registration of at least one ‘complete cycle’ of vibration. All selected videos were similar in length and clarity.
Visuoperceptual ordinal and nominal videolaryngostroboscopic variables were derived from reports of the Phonosurgery Committee of the European Laryngological Society and scored by a panel of two observers (glottic closure, periodicity of vibratory cycles, vocal fold amplitude and symmetry of mucosal displacement).Reference Brunings, Vanbelle, Akkermans, Heemskerk, Kremer and Stokroos28–Reference Gamboa, Jimenez-Jimenez, Nieto, Montojo, Orti-Pareja and Molina30 For further details see the supplementary material, available on The Journal of Laryngology & Otology website. All of these variables were scored for each videolaryngostroboscopic recording using varying speed (slow motion, normal, up to frame-by-frame) and repeated as often as necessary. The videolaryngostroboscopic recordings were randomly selected, and both observers were blinded to the patients’ identity, medical history and for the measurement moment (baseline vs post-treatment). Prior to the assessment, the observers underwent consensus training for these measurements, as described previously.Reference Brunings, Vanbelle, Akkermans, Heemskerk, Kremer and Stokroos28 In order to determine the intra-panel observer agreement level, 33 per cent of the videolaryngostroboscopic recordings were rated twice by the panel of observers, again blinded and in randomised order. This multidimensional voice protocol was deemed appropriate to measure treatment effects in the present study. Details on statistical analysis are included in the online only supplementary material.
Patient characteristics
The study included 109 mentally competent patients with a diagnosis of idiopathic Parkinson's disease and dysphonic complaints. Twenty-five patients were excluded during the study because of change of anti-Parkinsonian medication (n = 21), dental surgery (n = 2) and unexpected co-morbidity not related to therapy (n = 2). None of the patients experienced adverse events as a result of the therapy. Adherence of the patients to therapy and their compliance for anti-Parkinsonian medication were ensured through a diary completed by the speech and language pathologists. Finally, each treatment group contained 28 patients (n = 84; 20 female and 64 male). Descriptive data analysis of patient characteristics was performed, and data normality was tested using Shapiro–Wilk tests. No significant differences were found in the baseline general characteristics between the three groups (Table 2), and the duration of the idiopathic Parkinson's disease was at least five years. All patients used levodopa except for two patients in group 1, three patients in group 2 and two patients in group 3. They did not use any anti-Parkinsonian medication. The median current intensity used for group 2 was 10.5 mA (25th; 75th percentile: 7.3; 14.0) versus 3.3 mA (25th; 75th percentile: 3.0; 4.4) for group 3.
Table 2. Baseline patient characteristics
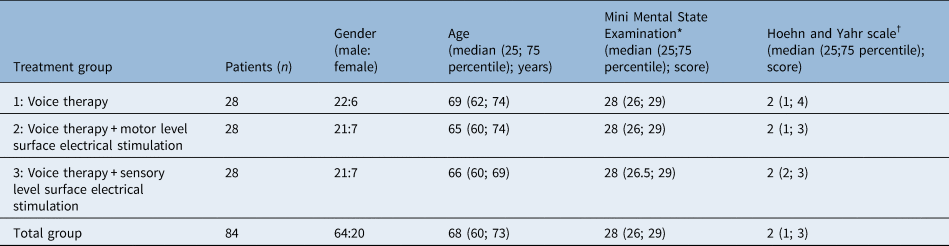
*Range, 0–30; †range, 1–5
Results
Voice handicap outcomes
Ninety-two per cent (n = 77) of the patients correctly completed the Voice Handicap Index questionnaire at baseline and 90 per cent (n = 76) after treatment. The mean (and standard deviation) Voice Handicap Index-Total score for the total group (n = 84) was 46.4 (21.39) at baseline and 51.2 (18.6) post-treatment. At the baseline, the floor or ceiling effect was considered negligible as few respondents got the lowest or highest possible Voice Handicap Index-Total score. Table 3 shows the descriptive statistics of the baseline data and the effect data of the Voice Handicap Index, the level of significance of the difference between post-treatment compared with baseline data for all groups (paired samples t-test), and the level of significance of post-treatment between-group differences (one way analysis of variance F-test for means). In group 2, a significant positive therapeutic effect for the Voice Handicap Index-Emotional (p = 0.006), Voice Handicap Index-Functional (p = .026) and Voice Handicap Index-Total (p = 0.033) subscales was found. Furthermore, in the total study population (n = 77), a significant positive therapeutic effect (post-treatment vs baseline) was observed for the Voice Handicap Index-Total (p = 0.003). However, when comparing post-treatment between group scores of each Voice Handicap Index subscale, no statistically significant difference between the three groups was found (Table 3).
Table 3. Descriptive statistics of the baseline and post-treatment VHI data
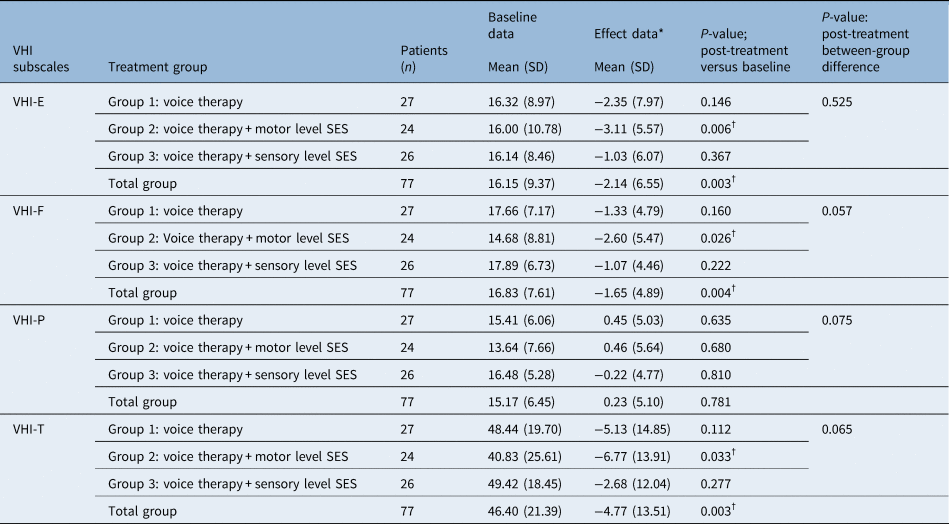
*Effect data = post-treatment minus baseline data; †statistically significant value. Table shows descriptive statistics of the baseline and post-treatment VHI data and the level of significance of the difference between post-treatment data compared with baseline data for all groups using the paired samples t-test. Furthermore, the level of significance of post-treatment between-group differences using the analysis of variance F-overall test for means. A p-value ≤ 0.05 was considered statistically significant. VHI = Voice Handicap Index; SD = standard deviation; SES = surface electrical stimulation; VHI-E = Voice Handicap Index-Emotional subscale; VHI-F = Voice Handicap Index-Functional subscale; VHI-P = Voice Handicap Index-Physical subscale, VHI-T = Voice Handicap Index-Total score
Videolaryngostroboscopy outcomes
The levels of intra-panel observer agreement for all videolaryngostroboscopic variables were determined. Agreement levels ranged from moderate to substantial (Cohens kappa coefficient more than 0.52–0.79). The frequency distribution of patients per category of the different videolaryngostroboscopic variables is shown in the online only supplementary material, providing an indication of the average baseline voice function of the study population. None of the patients showed anatomical changes as a result of vocal fold pathology of organic origin, such as polyps, vocal fold nodules, cysts and so on.
In addition to the complete case analysis, a mixed effects binary logistic model was used for the binary outcomes that were measured repeatedly. For this statistical method, the patients were divided into two clinical patient labels: a normal ‘0’ versus abnormal ‘1’ videolaryngostroboscopic status. Patients received the clinical label of ‘abnormal’ videolaryngostroboscopic status if their videolaryngostroboscopic examination was scored as impaired (score 1 or higher) in one or more of the measured videolaryngostroboscopic variables (see online only supplementary material).
Table 4 shows the baseline versus post-treatment videolaryngostroboscopic status for each treatment group. In total, 63 patients were included in the logistic regression complete case analysis for the videolaryngostroboscopic outcome (group 1: voice therapy, n = 23; group 2: voice therapy + motor level surface electrical stimulation, n = 21; group 3: voice therapy + sensory level surface electrical stimulation, n = 19). In order to assess the group effect, an interaction term with the videolaryngostroboscopic variables at baseline was included, which did not show any baseline group differences, even for the missing values. Thirty (47.6 per cent) patients had an abnormal videolaryngostroboscopic status at baseline, and 22 patients (34.9 per cent) had an abnormal status after treatment (odds ratio = 0.194; 95 per cent confidence interval (CI) = 0.06 to 0.607; p = 0.003). The group effect was not statistically significant (p = 0.845). Taking into account the missing values, the mixed effects binary logistic regression analysis produced an odds ratio of 0.470 (95 per cent confidence interval = 0.221 to 0.997; p = 0.049). Fourteen patients (22.2 per cent) showed a positive treatment effect where the videolaryngostroboscopic status abnormal (1) at baseline changed into a normal status (0) post-treatment. After adjustment for age, gender, and the Hoehn and Yahr score in the logistic regression analysis, no statistically significant between-group differences in the videolaryngostroboscopic outcome were found. Furthermore, 6 patients (9.5 per cent) showed a negative treatment effect meaning that the videolaryngostroboscopic normal status of ‘0’ at baseline changed into an abnormal status of ‘1’ post-treatment. This ‘reversed effect’ was equally distributed over the three treatment groups (n = 2 per group). In order to account for missing values, multiple imputation was performed. This technique produced a crude odds ratio of 0.245 (95 per cent CI = 0.081 to 0.741; p = .002) and an adjusted odds ratio of 0.184 (95 per cent CI = 0.054 to 0.633; p = .007). Sensitivity analyses, which were performed to test the effect of the treatment group, age, gender, and the Hoehn and Yahr score, showed similar results for both the crude and adjusted logistic regression analyses.
Table 4. Descriptive statistics in absolute numbers of the baseline and the post-treatment videolaryngostroboscopic status (0 versus 1) per treatment group and for the total group (n = 63)
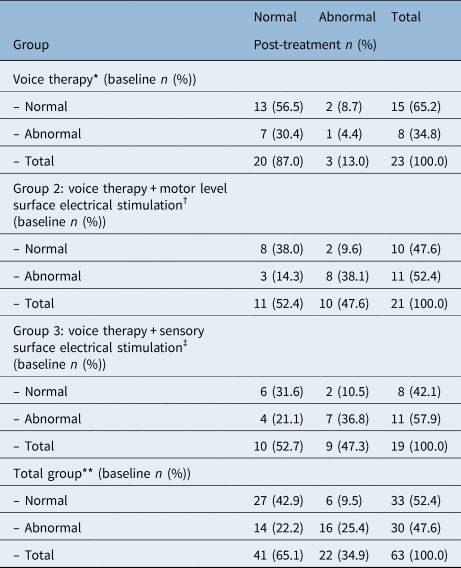
*Improved: 7 of 23 (30.5 per cent), deteriorated: 2 of 23 (8.7 per cent); †improved: 3 of 21 (14.3 per cent), deteriorated: 2 of 21 (9.5 per cent); ‡improved: 4 of 19 (21.1 per cent), deteriorated: 2 of 19 (10.5 per cent); **improved: 14 of 63 (22.2 per cent), deteriorated: 6 of 63 (9.5 per cent)
Discussion
In the present study, the effect of surface electrical stimulation as an adjunct to voice therapy (usual care) was investigated in dysphonic idiopathic Parkinson's disease patients. It was explored whether surface electrical stimulation of the suprahyoid region changes videolaryngostroboscopic outcome scores and patients’ self-assessment of voice impairment in daily life. Safety, feasibility and acceptability of surface electrical stimulation for dysphonia in idiopathic Parkinson's disease were high as none of the patients left the trial because of adverse events or non-compliance to therapy.
The pathophysiology of dysphonia in idiopathic Parkinson's disease is complex. It depends on the coordination of factors in both the peripheral and the central nervous system. Dysphonia can be caused by uncoordinated or disrupted signals along the dopaminergic and non-dopaminergic neural pathways.Reference Jankovic1,Reference Herd, Tomlinson, Deane, Brady, Smith and Sackley3 Previous studies described that idiopathic Parkinson's disease patients experience progressive voice impairment with the progression of their disease.Reference Gamboa, Jimenez-Jimenez, Nieto, Montojo, Orti-Pareja and Molina30–Reference van Hooren, Baijens, Vos, Pilz, Kuijpers and Kremer32 In this context, well-known voice characteristics of idiopathic Parkinson's disease are, among others, breathiness and reduced loudness because of vocal fold bowing or atrophy, vocal fold tremor or rigidity, and weakened diaphragmatic breathing.Reference Zhang, Jiang and Rahn33 On the grounds of clinical experience and the literature, we assumed that adding a peripheral stimulus at a sufficient intensity over the suprahyoid triangle with suprahyoid muscles and innervations from cervical and cranial nerves such as the trigeminal, hypoglossal with ansa cervicalis and superior laryngeal nerves (internal and external branches) originating from the vagal nerve could alter the videolaryngostroboscopic characteristics and the idiopathic Parkinson's disease patients' self-assessment of voice.Reference Lagorio, Carnaby-Mann and Crary13,Reference Humbert, Poletto, Saxon, Kearney and Ludlow23,Reference Seifpanahi, Izadi, Jamshidi and Shirmohammadi34
A significantly positive therapeutic effect within group 2 for the Voice Handicap Index-Emotional, Voice Handicap Index-Functional and Voice Handicap Index-Total (sub)scales was found. However, when comparing this therapeutic effect of group 2 with the Voice Handicap Index outcomes of group 1 and 3, the motor level surface electrical stimulation did not have a significant additional therapeutic effect. The improvement (baseline vs post-treatment) on the Voice Handicap Index-Total score in the total group suggests that intensive voice therapy does have a significant positive treatment effect. This positive therapeutic effect was also seen in the videolaryngostroboscopic results where 14 patients (22.2 per cent) showed an improved videolaryngostroboscopic status following treatment. Nevertheless, after adjustment for age, gender, and the Hoehn and Yahr score in the logistic regression analysis, no statistically significant between-group differences in the videolaryngostroboscopic outcome were found. Furthermore, six patients, equally distributed over the three groups, showed a deterioration of the videolaryngostroboscopic status following treatment. Reasons for this may include spontaneous disease progression of the idiopathic Parkinson's disease or other variables not measured in our protocol, such as pulmonary function parameters. The findings of the present study confirm the results of previous studies showing the benefits of voice therapy in idiopathic Parkinson's disease.Reference Ramig, Sapir, Fox and Countryman7,Reference Ramig, Fox and Sapir8,Reference Sackley, Smith, Rick, Brady, Ives and Patel35
Thus, no enhancing effect of adjunctive surface electrical stimulation was observed in the present study. The absence of a therapeutic effect of surface electrical stimulation in this study might be explained as follows. According to other authors, excitability depends on the stimulation parameters applied.Reference Fraser, Rothwell, Power, Hobson, Thompson and Hamdy36–Reference Bidus, Thomas and Ludlow39 The fixed stimulation variables (frequency 80 Hz, pulse width 700 μs, current intensity 0 to 25 mA) of the VitalStim appliance may not have been appropriate to induce any therapeutic effect during 15 days of surface electrical stimulation in dysphonic idiopathic Parkinson's disease patients. Another reason for the absence of group differences because of surface electrical stimulation may be that snap skin electrodes are not a precisely targeted method of electrical stimulation for suprahyoid muscles and nerves. However, a previous study in 32 healthy participants without any vocal pathology, with a similar placement of the electrodes as in the present study, did result in increased vocal fold adduction during stimulation at rest.Reference Seifpanahi, Izadi, Jamshidi and Shirmohammadi34 Perhaps other anatomical subsites of the neck are more susceptible to the reception and transduction of electrical stimuli for voice rehabilitation in idiopathic Parkinson's disease patients.Reference Bidus, Thomas and Ludlow39 Furthermore, the body of literature on studies using surface electrical stimulation in the context of voice rehabilitation is poor and does not allow a direct comparison with our results. These studies were conducted mainly on healthy patients or in patient groups that were not comparable with the current idiopathic Parkinson's disease group. Their study designs with regard to the applied type of electrical stimulator, stimulation paradigm and voice assessment protocol were also not comparable.Reference Lagorio, Carnaby-Mann and Crary13,Reference Seifpanahi, Izadi, Jamshidi and Shirmohammadi34,Reference Gorham-Rowan and Morris40,Reference Ko, Park, Hyun, Seo and Kim41
A central cueing effect of the motor- or sensory level stimulus helping the patient to improve the vocal function was expected but was ultimately not found in the present idiopathic Parkinson's disease sample.Reference Pinnington, Muhiddin, Ellis and Playford17,Reference Hirsch and Hammond42 In idiopathic Parkinson's disease, a deficit in the basal ganglia can result in disturbed internal cueing of automatic, sequential movements, such as gait, voice or swallowing. External cues provide temporal (timing) or spatial (size) stimuli associated with the initiation and ongoing facilitation of motor activity.Reference Hirsch and Hammond42 External cues can be applied in the form of visual, auditory and tactile stimuli that can trigger movements or that can provide rhythmic or spatial support to the central nervous system improving the quality and timing of movements. Thus, the explanation that external cue training using surface electrical stimulation reroutes the movement through a non-automatic pathway, removing it from the automatic basal ganglia pathway, could not be used as a hypothesis in the present study.Reference Hirsch and Hammond42,Reference Martens and Almeida43
• Dysphonia is a common symptom in idiopathic Parkinson's disease
• Surface electrical stimulation can improve muscle strength
• Voice therapy improves Voice Handicap Index and videolaryngostroboscopy results
• Surface electrical stimulation does not seem to improve Voice Handicap Index or videolaryngostroboscopic results in idiopathic Parkinson's disease
Previously, in a small case series of patients (without idiopathic Parkinson's disease) with chronic dysphonia as a result of vocal fold bowing, surface electrical stimulation applied over the superior laryngeal nerves and the cricothyroid muscles did significantly improve Voice Handicap Index scores.Reference Lagorio, Carnaby-Mann and Crary13 This study inspired us to design the present larger quasi-randomised study for idiopathic Parkinson's disease patients. Likewise, in our study, a therapeutic effect was found, as indicated by improved videolaryngostroboscopic and Voice Handicap Index scores for all three groups together. However, this effect cannot be attributed to surface electrical stimulation as we did not find any significant post-treatment between-group differences for the videolaryngostroboscopic and Voice Handicap Index scores. Instead, we can attribute the improvement in the three groups to exercises, since all groups received voice therapy. In itself, this is a valuable finding that can confirm the added value of voice therapy (usual care) for dysphonia in idiopathic Parkinson's disease patients. The present study results are preliminary and explorative, making it necessary to have further investigation that also considers sham stimulation.
Conclusion
This quasi-randomised, controlled study showed that intensive voice therapy (usual care) improved idiopathic Parkinson's disease patients' self-assessment of voice impairment and the videolaryngostroboscopic outcome score. However, surface electrical stimulation did not improve idiopathic Parkinson's disease patients' self-assessment of voice impairment using the Voice Handicap Index questionnaire or the videolaryngostroboscopic outcome score. The application of surface electrical stimulation for dysphonic complaints in idiopathic Parkinson's disease patients is unprecedented, and these explorative conclusions are preliminary.
Supplementary material
The supplementary material for this article can be found at https://doi.org/10.1017/S0022215122002031.
Acknowledgements
The authors thank R Biesheuvel for his contributions to the artwork.
Competing interests
None declared









