Article contents
Catalytic influence of Ni-based additives on the dehydrogenation properties of ball milled MgH2
Published online by Cambridge University Press: 26 August 2011
Abstract
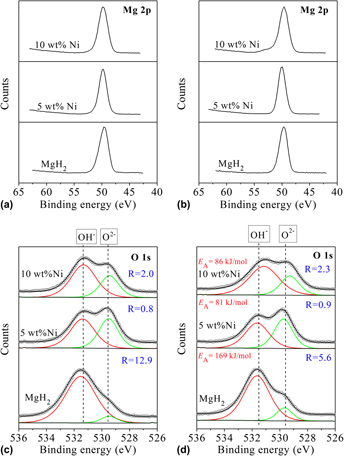
The catalytic influence of Ni, Zr2Ni5, and LaNi5 on the dehydrogenation properties of milled MgH2 was investigated. MgH2 milled in the presence of Ni (5 wt%) and Zr2Ni5 (5 wt%) catalysts for 2 h showed apparent activation energies, EA, of 81 and 79 kJ/mol, respectively, corresponding to ∼50% decrease in EA and a moderate decrease (∼100 °C) in the decomposition temperature (Tdec). A further 27 °C decrease in Tdec was observed after milling with 10 wt%Ni. Based on the EA values, the catalytic activity decreased in the following order: Ni ≈ Zr2Ni5 > LaNi5. X-ray photoelectron spectroscopy analysis of the milled and dehydrogenated states of the hydrides modified with Ni catalyst revealed that the observed reduction in EA may be due to the ability of Ni catalyst to decrease the amount of oxygen atoms in defective positions that are capable of blocking catalytically active sites thereby enhancing the dehydrogenation kinetics. In particular, our results reveal a strong correlation between the type of oxygen species adsorbed on Ni-modified MgH2 and the EA of the dehydrogenation reaction.
- Type
- Articles
- Information
- Copyright
- Copyright © Materials Research Society 2011
References
REFERENCES
- 9
- Cited by




