Article contents
Comparative and quantitative investigation of cell labeling of a 12-nm DMSA-coated Fe3O4 magnetic nanoparticle with multiple mammalian cell lines
Published online by Cambridge University Press: 17 January 2011
Abstract
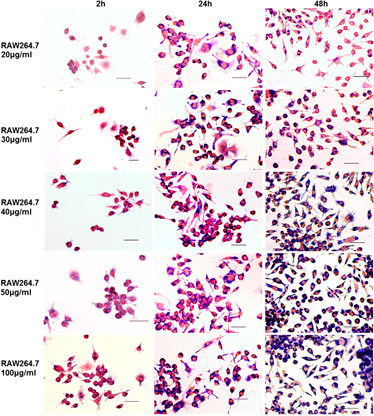
This work investigated the cell labeling of 12-nm meso-2,-3-dimercaptosuccinic acid (DMSA)-coated Fe3O4 magnetic nanoparticles with multiple mammalian cells. Six different cells, including RAW264.7, Hepa1-6, THP-1, HepG2, HeLa, and HL-7702, were treated with the nanoparticles at various concentrations (20~100 μg/mL) for different times (2~72 h), and the labeling effect was evaluated by observing the intracellular internalization of the nanoparticles with Prussian blue staining and measuring the corresponding cellular iron loading with colorimetric assay. The results demonstrated that the nanoparticles could label all cells studied. However, the labeling efficiency was not the same between different cells, which depended on the cell types, the nanoparticles’ concentration, and the time of treating cells with the nanoparticles. In comparison, RAW264.7 was labeled more effectively than other cells at any concentration of the nanoparticles. The iron loading of RAW264.7 significantly increased with the concentration of the nanoparticles and the treatment time. However, both human liver cells (HepG2 and HL-7720) were labeled with the lowest iron loading. The measurement of cell viability revealed that the growth of all cells was not affected by the nanoparticles at a common in vivo application dose of iron nanoparticles (30 μg/mL), demonstrating that the nanoparticles have better biocompatability.
Keywords
- Type
- Articles
- Information
- Copyright
- Copyright © Materials Research Society 2011
References
REFERENCES
- 9
- Cited by




