Introduction
The Late Triassic saw several periods of major biodiversity turnover, culminating in the End Triassic Extinction (ETE), described as one of the five largest mass extinction events in Earth history. Studies have suggested that >50% of genera from marine and terrestrial environments (Deenan et al., Reference Deenan, Ruhl, Bonis, Krijgsman, Kuerschner, Reitsma and Van Bergen2010), 23.4% of marine families, and 21.7% of terrestrial families (McGhee et al., Reference McGhee, Sheehan, Bottjer and Droser2004) went extinct (although insects were not counted in these studies). Nevertheless, there are current discussions on the severity of this event and whether it was one event or drawn out throughout the Rhaetian. The effects of this period on insect diversity are not well studied and large-scale datasets of insect diversity through time tend not to show an extreme event at the Triassic-Jurassic Boundary (TJB) (see Nicholson et al., Reference Nicholson, Mayhew and Ross2015; Condamine et al., Reference Condamine, Clapham and Kergoat2016). This paper is part of a larger study investigating the changes in insect diversity across the TJB to assess the impact of the ETE on insect diversity. Because there are few currently available rich insect outcrops, a large part of this project is the taxonomic revision of historical fossil insect collections from the Late Triassic to Early Jurassic at the species level to bring the taxonomy to current understanding. Herein, we revise Liassophlebiidae Tillyard, Reference Tillyard1925, a family of damsel-dragonflies known from the Late Triassic and Early Jurassic of Europe, Central Asia, China, and Antarctica.
Liassophlebiidae is a small extinct family of damsel-dragonflies known from the early Mesozoic of western Europe, Central Asia, and Antarctica described in the suborder Epiprocta, which contains the extant dragonflies, similar in form to the other extant odonate suborder Zygoptera (damselflies). Most species are described from isolated wings or abdominal segments of adults and there are no known larval specimens, although it is difficult to compare adult and larval fossil specimens because they are so different morphologically. Only Liassophlebia Tillyard, Reference Tillyard1925 and Petrophlebia Tillyard, Reference Tillyard1925 (type species P. anglicana Tillyard, Reference Tillyard1925) were included in the family by Tillyard (Reference Tillyard1925). Liassophlebia originally comprised four species based on wings: L. magnifica Tillyard, Reference Tillyard1925, L. batheri Tillyard, Reference Tillyard1925, L. withersi Tillyard, Reference Tillyard1925, and L. westwoodi (Hagen, Reference Hagen1850), and two possible species based on partial abdomens: L. (?) clavigaster Tillyard, Reference Tillyard1925 and L. (?) hopei (Brodie, Reference Brodie1845). Liassophlebia westwoodi was described from a specimen previously figured by Brodie (Reference Brodie1845, pl. 10, fig. 8) and named Heterophlebia westwoodi by Hagen (Reference Hagen1850, p. 359); it was later transferred to Tarsophlebia Hagen, Reference Hagen1866 by Hagen (Reference Hagen1866). Liassophlebia (?) hopei was described from a specimen figured and named Libellula hopei by Brodie (Reference Brodie1845, pl. 10, fig. 3); it was later transferred to Heterophlebia Westwood, Reference Westwood1849 by Selys-Longchamps and Hagen (Reference Selys-Longchamps and Hagen1850) and then ‘(Anisozygopteron?)’ by Handlirsch (Reference Handlirsch1906). Petrophlebia was transferred to Architemistidae Tillyard, Reference Tillyard1917 by Fraser (Reference Fraser1957) and then to Campterophlebiidae Handlirsch, Reference Handlirsch1920 by Nel et al. (Reference Nel, Martínez-Delclòs, Paicheler and Henrotay1993). Although Bechly (Reference Bechly2016) proposed to transfer it back to Architemistidae, its position in the Campterophlebiidae was confirmed by revision of the family by Kelly and Nel (Reference Kelly and Nel2017).
The diagnosis of Liassophlebiidae was slightly emended by Zeuner (Reference Zeuner1962) to include species with a discoidal cell closed basally. Petrophlebia anglicanopsis Zeuner, Reference Zeuner1962, Liassophlebia jacksoni Zeuner, Reference Zeuner1962, and L. gigantea Zeuner, Reference Zeuner1962 were also described. Additional specimens were identified as L. magnifica from the Jackson collection of the lower Lias of Dorset. The Jackson collection was re-examined by Whalley (Reference Whalley1985) who altered some of Zeuner’s species descriptions, transferred P. anglicanopsis to Liassophlebia and described L. pseudomagnifica Whalley, Reference Whalley1985 from one of Zeuner’s L. magnifica specimens. Liassophlebia anglicanopsis was considered an uncertain taxon in Liassophlebiidae by Nel et al. (Reference Nel, Martínez-Delclòs, Paicheler and Henrotay1993).
Additional genera were included from continental Europe, Central Asia, China, and Antarctica, which are not known from the British deposits: (1) Caraphlebia Carpenter, Reference Carpenter1969 from South Victoria Land, Antarctica was considered possibly referable to Turanothemistidae Pritykina, Reference Pritykina1968 by Nel et al. (Reference Nel, Martínez-Delclòs, Paicheler and Henrotay1993) but was transferred to Selenothemistidae Handlirsch, Reference Handlirsch1939 by Bechly (Reference Bechly2016); (2) Ferganophlebia Pritykina, Reference Pritykina1970 from the Early Jurassic of Kyrgyzstan; (3) Paraliassophlebia Hong, Reference Hong1983 from the Middle Jurassic of China was considered as Epiprocta incertae sedis by Nel et al. (Reference Nel, Martínez-Delclòs, Paicheler and Henrotay1993) but was tentatively transferred to Selenothemistidae by Bechly (Reference Bechly2016); and (4) Grimmenopteron Ansorge, Reference Ansorge1996 and Bavarophlebia Nel and Petrulevičius, Reference Nel and Petrulevičius2005, both from the Toarcian of Germany, each with one associated species. A further specimen was identified as Liassophlebia sp. from the Early Jurassic of Austria by Kohli et al. (Reference Kohli, Ware and Bechly2016).
Liassophlebiidae was reported to be known from the Early Jurassic, Hettangian-Toarcian, by Nicholson et al. (Reference Nicholson, Mayhew and Ross2015, supplementary data) but this disregards the four species described by Tillyard (Reference Tillyard1925) from the Penarth Group of Strensham, England, which is Late Triassic: Rhaetian. This locality was reported as Rhaetian by Nicholson et al. (Reference Nicholson, Mayhew and Ross2015) for other families but Tillyard’s book was not cited so these species must have been missed. The inclusion of this omission changes the dynamics of the family across the TJB because including these four species extends the family to before the boundary, indicating that the family survived the extinction event rather than originating in the subsequent stage.
Geological setting
The specimens discussed herein are from the Late Triassic to Early Jurassic of England (Fig. 1). They are mostly found in shallow marine limestones; these rocks are fine-grained enough to generally preserve insects well. The living insects were not necessarily associated with the marine environment but were transported from their terrestrial or freshwater habitat through fluvial systems to their final deposition in the marine shallows. The Late Triassic material was collected from bed 18 of the Penarth Group at the historical locality of Strensham in the county of Worcestershire (Brodie, Reference Brodie1845) and was updated to current geological nomenclature by Kelly et al. (Reference Kelly, Ross and Davidson2017) who found the horizon to lie within the Lilstock Formation, Cotham Member, which is Rhaetian in age. The Early Jurassic fossils were collected from three localities Binton in the county of Warwickshire, another historical locality, exposed several insect-bearing horizons, which Brodie (Reference Brodie1845) originally described and Kelly et al. (Reference Kelly, Ross and Jarzemowski2018) found to correspond to the Planorbis Chronozone (Blue Lias Formation, Wilmcote Limestone Member) of the Hettangian. The other two localities—Stonebarrow and Catherston Lane—are very similar and are found at or near the Dorset coast. Stonebarrow is still actively collected from and Catherston Lane was active for a short time during the construction of the Charmouth bypass. There are several insect-bearing horizons; the insects discussed herein were collected from the ‘flatstones’ (bed 83/83h), which is a local name for a horizon found in the Obtusum Chronozone, Obtusum Subchronozone (Charmouth Mudstone Formation, Black Ven Mudstone Member) (Page, Reference Page2010; Kelly et al., Reference Kelly, Ross and Davidson2017).
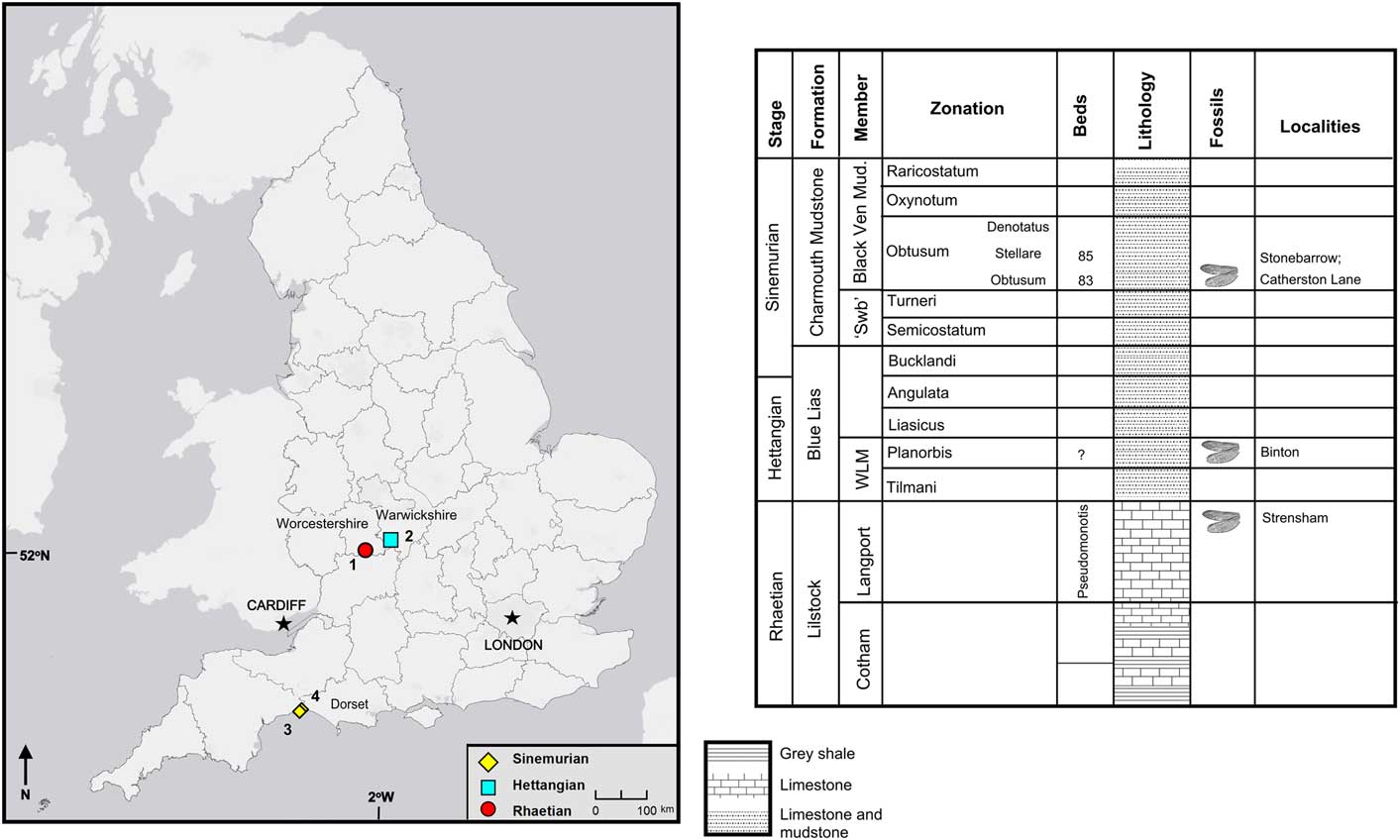
Figure 1 Locality map and stratigraphic chart for English specimens described herein. 1 = Strensham, Gloucestershire; 2 = Binton, Warwickshire; 3 = Stonebarrow, Dorset; 4 = Catherston Lane, Dorset; ‘Swb’ = ‘shales-with-beef’; WLM = Wilmcote Limestone Member.
Caraphlebia antarctica Carpenter, Reference Carpenter1969 was described from the Jurassic ‘Mawson Tillite’ on Carapace Nunatak, South Victoria Land, Antarctica (Carpenter, Reference Carpenter1969). Mawson Tillite is a historical name for the Mawson Diamictite of the Ferrar Group (Balance and Watters, Reference Balance and Watters1971), now known as the Mawson Formation (Elliot and Hanson, Reference Elliot and Hanson2001). Based on U/Pb and 40Ar/39Ar analysis of volcanic rocks in North Victoria Land, Musumeci et al. (2004) concluded that the pyroclastic event that led to the formation of the Prebble, Mawson, and Exposure Hill formations occurred between the Hettangian and lower Pliensbachian. However, given the sizable hiatus of Lower Jurassic rocks in South Victoria Land (Ribecai, Reference Ribecai2007; Schöner et al., Reference Schöner, Bomfleur, Schneider and Viereck-Goette2011), any fossils from the Mawson Formation in this area are likely to have been deposited later within this estimation.
The Mawson Formation is unconformably overlain by the Carapace Sandstone Formation, which is not present in all areas (Ribecai, Reference Ribecai2007). However, the insect was collected on Carapace Nunatak where the formation is present and has been estimated to have been deposited during the upper Sinemurian to lower Pliensbachian (Ribecai, Reference Ribecai2007). This means that the age estimation for the Mawson Formation in this area could lie within the later Hettangian to early late Sinemurian, making them of similar age to the English specimens.
Materials and methods
Collections of English material were examined from The Natural History Museum, London (NHMUK), the National Museum of Wales, Cardiff (NMW), and the Oxford University Museum of Natural History (OUMNH). Photographs of the Antarctic specimen were examined from the National Museum of Natural History (United States National Museum), Smithsonian Institution, Washington, DC (USNM). All specimens from the English Midlands (Warwickshire and Worcestershire) were collected by the Rev. Peter Bellinger Brodie in the nineteenth century except for the OUMNH specimen, which was collected by the Rev. Frederick William Hope; all of the Dorset specimens were collected by James Frederick Jackson in the twentieth century except for the one collected from Catherston Lane, which was collected by a team of volunteers led by Kevin Page.
All specimens (except the holotype of Caraphlebia antarctica) were examined in person by the primary author and remotely via photographs by the co-author. Specimens were examined using the microscope equipment available at each museum and photographs were taken with a stand supporting a Nikon D3300 camera with AF-S Micro Nikkor 40-mm macrolens. Measurements were taken from photographs using the software package ImageJ (National Institutes of Health, ver. 1.51) and the scale of each image was calibrated using a standard ruler. Taxonomic figures were constructed in DrawPlus (Serif, version X8).
Venation nomenclature is based on the interpretations of Riek and Kukalová-Peck (Reference Riek and Kukalová-Peck1984), as modified by Nel et al. (Reference Nel, Martínez-Delclòs, Paicheler and Henrotay1993) and Bechly (Reference Bechly1996). Abbreviations are as follows: AA = anterior anal; AP = posterior anal; Arc = arculus; Ax = primary antenodal crossvein; Ax0 = first branch of primary antenodal crossvein; Ax1 = second branch of primary antenodal crossvein; Ax2 = third branch of primary antenodal crossvein; C = costal vein; Cu = cubitus; CuA = anterior cubitus; CuA1 = distal branch of anterior cubitus; CuA2 = proximal branch of anterior cubitus; CuP = posterior cubitus; DC = discoidal cell; Ht = hypertriangle; IM = intercalary medial vein; IR = intercalary radial vein; IR1 = intercalary radial vein 1; IR2 = intercalary radial vein 2; MA = anterior median; MA1 = anterior branch of anterior median; MA2 = posterior branch of anterior median; MP = posterior median; N = nodus; ‘O’ = oblique vein; Pt = pterostigma; RA = anterior radius; RP = posterior radius; RP1 = first branch of posterior radius; RP2 = second branch of posterior radius; RP3/4 = third/fourth branch of posterior radius; ScP = posterior subcostal; T = triangle. The higher classification of fossil and extant Odonatoptera is based on the phylogenetic system of Bechly (Reference Bechly1996, Reference Bechly2016).
Repositories and institutional abbreviations
NHMUK, The Natural History Museum, London, UK; NMW, National Museum of Wales, Cardiff, UK; OUMNH, Oxford University Museum of Natural History, Oxford, UK; USNM, National Museum of Natural History, Smithsonian Institution, Washington, DC, USA.
Systematic paleontology
Order Odonata Fabricius, Reference Fabricius1793
Suborder Epiprocta Lohmann, Reference Lohmann1996
Superfamily Heterophlebioidea Needham, Reference Needham1903
Family Liassophlebiidae Tillyard, Reference Tillyard1925
Type genus
Liassophlebia Tillyard, Reference Tillyard1925.
Other genera
Ferganophlebia Pritykina, Reference Pritykina1970; Grimmenopteron Ansorge, Reference Ansorge1996; Bavarophlebia Nel and Petrulevičius, Reference Nel and Petrulevičius2005; Rossiphlebia new genus.
Emended diagnosis
Discoidal cell basally closed in hindwing, sometimes with incomplete veinlet between ‘hypertriangle’ and discoidal triangle; discoidal cell open in forewing; subdiscoidal cell closed in both; subdiscoidal cell widened in forewing, with convex posterior margin; MP+CuA with very strong posterior curve in discoidal cell, so that subdiscoidal space is rather transverse; hindwings with unicellular anal loop enlarged; few, if any, antefurcal crossveins between RP and MA from arculus to midfork; no secondary antenodal crossveins between C and ScP (first row).
Remarks
A nearly straight, long secondary vein in the postdiscoidal space slightly distal of the triangle is present in Liassophlebia and Rossiphlebia n. gen., but this vein is absent in Grimmenopteron, Ferganophlebia, and Caraphlebia.
Genus Liassophlebia Tillyard, Reference Tillyard1925
Type species
Liassophlebia magnifica Tillyard, Reference Tillyard1925.
Emended diagnosis
Cubito-anal area of hindwing large and broad, with 5 or 6 rows of cells between CuA and posterior wing margin; subdiscoidal space not divided into two large cells by anterior branch of AA that ends on CuA, but divided into small cells; wings very large.
Liassophlebia magnifica Tillyard, Reference Tillyard1925
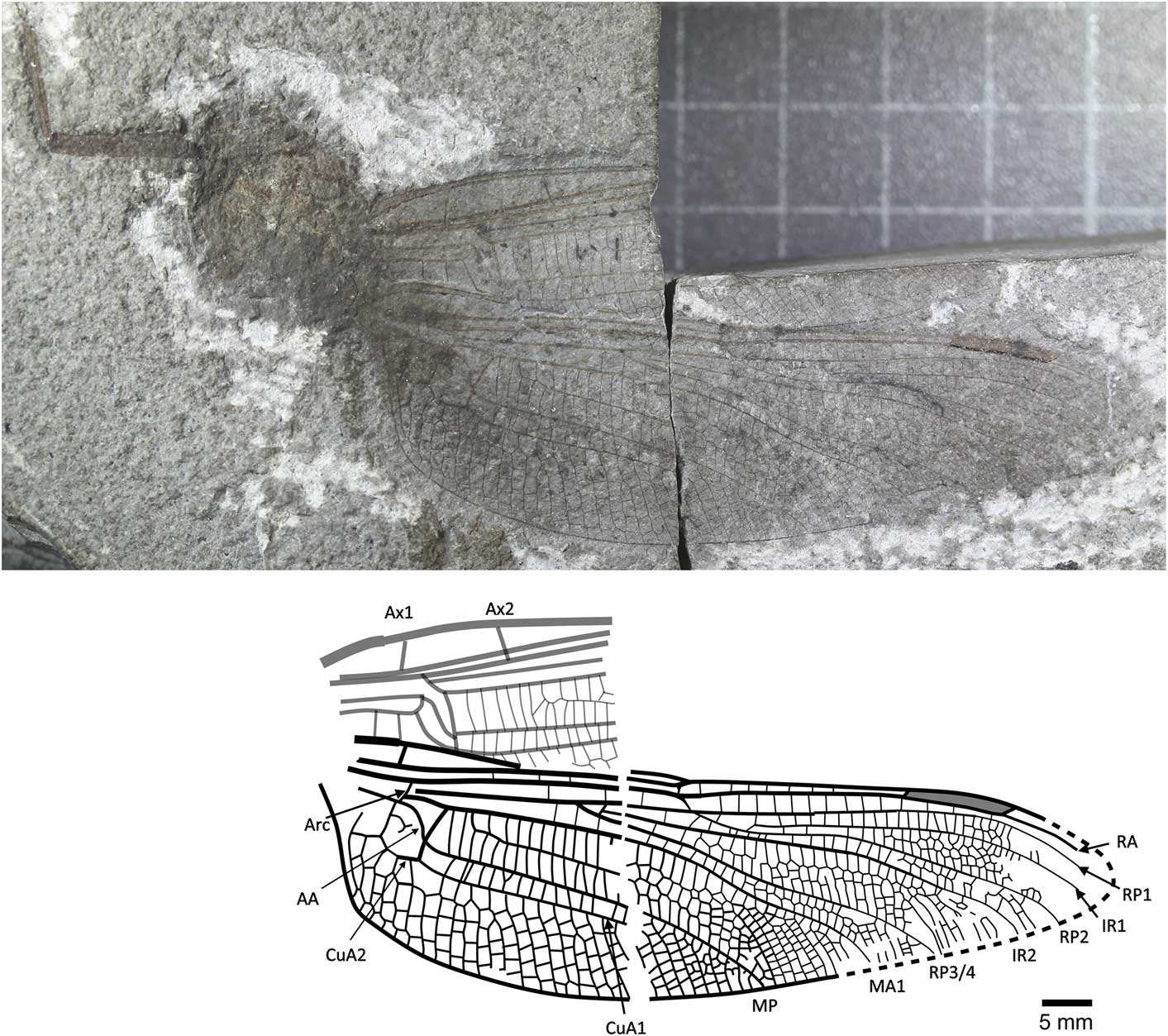
Figure 2 Holotype of Liassophlebia magnifica Tillyard, Reference Tillyard1925 (NHMUK I.6648), Binton, Warwickshire (Hettangian), photograph and reconstruction. AA = anterior anal; Arc = arculus, Ax1 = second branch of primary antenodal crossvein; Ax2 = third branch of primary antenodal crossvein; CuA1 = distal branch of anterior cubitus; CuA2 = proximal branch of anterior cubitus; IR1 = intercalary radial vein 1; IR2 = intercalary radial vein 2; MA1 = anterior branch of anterior median; MP = posterior median; RA = anterior radius; RP1 = first branch of posterior radius; RP2 = second branch of posterior radius; RP3/4 = third/fourth branch of posterior radius.
1925 Liassophlebia magnifica Reference TillyardTillyard, p. 15, pl. 1, fig. 3, pl. 2, fig. 4, text-figs. 3, 4.
1939 Liassophlebia magnifica; Reference HandlirschHandlirsch, p. 23.
1957 Liassophlebia magnifica; Reference AsahinaAsahina, p. 1, figs. 1, 3.
1962 Liassophlebia magnifica; Reference ZeunerZeuner, p. 162, pl. 27, fig. 1.
1993 Liassophlebia magnifica; Reference Nel, Martínez-Delclòs, Paicheler and HenrotayNel et al., p. 139, fig. 107.
1995 Liassophlebia magnifica; Reference BechlyBechly, p. 16.
1996 Liassophlebia magnifica; Reference TruemanTrueman, p. 69.
2003 Liassophlebia magnifica; Reference Fleck, Bechly, Martínez-Delclòs, Jarzembowski, Coram and NelFleck et al., p. 56, 86.
2003 Liassophlebia magnifica; Reference RehnRehn, p. 212.
Holotype
NHMUK I.6648/I.10462 (Fig. 2), ‘Insect limestone’ of the Planorbis Chronozone (Lias Group, Blue Lias Formation, Wilmcote Limestone Member); Early Jurassic, Hettangian; Binton, Warwickshire. Female according to lack of anal angle and anal triangle.
Emended diagnosis
Female hindwing, anal branch forks into subdiscoidal space forming distinct structure (also seen in Liassophlebia pseudomagnifica, but see below).
Remarks
The specimen NHMUK I.11089 was also attributed to this species (Tillyard, Reference Tillyard1925) but only a partial abdomen is preserved and so it is impossible to link it with any species described from wings. Zeuner (Reference Zeuner1962) also attributed NHMUK In.64000, In.59106, and In.49213 to this species, but the first is the holotype of L. pseudomagnifica (see below); the second is the holotype of Hypsothemis fraseri Whalley, Reference Whalley1985, and the last is a fragment of the anterior margin of a wing and is not identifiable at the species level.
Liassophlebia withersi Tillyard, Reference Tillyard1925
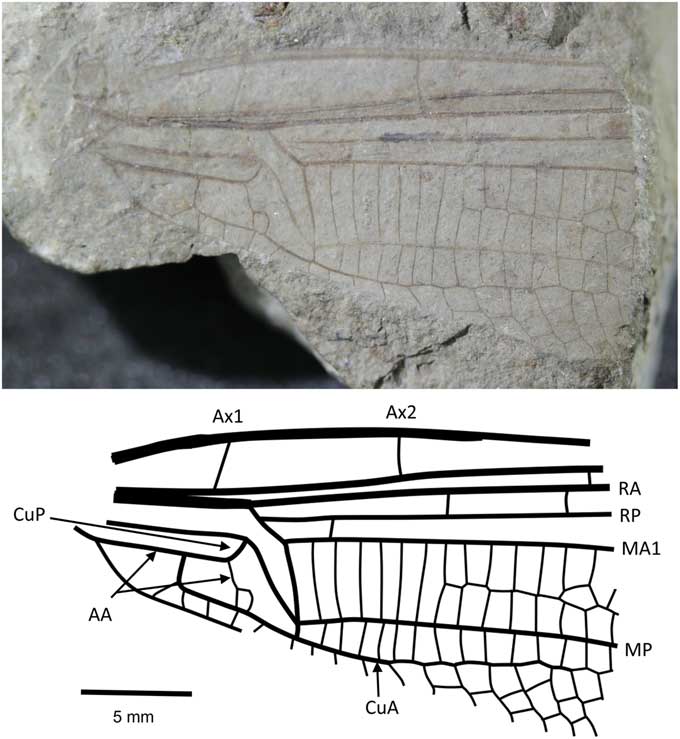
Figure 3 Holotype of Liassophlebia withersi Tillyard, Reference Tillyard1925 (NHMUK I.10697), Strensham, Worcestershire (Rhaetian), photograph and reconstruction. AA = anterior anal; Ax1 = second branch of primary antenodal crossvein; Ax2 = third branch of primary antenodal crossvein; CuA = anterior cubitus; CuP = posterior cubitus; MA1 = anterior branch of anterior median; MP = posterior median; RA = anterior radius; RP = posterior radius.
1925 Liassophlebia withersi Reference TillyardTillyard, p. 17, pl. 3, fig. 8.
1939 Liassophlebia Withersi; Reference HandlirschHandlirsch, p. 23.
1962 Liassophlebia withersi; Reference ZeunerZeuner, p. 164.
1993 Liassophlebia withers; Reference Nel, Martínez-Delclòs, Paicheler and HenrotayNel et al., p. 142, fig. 106.
Holotype
NHMUK I.10697 (Fig. 3), ‘Insect limestone’ of the Pseudomonotis beds (Penarth Group, Lilstock Formation); Late Triassic, Rhaetian; Strensham, Worcestershire.
Diagnosis
Similar to Liassophlebia magnifica but smaller (distance from arculus to distal acute angle of discoidal cell = 5.6 mm, compare to >6 mm in L. magnifica). Two anal cells, compared to three in L. magnifica.
Additional material
NHMUK I.10528, from Strensham.
Remarks
The two specimens discussed here are forewings, whereas the other described species are known mostly from hindwings, except for the partial forewing in Liassophlebia magnifica. The differences between this partial forewing and that of the holotype of L. withersi are few and better-preserved specimens could lead to the synonimization of this species with L. magnifica, or with one of the other species currently only described from hindwings. NHMUK I.10528 is a forewing originally attributed to L. batheri (which is herein considered nomen dubium, see below). Upon examination, it is clear that there are few differences between this specimen and the holotype of L. withersi except for the aberration in the anal vein of the holotype NHMUK I.10697 and an additional crossvein in the area immediately basal to the subdiscoidal cell. There is also a size difference; NHMUK I.10697 is 10.1 mm in width when measured level with the distal point of the discoidal triangle, and I.10528 is 11.3 mm. The distance from the distal point of the discoidal triangle to the point at which the arculus meets the radial vein in I.10697 is 5.1 mm and in I.10528 is 6.4 mm. Upon further material being described, they might be recognized as separate species, but there is little justification to split them with the evidence available.
Liassophlebia pseudomagnifica Whalley, Reference Whalley1985
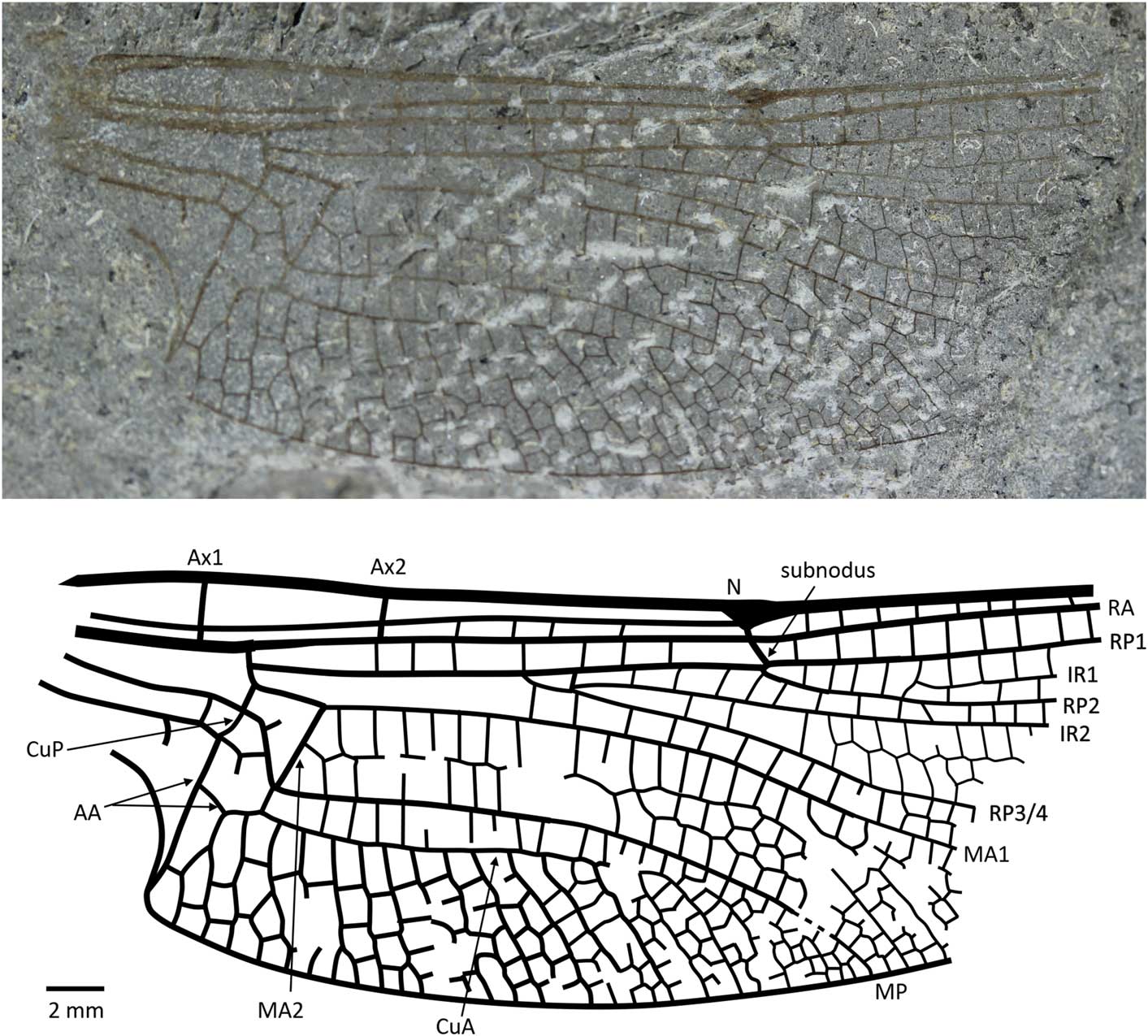
Figure 4 Holotype of Liassophlebia pseudomagnifica Whalley, Reference Whalley1985 (NHMUK I.64000); Stonebarrow, Dorset (Sinemurian); photograph and reconstruction. AA = anterior anal; Ax1 = second branch of primary antenodal crossvein; Ax2 = third branch of primary antenodal crossvein; CuA = anterior cubitus; CuP = posterior cubitus; IR1 = intercalary radial vein 1; IR2 = intercalary radial vein 2; MA1 = anterior branch of anterior median; MA2 = posterior branch of anterior median; MP = posterior median; N = nodus; RA = anterior radius; RP1 = first branch of posterior radius; RP2 = second branch of posterior radius; RP3/4 = third/fourth branch of posterior radius.
1962 Liassophlebia magnifica Tillyard; Reference ZeunerZeuner, p. 162, pl. 27, fig. 1.
1985 Liassophlebia pseudomagnifica Reference WhalleyWhalley, p. 120, fig. 5a, b.
1993 Liassophlebia pseudomagnifica; Reference Nel, Martínez-Delclòs, Paicheler and HenrotayNel et al., p. 139, figs. 104, 105.
2003 Liassophlebia pseudomagnifica; Reference Fleck, Bechly, Martínez-Delclòs, Jarzembowski, Coram and NelFleck et al., p. 56.
Holotype
NHMUK In.64000 (Fig. 4), ‘Flatstones’ (bed 83) of the Obtusum Chronozone, Obtusum Subchronozone (Lias Group, Charmouth Mudstone Formation, Black Ven Mudstone Member); Early Jurassic, lower Sinemurian; Stonebarrow, Dorset.
Diagnosis
Hindwing anal branch forking into subdiscoidal space similar to that seen in Liassophlebia magnifica; anal angle and triangle present.
Description
See previous redescription by Nel et al. (Reference Nel, Martínez-Delclòs, Paicheler and Henrotay1993).
Remarks
This species was split from Liassophlebia magnifica based on a difference in the number of rows of cells between ‘M and Cu’ (Whalley, Reference Whalley1985), but it is clear from re-examination of the types that the numbers of rows are the same in the two specimens. Also, the peculiar shape of the anal vein in the subdiscoidal space is shared by L. magnifica and L. pseudomagnifica; this shape is not seen in any other species of liassophlebiid. The main differences between the two types of L. magnifica and L. pseudomagnifica are in the shape of the anal area, which is related to sexual dimorphism (presence of an anal angle and triangle in males), present in all Epiprocta (= ‘Anisozygoptera’ + Anisoptera). Another difference concerns the supplementary longitudinal vein in the basal part of the postdiscoidal area that begins three cells distal of the discoidal triangle in L. magnifica whereas it begins very close to it in L. pseudomagnifica. This difference could be due to intraspecific variation. There is no way to determine whether they belong to the same species until better preserved specimens are found.
Liassophlebia batheri Tillyard, Reference Tillyard1925, nomen dubium
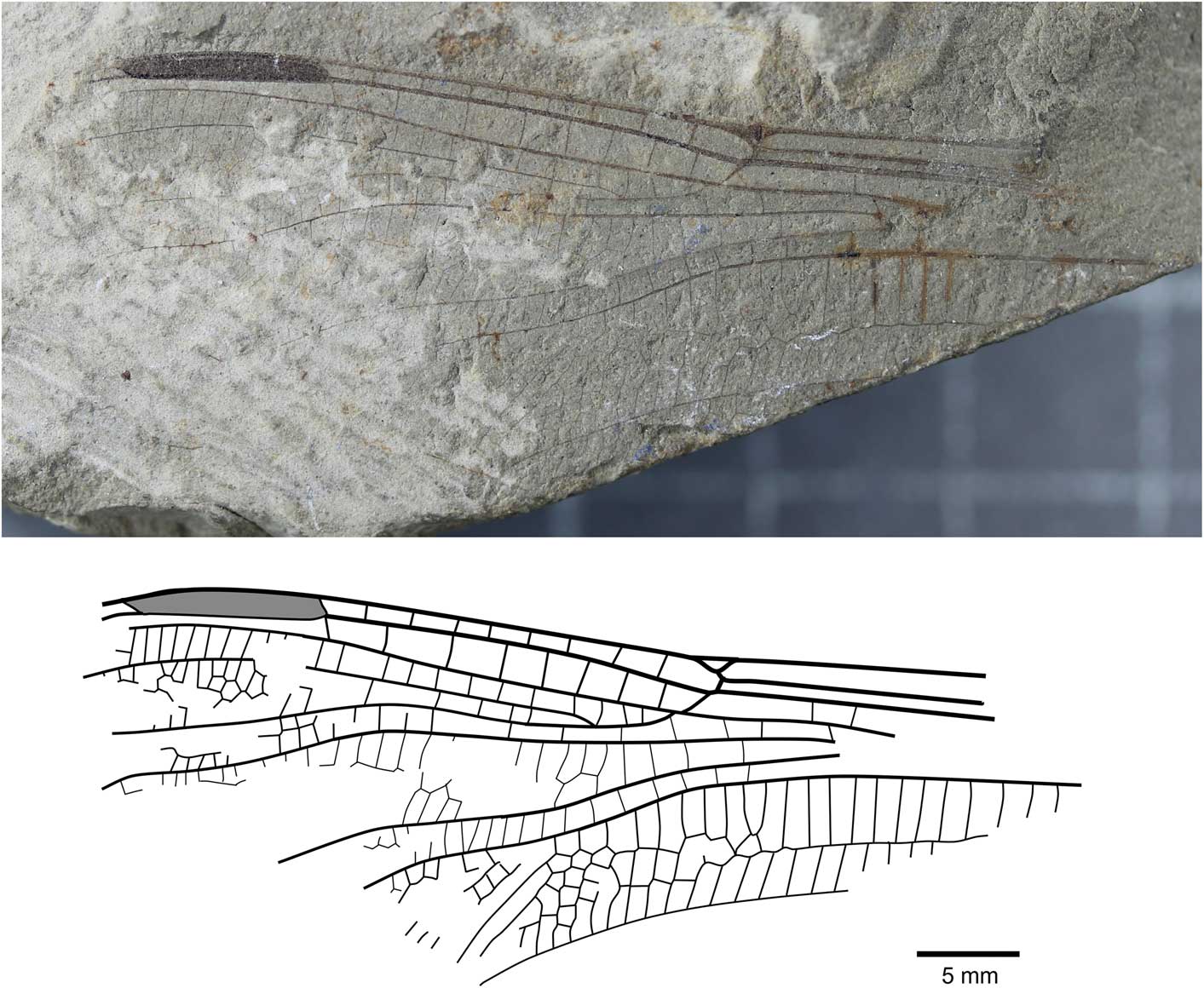
Figure 5 Holotype of Liassophlebia batheri Tillyard, Reference Tillyard1925 (NHMUK I.10434), Strensham, Worcestershire (Rhaetian), photograph and reconstruction.
1925 Liassophlebia batheri Reference TillyardTillyard, p. 16, pl. 2, figs. 5, 6, pl. 3, fig. 7.
1939 Liassophlebia batheri; Reference HandlirschHandlirsch, p. 23.
1993 Liassophlebia batheri; Reference Nel, Martínez-Delclòs, Paicheler and HenrotayNel et al., p. 142.
Holotype
NHMUK I.10434/10435 (Fig. 5), ‘Insect limestone’ of the Pseudomonotis beds (Penarth Group, Lilstock Formation); Late Triassic, Rhaetian; Strensham, Worcestershire.
Remarks
The holotype of this species is referable to Liassophlebiidae based on similarity to the holotype of the type species but there are few other diagnostic characters to separate this specimen from other liassophlebiids from the UK. Additionally, there are no comparable characters between this specimen and the forewing specimen previously attributed to this species, so it is impossible to say whether they are the same species. NHMUK I.10528 was found to be very similar to Liassophlebia withersi and has been attributed to this species herein. Compared to L. magnifica (the type species of Liassophlebia), the pterostigma of I.10434 is similar; there are two or three fewer postnodal veins and several crossveins can be slightly more or less oblique, leading to an assumption that they are similar. However, the diagnostic characters of L. magnifica are not present and so it is impossible to accurately attribute this specimen to that, or any other, species. Given the lack of diagnostic characters and the removal of the forewing from the type series, it is clear that this species name should be considered nomen dubium.
Genus Rossiphlebia new genus
Type species
Liassophlebia jacksoni Zeuner, Reference Zeuner1962.
Diagnosis
Male hindwing characters known. Strong anterior branch of AA ending on CuA and splitting subdiscoidal cell into two relatively large cells; cubital area wide (six or seven rows of cells); anal triangle split into two large cells in male; base of RP2 aligned with subnodus. Well-defined supplementary longitudinal vein in basal part of postdiscoidal area, beginning very close to discoidal triangle (also present in Liassophlebia pseudomagnifica).
Etymology
Named for Dr. Andrew Ross, British palaeoentomologist and supervisor to the senior author.
Remarks
This genus is very interesting because it seems to exhibit characters of both Liassophlebiidae and Heterophlebiidae. It is a liassophlebiid according to the rudimentary discoidal triangle and the presence of a well-defined supplementary longitudinal vein in the basal part of the postdiscoidal area, but the division of the subdiscoidal cell is similar to that of heterophlebiids, with a strong anterior branch of AA, but in male heterophlebiids, the anal triangle is split into three cells. The new genus is based on hindwing characters, thus it is difficult to compare with Ferganophlebia and Grimmenopteron, which are based on forewings only (Pritykina, Reference Pritykina1970; Ansorge, Reference Ansorge1996). Nevertheless, it differs from both (and from Caraphlebia) in the presence of a well-defined supplementary longitudinal vein in the basal part of the postdiscoidal area. It is not comparable to Bavarophlebia, which is based on a forewing, but in the latter, the base of RP2 is distinctly distal to the subnodus, whereas it is well aligned with it in Rossiphlebia n. gen.
Rossiphlebia jacksoni (Zeuner, Reference Zeuner1962)
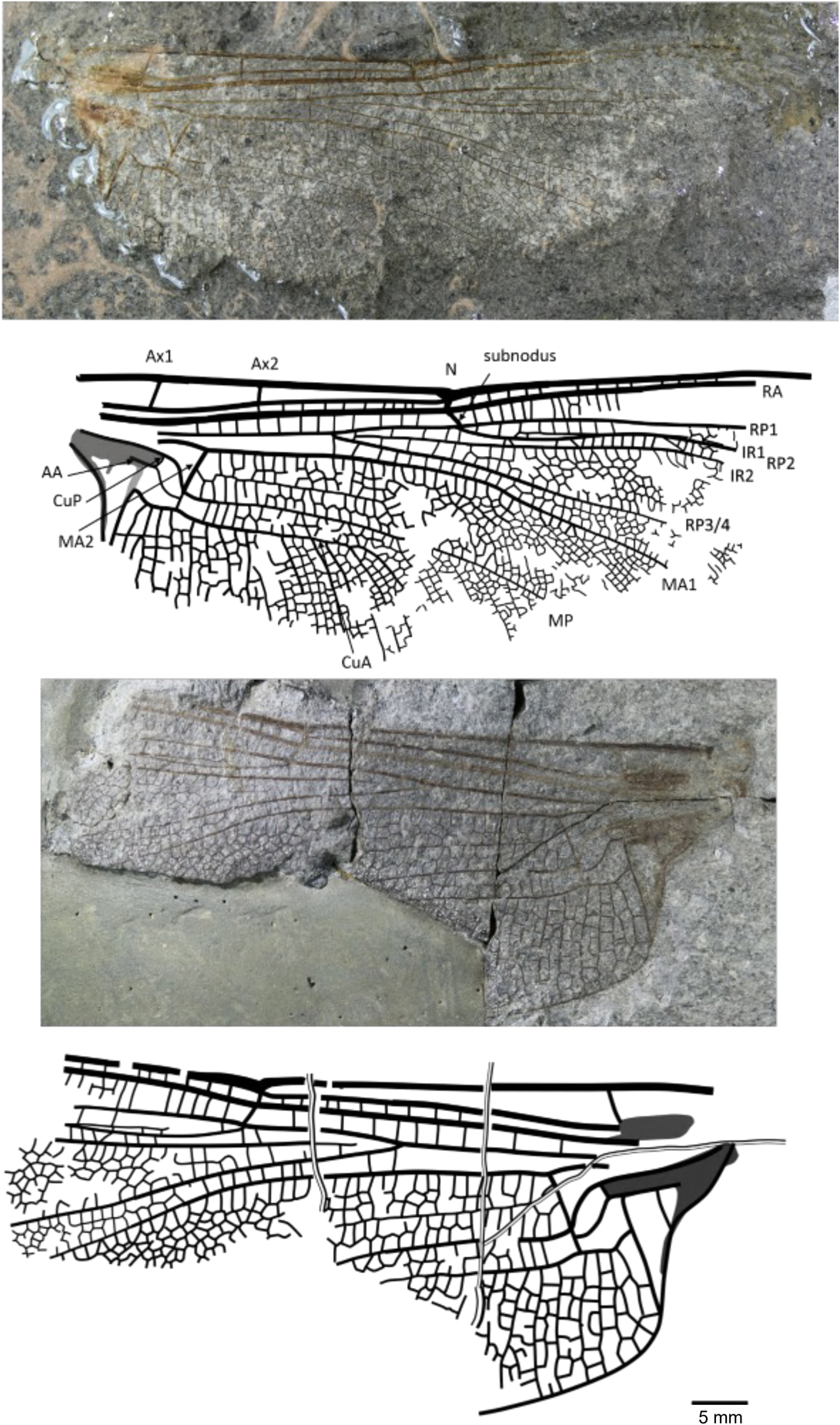
Figure 6 Holotype of Rossiphlebia jacksoni (Zeuner, Reference Zeuner1962) (NHMUK In.53999, part and counterpart), Stonebarrow, Dorset (Sinemurian), photograph and reconstruction. AA = anterior anal; Ax1 = second branch of primary antenodal crossvein; Ax2 = third branch of primary antenodal crossvein; CuA = anterior cubitus; CuP = posterior cubitus; IR1 = intercalary radial vein 1; IR2 = intercalary radial vein 2; MA1 = anterior branch of anterior median; MA2 = posterior branch of anterior median; MP = posterior median; N = nodus; RA = anterior radius; RP1 = first branch of posterior radius; RP2 = second branch of posterior radius; RP3/4 = third/fourth branch of posterior radius.
1962 Liassophlebia jacksoni Reference ZeunerZeuner, p. 162, pl. 25, figs. 1, 2.
1985 Liassophlebia jacksoni; Reference WhalleyWhalley, p. 121, fig. 6.
1993 Liassophlebia jacksoni; Reference Nel, Martínez-Delclòs, Paicheler and HenrotayNel et al., p. 139, figs. 102, 103.
Holotype
NHMUK In.53999, part and counterpart (Fig. 6), ‘Flatstones’ (bed 83) of the Obtusum Chronozone, Obtusum Subchronozone (Lias Group, Charmouth Mudstone Formation, Black Ven Mudstone Member); Early Jurassic, lower Sinemurian; Stonebarrow, Dorset.
Diagnosis
Nine antenodal crossveins of second row; two or three rows of cells between MA1 and the supplementary longitudinal vein.
Description
See original description by Zeuner (Reference Zeuner1962).
Additional material
NMW 91.14G.1, Catherston Lane.
Remarks
This species is transferred to the new genus Rossiphlebia based on hindwing characters; no forewings are yet known.
Family Anglophlebiidae new family
Type genus
Anglophlebia new genus.
Diagnosis
As for the type species, by monotypy.
Etymology
Anglo, the Latin prefix for England, and phlebiidae, a common suffix for Liassic dragonfly families.
Genus Anglophlebia new genus
Type species
Liassophlebia gigantea Zeuner, Reference Zeuner1962.
Diagnosis
As for the type species.
Etymology
Anglo, the Latin prefix for England, and phlebia, a common suffix for Liassic dragonfly genera.
Anglophlebia gigantea (Zeuner, Reference Zeuner1962)
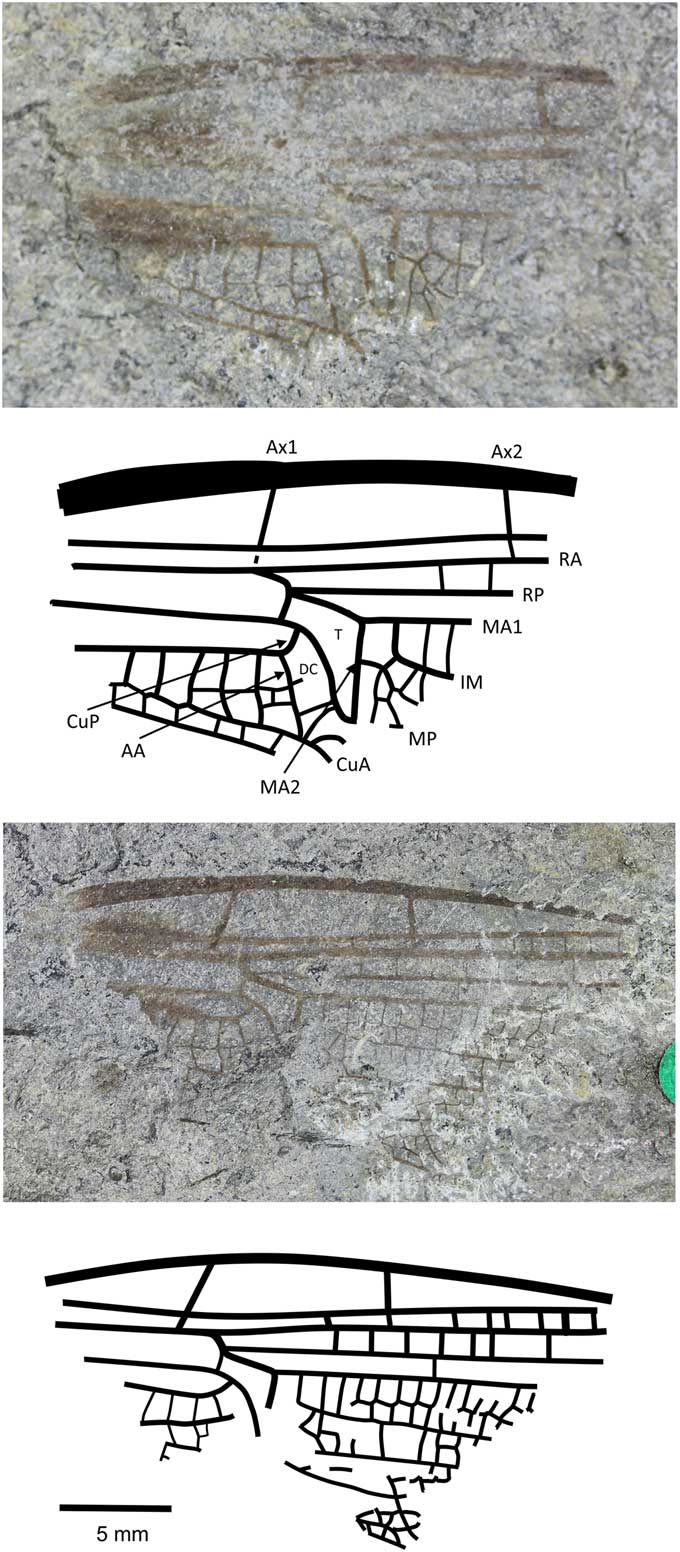
Figure 7 Holotype of Anglophlebia gigantea (Zeuner, Reference Zeuner1962) (NHMUK In.51030 pt and cpt); Stonebarrow, Dorset (Sinemurian); photograph and reconstruction. AA = anterior anal; Ax1 = second branch of primary antenodal crossvein; Ax2 = third branch of primary antenodal crossvein; CuA = anterior cubitus; CuP = posterior cubitus; DC = discoidal cell; IM = intercalary medial vein; MA1 = anterior branch of anterior median; MA2 = posterior branch of anterior median; MP = posterior median; RA = anterior radius; RP = posterior radius; T = triangle.
1962 Liassophlebia gigantea Reference ZeunerZeuner, p. 163, pl. 27, fig. 2.
1985 Liassophlebia gigantea; Reference WhalleyWhalley, p. 122, fig. 7a, b.
1993 Liassophlebia gigantea; Reference Nel, Martínez-Delclòs, Paicheler and HenrotayNel et al., p. 139, fig. 108.
Holotype
NHMUK In.51030, part and counterpart (Fig. 7), ‘Flatstones’ (bed 83) of the Obtusum Chronozone, Obtusum Subchronozone (Lias Group, Charmouth Mudstone Formation, Black Ven Mudstone Member); Early Jurassic, lower Sinemurian; Stonebarrow, Dorset.
Diagnosis
Narrow wing with characters of both fore- and hindwings of the Liassophlebiidae: AA strongly curved posteriorly and straight (typical of forewings); posterior margin of subdiscoidal cell not convex and forking, with Cu appearing to extend toward posterior margin; subdiscoidal cell widened distally as in Liassophlebiidae; discoidal cell basally closed, with shape reminiscent of that in liassophlebiid hindwings; subdiscoidal space divided into three or four cells; at least six cells between subdiscoidal cell and wing base; cubital area very narrow (forewing character); intercalary medial vein beginning at first crossvein after distal margin of discoidal triangle (MA2) and stronger and smoother than that of liassophlebiids; also more cells in postdiscoidal area with possible second posterior intercalary vein.
Remarks
This is a peculiar specimen because it seems to exhibit characters traditionally indicative of both fore- and hindwings. With the shape of the discoidal cell and the presence of the intercalary vein in the postdiscoidal area, it could be identified as a hindwing, as indeed it was by Zeuner (Reference Zeuner1962). However, in the counterpart, the cubito-anal area is better preserved and exhibits characters of a forewing (AA curving strongly posteriorly; shape of anal area; narrow cubital area) and of a hindwing (discoidal cell basally closed; shape of discoidal cell; intercalary vein in postdiscoidal area; subdiscoidal cell divided).
There are two possible hypotheses based on the evidence: (1) that we have a hindwing with a reduced cubito-anal area, as in some Campterophlebiidae (Nel et al., Reference Nel, Huang and Lin2008), or (2) that we have a forewing with some hindwing characters (as in the Liassogomphidae Tillyard, Reference Tillyard1935 and modern Anisoptera in which the discoidal cell is divided into a hypertriangle and a discoidal triangle in the forewings, compared to the Heterophlebiidae Handlirsch, Reference Handlirsch1906, in which this is only true in the hindwings). Either way, and although the specimen is only the basal fragment, it is unique among the British materials (checked at all known repositories of British Late Triassic–Early Jurassic material in the UK and the USA), the Liassic material held in Paris at the Muséum National d’Histoire Naturelle (examined by AN), the Late Triassic–Early Jurassic Russian and Central Asian material (checked at the Paleontological Institute of the Russian Academy of Sciences in Moscow by RSK and Dmitry Vassilenko), and the Late Triassic–Early Jurassic material from China held at the Nanjing Institute of Geology and Palaeontology, Chinese Academy of Sciences (examined by RSK). The specimen does not fit with any family diagnosis already described and so we tentatively erect a new family for this species in the hope that further, better-preserved specimens will be found and described in the future.
Clade Isophlebioptera Bechly, Reference Bechly1996
Family Selenothemistidae Handlirsch, Reference Handlirsch1939
Genus Caraphlebia Carpenter, Reference Carpenter1969
Type species
Caraphlebia antarctica Carpenter, Reference Carpenter1969.
Diagnosis
A very well-defined CuA2 directed toward posterobasal side of hindwing.
Caraphlebia antarctica Carpenter, Reference Carpenter1969
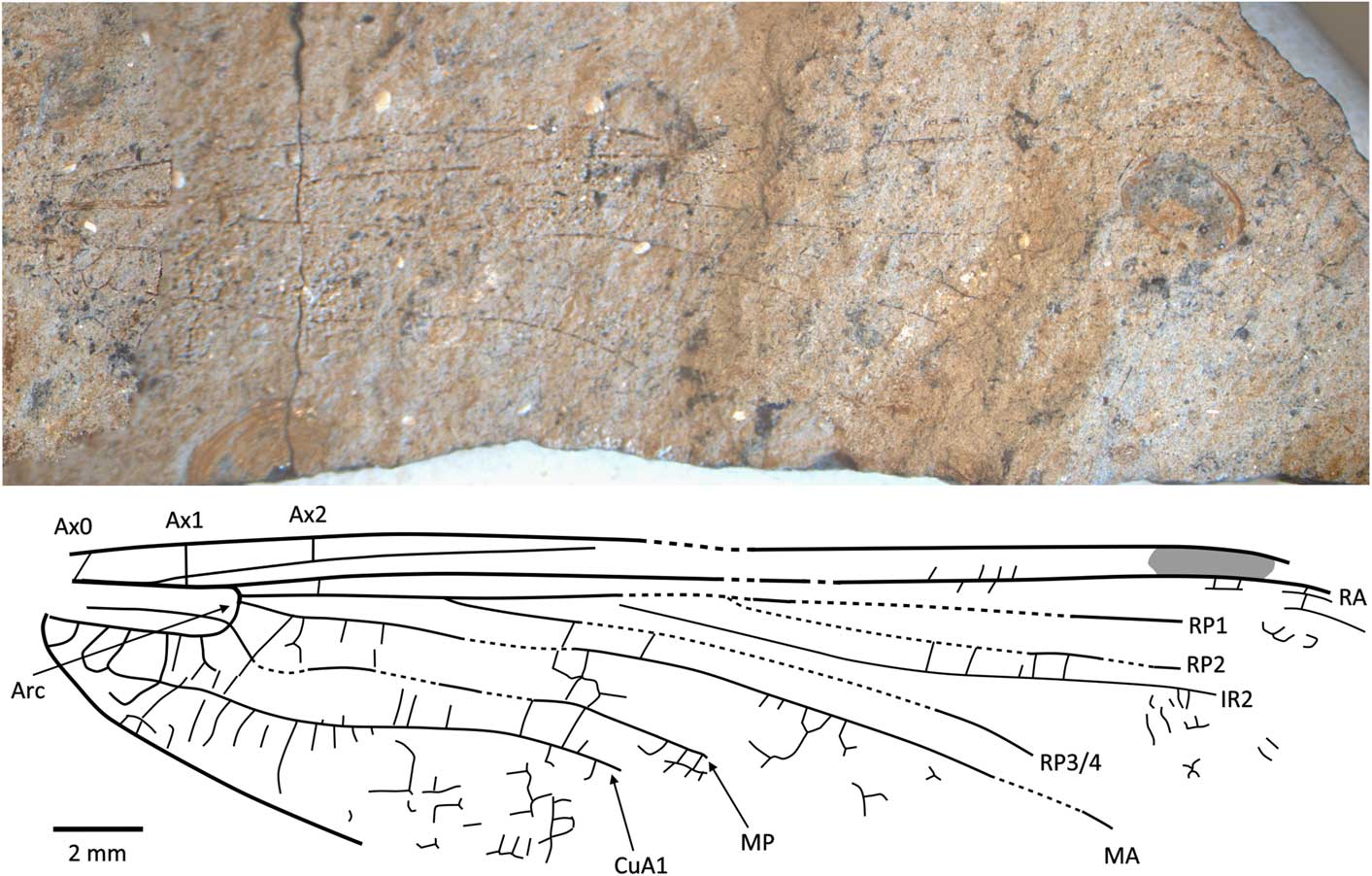
Figure 8 Holotype of Caraphlebia antarctica Carpenter, Reference Carpenter1969 (USNM 165874), Carapace Nunatak (Hettangian to Sinemurian), photograph and reconstruction. Ax0 = first branch of primary antenodal crossvein; Ax1 = second branch of primary antenodal crossvein; Ax2 = third branch of primary antenodal crossvein; Arc = arculus; CuA1 = distal branch of anterior cubitus; IR2 = intercalary radial vein 2; MA = anterior median; MP = posterior median; RA = anterior radius; RP1 = first branch of posterior radius; RP2 = second branch of posterior radius; RP3/4 = third/fourth branch of posterior radius.
1969 Caraphlebia antarctica Reference CarpenterCarpenter, p. 419, fig. 1.
1993 Caraphlebia antarctica; Reference Nel, Martínez-Delclòs, Paicheler and HenrotayNel et al., p. 259, fig. 221.
Holotype
USNM 165874, part and counterpart (Fig. 8), Ferrar Group, Mawson Formation; Early Jurassic, lower Lias (possibly Hettangian−Sinemurian); Carapace Nunatuk, South Victoria Land, Antarctica.
Diagnosis
Hindwing CuA2 quite distinct, directed toward posterobasal margin of wing.
Description
Primary antenodals present, Ax1 basal to arculus, Ax2 distal to it, arculus closer to Ax1 than to Ax2. Crossveins between RA and RP basal to expected position of subnodus; postnodal crossveins not visible. Pterostigma very long and narrow, but apparently distally incomplete. Postdiscoidal area not distally narrowed. Area between RP3/4 and IR2 apparently distally broadened. Oblique veins not preserved. Nodal structures very poorly preserved. RP2 and IR1 not discernible. Discoidal cell poorly preserved, but quite short and with distal side MA2 twice as long as basal side (without any trace of subdivision into hypertriangle and triangle, as figured by Carpenter, Reference Carpenter1969). Subdiscoidal space broad, with posterior side (AA and CuA) convex. Basal part of CuA short, CuA2 quite distinct, directed toward posterobasal margin of wing, with AA ending within it. Cubital area broad with ~5 or 6 rows of cells between CuA and posterior wing margin. Anal area broad with two rows of large cells. Anal margin rounded, without any angle; no anal triangle (female).
Remarks
The original description and figure (Carpenter, Reference Carpenter1969) shows an almost complete and supposedly well-preserved fossil. However, the type specimen (Fig. 8) is not as well-preserved with most of the apical half of the wing missing, although fortunately most of the major veins are preserved though slightly warped by the structure of the rock. The numerous secondary antenodal crossveins figured by Carpenter (Reference Carpenter1969, fig. 1) are not discernible. The pattern of the longitudinal veins, i.e., postdiscoidal space not narrowed and RP3/4 not parallel to IR2, corresponds to Liassophlebiidae and Selenothemistidae. However, the shape of the discoidal cell differs from that of liassophlebiids in that it is relatively short and without any trace of division into a hypertriangle and discoidal triangle. The probable absence of secondary antenodal crossveins and veins in the basal area between RA and RP also supports attribution to the Selenothemistidae. The quite well-defined CuA2, curved toward the posterobasal side of the wing, is very particular and probably is an autapomorphy of the genus.
Odonata incertae sedis, fam. indet.
‘Liassophlebia’ clavigaster Tillyard, Reference Tillyard1925

Figure 9 Holotype of ‘Liassophlebia’ clavigaster Tillyard, Reference Tillyard1925 (NHMUK I.10433), Strensham, Worcestershire (Rhaetian), photograph.
1925 Liassophlebia (?) clavigaster Reference TillyardTillyard, p. 19, pl. 3, fig. 9, text-fig. 5.
1939 (Anisozygopteron) clavigaster; Reference HandlirschHandlirsch, p. 29.
1978 Liassophlebia clavigaster; Reference LindleyLindley, p. 344.
1993 Liassophlebia (?) clavigaster; Reference Nel, Martínez-Delclòs, Paicheler and HenrotayNel et al., p. 142, fig. 109.
Holotype
NHMUK I.10433 (Fig. 9), ‘Insect limestone’ of the Pseudomonotis beds (Penarth Group, Lilstock Formation); Late Triassic, Rhaetian; Strensham, Worcestershire.
Remarks
This specimen and the other identified as this species (NHMUK I.475) are known only from abdominal segments. Liassophlebiid higher taxonomy is based on wings so it is impossible to attribute this specimen to a genus or family. Therefore, we consider this species to be incertae sedis at those levels until a better-preserved specimen allows identification.
‘Liassophlebia’ hopei (Brodie, Reference Brodie1845)

Figure 10 Holotype of ‘Liassophlebia’ hopei (Brodie, Reference Brodie1845) (OUMNH J.55084); Strensham, Worcestershire (Rhaetian); photograph.
1845 Libellula hopei Reference BrodieBrodie, p. 71, pl. 10, fig. 3.
1892 Libellula hopei; Reference WoodwardWoodward, p. 195.
1879 Libellula hopei; Reference GossGoss, p. 129.
1850 Petalura liassina (Strickland); Reference HagenHagen, p. 359.
1850 Heterophlebia hopei; Reference Selys-Longchamps and HagenSelys-Longchamps and Hagen, p. 359 (footnote).
1906 (Anisozygopteron ?) hopei; Reference HandlirschHandlirsch, p. 470.
1939 (Anisozygopteron) hopei; Reference HandlirschHandlirsch, p. 29.
1925 Liassophlebia (?) hopei; Reference TillyardTillyard, p. 19.
1993 Liassophlebia hopei; Reference Nel, Martínez-Delclòs, Paicheler and HenrotayNel et al., p. 143.
Holotype
OUMNH J.55084 a and b, ‘Insect Limestone’ of the Pseudomonotis beds (Penarth Group, Lilstock Formation); Rhaetian; Strensham, Worcestershire.
Remarks
Same reasoning as for ‘Liassophlebia’ clavigaster, above.
Discussion
Examination of the fossil material from the Late Triassic and Early Jurassic of England has led to several major changes to the taxonomy of Liassophlebiidae and changes to the diversity estimates of insects across the TJB. The new family Anglophlebiidae is tentatively erected for a specimen previously attributed to the liassophlebiid species Liassophlebia gigantea by Zeuner (Reference Zeuner1962). This specimen is only a fragment of a wing, and it is expected that future changes to the taxonomy of this family will be required as and when better-preserved specimens are found. However, the characters that are preserved are of sufficient uniqueness that it is important to draw attention to the specimen. This taxon is of particular interest because it appears to exhibit characters traditionally attributed to both fore- and hindwings, respectively narrow cubito-anal area vs. discoidal cell basally close and nearly divided into two parts by an incomplete crossvein.
Rossiphlebia n. gen. is erected for a species previously attributed to Liassophlebia jacksoni by Zeuner (Reference Zeuner1962). This species is clearly not attributable to Liassophlebia because the subdiscoidal space is subdivided into two large cells by a branch of AA. This genus is particularly interesting because it seems to exhibit characters of both Liassophlebiidae and Heterophlebiidae. As the taxonomy of these groups is revised, it is also important to consider the bigger picture and the effects of such findings on previous phylogenies (e.g., Nel et al., Reference Nel, Martínez-Delclòs, Paicheler and Henrotay1993). With the new characters described since 1993, the topography of a Jurassic Odonata phylogeny would probably look much different.
Two specimens—a forewing and a hindwing—were originally attributed to Liassophlebia batheri by Tillyard (Reference Tillyard1925). Because these are isolated wings, it is impossible to ascertain whether they belong to the same species without a specimen that preserves both fore- and hindwings together. Moreover, the forewing was found to be very similar to the holotype of L. withersi, which is described from a forewing. The only real difference is an aberration in the anal area of the holotype and a size difference that is not sufficient to split them into separate species, so the forewing previously attributed to L. batheri is herein transferred to L. withersi. This leaves only the hindwing holotype of L. batheri, which is not of sufficient preservation for description and so the species is herein considered nomen dubium.
Two speciesLiassophlebia (?) clavigaster and L. (?) hopei—were described from isolated abdominal segments. However, liassophlebiid higher taxonomy is described from isolated wings and there are yet to be any specimens described with both wing and abdomenal characters, so it is impossible to attribute isolated abdomens to the same taxa. The taxonomy of these specimens is not clear higher than family level because members of Stenophlebiidae also have apically widened abdomens similar to L. (?) clavigaster; it is therefore not yet possible to attribute these fossils to a precise higher group (e.g., Stenophlebiomorpha, Heterophlebioptera, etc.).
Taxonomic revision of historical collections is important because it increases our understanding of past diversity and allows us to better reconstruct palaeoentomofaunas. There are also implications for our understanding of insect phylogenies because several of the species described herein exhibit characters from traditionally separate families. The current revision did not however have much of an effect on our understanding of the impact of the ETE on insect diversity. We consider L. batheri to be nomen dubium and we transferred the other specimen identified as this species to L. withersi; this reduced the species richness of insects in the Rhaetian by one (L. batheri) but did not affect the ranges of other species or the genus. There were no taxonomic changes to L. magnifica and no further specimens were found, so there were no changes to the range of this species and so it still seems to have originated in the Hettangian following the ETE. Selenothemistidae was previously only known from the Toarcian of Germany (Handlirsch, Reference Handlirsch1939) so the confirmation of Caraphlebia in this family increases the range of the family back to the Hettangian or Sinemurian, indicating that the family originated closer to the TJB and prior to the early Toarcian mass extinction. Rossiphlebia n. gen. and Anglophlebiidae n. fam. are newly described but are only known from the Sinemurian. Their description increases the diversity of insects in the Sinemurian but does not affect the range of Liassophlebiidae or Liassophlebia across the TJB.
Acknowledgments
The lead author would like to acknowledge the support of his Ph.D. supervisors M. Benton at the University of Bristol and A. Ross at the National Museum of Scotland for their advice and support throughout his Ph.D. project for which this was a side project. The authors would also like to thank C. Mellish, the curator of invertebrate paleontology at NHMUK, for continued access to the collections in London; to E. Howlett for access to OUMNH collections and photographs; and to C. Howells for access to NMW collections. We would also like to thank F. Marsh from the USNM for taking and sending photographs of the specimen from Antarctica. Also, thanks to the Natural Environment Research Council (NERC) for funding the lead author’s Ph.D. project from which this study was made possible (studentship number NE/L002434/1).
Data statement.--- This study did not involve any underlying data. All specimens are available for re-study in the public institutions indicated in the text.














