Introduction
Since the late 20th century, concerns over the condition of the bottlenose dolphin (Tursiops truncatus) Mediterranean sub-population, listed as vulnerable on the IUCN Red List (IUCN, 2017), have increased. While T. truncatus is perhaps one of the most extensively studied dolphin species due to its cosmopolitan distribution, their population numbers in certain areas have decreased significantly since the 1940s (Folkens & Randall, Reference Folkens and Randall2002). Due to the species’ predominantly coastal range, this decline is often attributed to anthropogenic factors (Wilson et al., Reference Wilson, Thompson and Hammond1997). Behavioural changes are associated with a combination of environmental cues and human pressures that can lead to changes in population-level dynamics (Nowacek et al., Reference Nowacek, Wells and Solow2001; Bas et al., Reference Bas, Christiansen, Öztürk, Öztürk and Watson2017). It is therefore important that we understand natural patterns of dolphin behaviour so any anthropogenic impacts can be mapped and quantified (Bearzi, Reference Bearzi2002). Indeed, bottlenose dolphin behaviour is known to be complex and extremely fluid both within and between populations (Shane et al., Reference Shane, Wells and Würsig1986; Hanson & Defran, Reference Hanson and Defran1993; Connor et al., Reference Connor, Wells, Mann, Read, Mann, Connor, Tyack and Whitehead2000; Gregory & Rowden, Reference Gregory and Rowden2001). Factors related to geographic and temporal variability and group size have the potential to affect behaviour both directly and indirectly (Miller et al., Reference Miller, Mackey, Hoffland, Solangi and Kuczaj2010; Hwang et al., Reference Hwang, Defran, Bearzi, Maldini, Saylan, Lang, Dudzik, Guzon-Zatarain, Kelly and Weller2014).
Various studies have revealed relations between group size and behaviour in this highly social species. For example, diving and foraging groups are significantly smaller than socializing groups (Bearzi et al., Reference Bearzi, Notarbartolo di Sciara and Politi1997) whilst travelling groups tend to be significantly smaller than foraging groups (Rogers et al., Reference Rogers, Brunnick, Herzing and Baldwin2004). It is still unclear whether it is group size that affects behavioural choices in bottlenose dolphins or whether the behaviours they engage in direct group size. Identifying the social processes influencing behaviour is central to understanding this species’ habitat use and associated distribution patterns.
Furthermore, temporal variation in environmental conditions has been shown to affect T. truncatus behaviour (Shane et al., Reference Shane, Wells and Würsig1986; Connor et al., Reference Connor, Wells, Mann, Read, Mann, Connor, Tyack and Whitehead2000). Although a conclusive daily behavioural cycle for bottlenose dolphins has yet to be determined (Bearzi et al., Reference Bearzi, Politi and di Sciara1999), other factors that change diurnally, such as tide, currents, wind and prey presence are known to affect their behaviour (Hanson & Defran, Reference Hanson and Defran1993; Bearzi et al., Reference Bearzi, Notarbartolo di Sciara and Politi1997; Gregory & Rowden, Reference Gregory and Rowden2001; Daura-Jorge et al., Reference Daura-Jorge, Wedekin, Piacentini and Simões-Lopes2005; Miller et al., Reference Miller, Mackey, Hoffland, Solangi and Kuczaj2010). For instance, bottlenose dolphins in the South Atlantic engage in resting behaviour in the morning, when schooling fish are unavailable, with levels of activity increasing in the afternoon as prey availability increases (Würsig and Würsig, Reference Würsig and Würsig1979). Linking diurnal changes to dolphin behaviour may point to further environmental variables that explain behavioural choices in bottlenose dolphins. Similarly, seasonal changes in water temperature, salinity and prey availability have been linked with changes in T. truncatus behaviour (Bräger, Reference Bräger1993; Hanson & Defran, Reference Hanson and Defran1993; Miller et al., Reference Miller, Mackey, Hoffland, Solangi and Kuczaj2010). Indeed, fluctuations in prey availability due to changes in sea temperatures with season are known to result in increased foraging or travelling behaviour as prey move in and out of the region (Bearzi et al., Reference Bearzi, Politi and di Sciara1999). Furthermore, identifying seasonal patterns in dolphin behaviour that correspond to changes in environmental and anthropogenic pressures can yield invaluable insight into local populations’ life history and help establish more efficient conservation strategies (Miller et al., Reference Miller, Mackey, Hoffland, Solangi and Kuczaj2010).
In addition to social and temporal factors, geographic features found in T. truncatus’ range affect its behaviour (Ballance, Reference Ballance1992; Acevedo-Gutiérrez & Parker, Reference Acevedo-Gutiérrez and Parker2000; Benoit-Bird & Au, Reference Benoit-Bird and Au2003; Hastie et al., Reference Hastie, Wilson, Wilson, Parsons and Thompson2004; Toth et al., Reference Toth, Hohn, Able and Gorgone2012; Hwang et al., Reference Hwang, Defran, Bearzi, Maldini, Saylan, Lang, Dudzik, Guzon-Zatarain, Kelly and Weller2014; Temple et al., Reference Temple, Tregenza, Amir, Jiddawi and Berggren2016). Typically, distance from shore is directly linked to bathymetry and a slight change in oceanic depth can affect the entire ecology of a region (Bell, Reference Bell1983). This heterogeneity of marine habitats plays a major role in the distributions and behaviour variations of bottlenose dolphins (Ballance, Reference Ballance1992; Hanson & Defran, Reference Hanson and Defran1993; Acevedo-Gutiérrez & Parker, Reference Acevedo-Gutiérrez and Parker2000; Hastie et al., Reference Hastie, Wilson, Wilson, Parsons and Thompson2004; Hwang et al., Reference Hwang, Defran, Bearzi, Maldini, Saylan, Lang, Dudzik, Guzon-Zatarain, Kelly and Weller2014). In T. truncatus, diving and foraging behaviour are associated with deeper waters whilst travelling and resting are observed more often at shallower depths, which offer protection from predators and navigation guidance (Ballance, Reference Ballance1992; Acevedo Gutiérrez & Parker, Reference Acevedo-Gutiérrez and Parker2000; Bearzi, Reference Bearzi2005). Indeed, dolphins found closer to shore, on average, spend more time travelling, followed by feeding and socializing (Hanson & Defran, Reference Hanson and Defran1993; Bearzi, Reference Bearzi2005).
Here, we focus on an under-studied population of bottlenose dolphins found in the South-eastern Adriatic Sea along the coastline of Montenegro. Despite the wide research on bottlenose dolphin behaviour globally, in Montenegro, peer-reviewed cetacean research is severely limited (Joksimović et al., Reference Joksimović, Mandić and Durović2013; Gaspari et al., Reference Gaspari, Scheinin, Holcer, Fortuna, Natali, Genov, Frantzis, Chelazzi and Moura2015; Durović et al., Reference Durović, Holcer, Joksimović, Mandić, Fortuna, Ikica and Vuković2016). The current study was established in September 2016 as the first long-term cetacean monitoring project in Montenegro. To further our understanding of the local dolphin population, our study aimed to investigate the relationship between behaviour and a set of spatial, environmental and social factors. Specifically, we tested the hypothesis that (i) proximity to shore and depth influenced behavioural choices, (ii) the local population displayed seasonal and diurnal patterns in behaviour and (iii) behaviour varied significantly with group size. The information collected will be crucial in providing baseline data on the behavioural patterns of bottlenose dolphins in Montenegro that will help in the development of local marine conservation initiatives.
Materials and methods
Study area
The entire coastline of Montenegro was divided into three regions to allow for an evenly distributed sampling effort. Five land survey stations were then randomly selected based on these regions, two in the South (Ulcinj and Utjeha), two in the centre (Bar and Petrovac) and one in the North (Herceg-Novi). These stations allowed for a total coverage of 137.5 km2 out of the 575.7 km2 making up Montenegro's coastal waters (Figure 1).
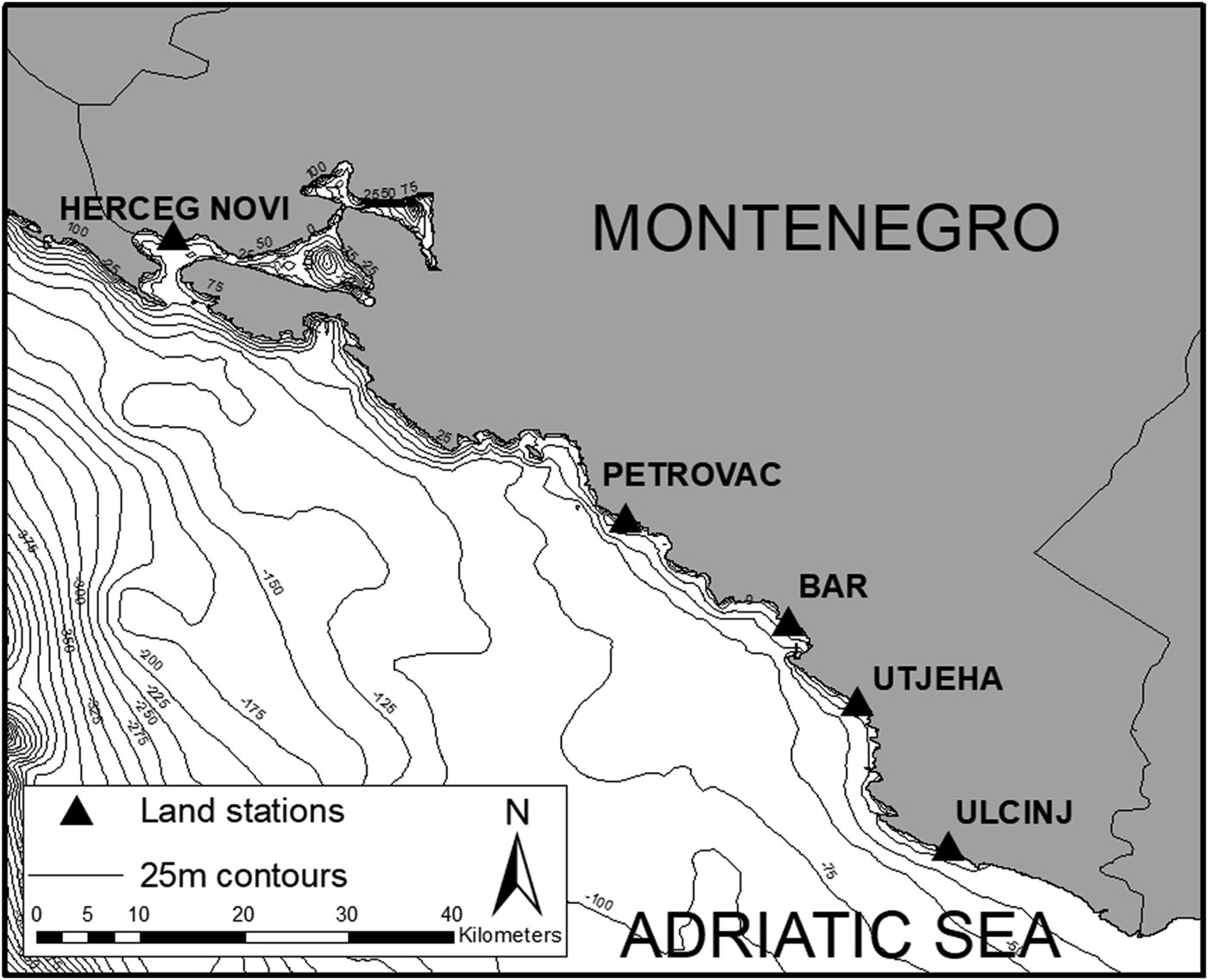
Fig. 1. Map of Montenegro's coastline and all survey stations used in this study: Ulcinj (19°12′38″E 41°55′29″N, 92 m above sea level), Utjeha (19°08′46″E 42°03′01″N, 78 m), Bar (19°04′19″E 42°07′11″N, 23 m), Petrovac (18°55′17″E 42°12′31″N, 148 m) and Herceg-Novi (18°32′25″E 42°27′11″N, 84 m). Bathymetry contours show 25 m increases in depth (data from: IOC, IHO & BODC, 1994).
Depth along the coastline of Montenegro ranged from 5 to 75 m. This depth range was maintained along most of the coastline with few deeper areas except in the Bay of Kotor area. These relatively shallow waters contrasted with some of the deepest waters of the Adriatic Sea, over 650 m, found further offshore (Figure 1).
Data collection
The coastal region of Montenegro was surveyed using land-based observation techniques to avoid potential behavioural changes as dolphins reacted to research vessels (Lemon et al., Reference Lemon, Lynch, Cato and Harcourt2006).
Each land station was visited, weather permitting, at least twice a month for an entire year from September 2016 to October 2017. Surveys, lasting from 3 to 4 h, were conducted from an elevated position on the shoreline via continuous scans using both 10 × 50 magnification binoculars and a Sokkia DT5A Electronic Theodolite. All observations recorded above 4000 m from shore were discarded as dolphin fins were not discernible from wave crests.
Environmental variables (Beaufort sea state, cloud cover and glare) were collected hourly and surveys were abandoned when sea state increased to 3 or more. Data were collected during daylight hours with surveys always starting and ending at sunrise or sunset. Time of day was classified as ‘morning’ when surveys started at sunrise and ‘afternoon’ when surveys ended at sunset. Seasons were defined by 3-month intervals: spring (March, April, May), summer (June, July, August), autumn (September, October, November) and winter (December, January, February).
Dolphin coordinate recordings gathered with the Theodolite were converted to geographic coordinates using Pythagoras software (Pythagoras 1.2; Würsig et al., Reference Würsig, Cipriano, Würsig, Pryor and Norris1991; Lerczak & Hobbs, Reference Lerczak and Hobbs1998), and information about depth and distance from shore were inferred using ArcGis (ESRI, 2011, version 10.3.1).
Focal group scan sampling was used to assess group behaviour, assuming that the surface behaviour was representative of that underwater (Lusseau, Reference Lusseau2003; Bas et al., Reference Bas, Christiansen, Öztürk, Öztürk and Watson2017). Behavioural states were recorded according to a study-specific ethogram (Table 1) along with group size at 5 min intervals upon dolphin sighting. Groups were defined as the number of individuals within 50 m of each other. The predominant behaviour at each 5 min interval (over 50% of animals from a defined group engaged) was recorded as the group behaviour. Whenever the group was not sighted at the end of the 5 min interval, the next sighting started the interval sampling anew. If the group was not observed for 20 min, the next sighting was categorized as a new group.
Table 1. Ethogram of T. truncatus behaviour based on previous studies: Lusseau (Reference Lusseau2003) and Bas et al. (Reference Bas, Christiansen, Öztürk, Öztürk and Watson2017)
During focal follows, one observer was tasked with continuous scanning of the area while two observers recorded behaviours and followed the group and one observer tracked the dolphins with the theodolite. If the focal group split or another group was sighted, continuous scanning stopped, and one observer followed each group whilst the remaining observer guided the theodolite user between groups. Continuous scans were restarted when one of the groups was not spotted for more than 15 min. Sightings were often short (less than 15 min), thus 5 min intervals were deemed long enough to avoid a bias from temporal autocorrelation of the data as few groups had more than two behaviour recordings.
Statistical analysis
We tested for the independence of all explanatory environmental variables (depth, time of day, distance from shore and season) and behaviour by using a series of Chi-square tests. In order to perform such tests, the data for distance from shore and depth were transformed into integer categories of 500 m increments for distance from shore and 10 m increments for depth, as environmental conditions were not expected to vary within these ranges (Bell, Reference Bell1983; Ballesteros, Reference Ballesteros1989; Micheli et al., Reference Micheli, Benedetti-Cecchi, Gambaccini, Bertocci, Borsini, Osio and Romano2005). Any explanatory variable failing to show a significant relationship (P > 0.05) with behaviour was discarded in further analyses. Additionally, we tested for correlation between depth and distance from shore.
Behaviour in this study was an unranked categorical variable with four levels (Table 1). Behaviour could thus be modelled against the chosen explanatory environmental variables using a multinomial distribution. Multinomial regression allowed for the estimation of the relative risk ratios for a unit change in predictor variables of both continuous and categorical types (Blizzard & Hosmer, Reference Blizzard and Hosmer2007). In turn, we estimated the probability of each behaviour outcome with respect to a baseline behaviour for any independent variable. Assuming our ethogram described all behavioural states observed in our population, the independence of irrelevant alternatives was not violated and made multinomial logistic regression an ideal tool to analyse the effect of the aforementioned variables on behavioural probabilities (Steckenreuter et al., Reference Steckenreuter, Möller and Harcourt2012). Furthermore, as most groups (50%) only accounted for 1–2 observations we considered the assumption of independence of data not to be broken. We thus ran a series of multinomial regressions from a fully saturated model, where all interaction and additive terms between explanatory variables were considered, to the simplest model, where only one explanatory variable was considered. We chose the best-fit model out of all combinations based on both AIC and BIC values.
Following the results of our multinomial regression, we further analysed the relationship between group size and behaviour considering that behaviour may have been affecting group size more than the inverse. Thus, we ran an analysis of variance (ANOVA) model to test for a specific effect of behaviour on group size. We then ran a post-hoc Tukey honest significant difference test to identify which behaviours, if any, were significantly affecting group size.
All analyses in this paper were conducted using the statistical software R (R Core Team, 2018) and multinomial regression was performed using the nnet package (Venables & Ripley, Reference Venables and Ripley2002).
Results
Sampling
A total of 180 surveys (537 h) were carried out and dolphins were sighted on 60 different surveys during the study period, totalling 51 followed groups, and 134 behavioural observations. The number of observations per group ranged between 1–10 with the median equal to 2. Of the total number of surveys, 31% were carried out in Ulcinj, 21% in Utjeha, 17% in Bar, 15% in Petrovac and 16% in Herceg-Novi. Dolphins were sighted most in Ulcinj (46% of observations), followed by Utjeha (24%), Bar (10%), Petrovac (9%) and Herceg-Novi (11%) (Figure 2).
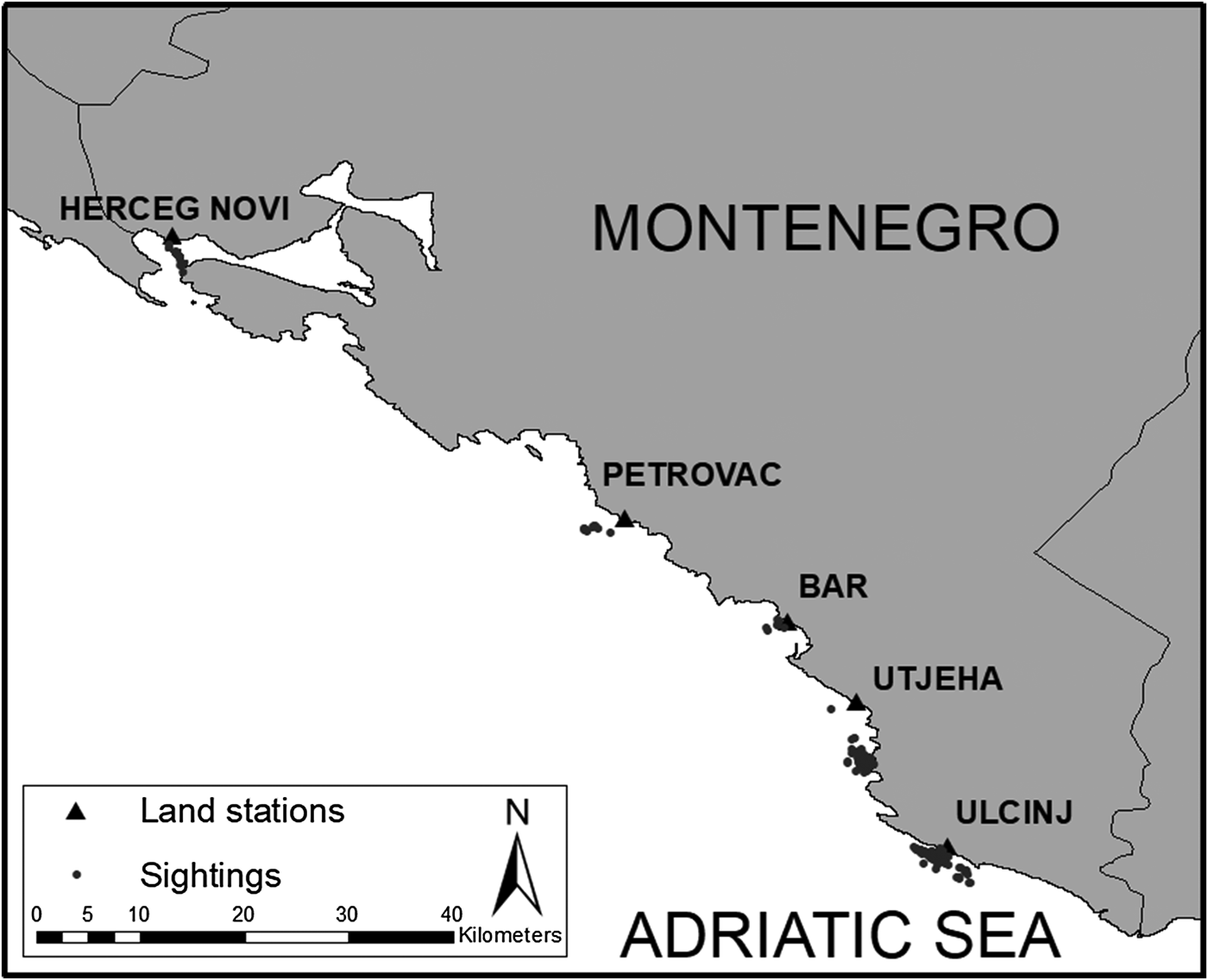
Fig. 2. Map of Montenegro's coastline and all individual dolphin sightings used in this study.
Group sizes varied from 1 to 9 with a mean and median of 4 individuals per group. The most commonly observed behaviour was found to be travelling (55% of the time) followed by diving (22%), socializing-resting (15%) and surface feeding (8%).
Of the total observations recorded, 44% occurred in the morning and 56% in the afternoon. More dolphin groups were observed in the autumn months (30%) followed by winter (28%), summer (25%) and spring (17%).
Dolphins were recorded at depths of 10–100 m over distances of 100–4000 m away from the shoreline, beyond which theodolite readings were discarded. Median recorded depth was 8 m and 75% of observed dolphins were found between 3 and 25 m deep. Median recorded distance from shore was 900 m and 75% of the observations were made between 600 and 1500 m.
Statistical analysis
Individual Chi-square test results showed that two of the four categorical or integer variables, season (χ2 = 36.10, P < 0.001) and distance from shore (χ2 = 34.47, P < 0.05), considered here were significantly not independent from behaviour. As behaviour was not found to significantly depend upon time of day or depth (P > 0.05), both variables were dropped from all subsequent analysis. Depth and distance from shore were found to be highly correlated (r = −0.88, t = −21.64, P < 0.001), supporting the use of distance from shore alone in further analysis.
Stepwise multinomial regression carried out with the remaining two explanatory environmental variables (season and distance from shore) and group size led us to reject all variables but one. Indeed, the best-fit model (AIC = 287.89 and BIC = 322.67) was that considering season as the sole explanatory variable (Figure 3). The model revealed a significant increase in surface-feeding (z = 2.13, SE = 0.42, P < 0.05) and a near significant increase in socializing (z = 1.91, SE = 0.31, P = 0.056) over the course of the year. This considerable increase in both surface-feeding and socializing behaviours came mainly at the expense of travelling (Figure 4), although this relationship was not found to be significant (P > 0.05).
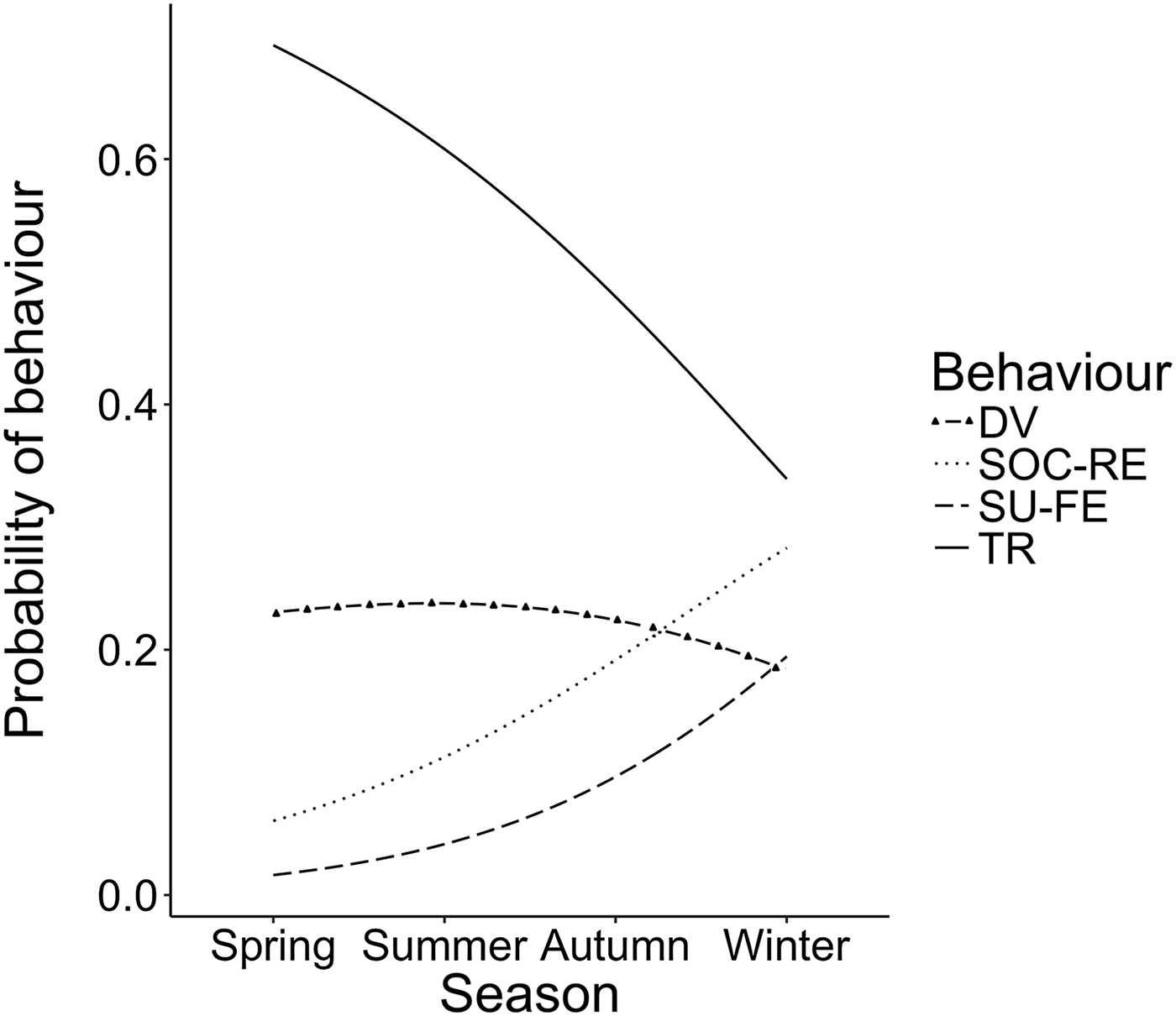
Fig. 3. Season model from stepwise multinomial regression of bottlenose dolphin behaviour.
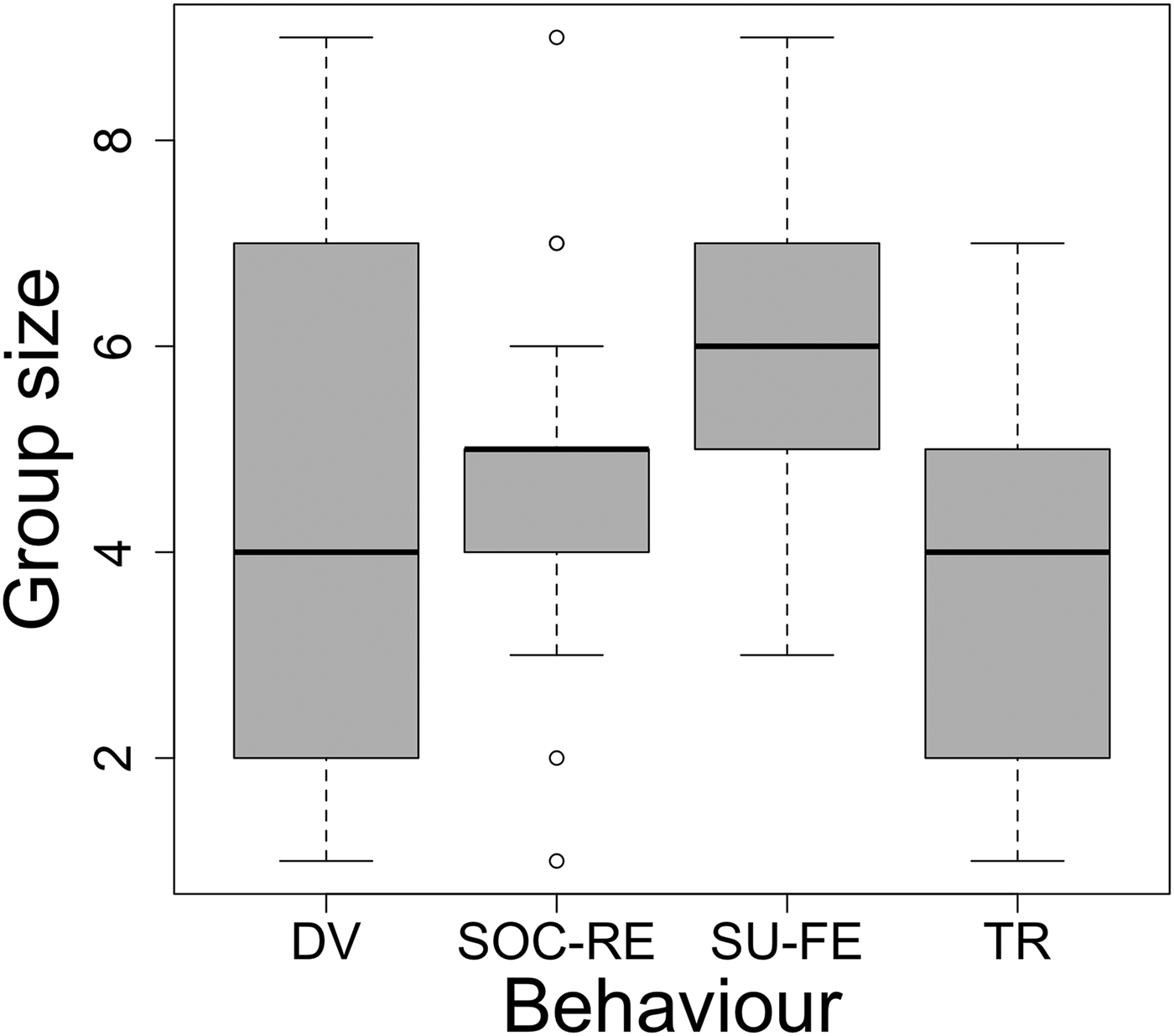
Fig. 4. Variation in group sizes among behaviour categories. Boxplots show median, quartile, minimum, maximum and outliers.
The second best model, including both season and group size as additive terms, was found to fit the data as well as the season model on AIC alone but not when BIC was considered alongside it (AIC = 287.60 and BIC = 331.07). Thus, we considered the effect behaviour may be having on group size. The ANOVA test result suggested that the variance in group size within each behaviour category was significantly different from the overall variance in group size (F = 4.33, df = 3, P < 0.01). The post-hoc Tukey test revealed that travelling groups were significantly smaller than those engaged in surface-feeding behaviour (difference = 2, P < 0.01). No other pairwise differences were found to be significant.
Discussion
Our results suggest that spatial variables and time of day do not have an effect on the behavioural patterns of coastal Montenegro's bottlenose dolphins while season explains most of the observed variation in behaviour. Indeed, depth and distance from shore failed to explain the observed variation in behaviour. Depth and distance from shore are highly correlated variables that we expect to have analogous effects on dolphin behaviour via their effect on habitat complexity (Sargeant et al., Reference Sargeant, Würsing, Heithaus and Mann2007). Montenegro is home to some of the deepest waters in the Adriatic Sea whilst its territorial waters are shallow (Blake & Topalović, Reference Blake and Topalović1996). In this study, no recordings were made at depths beyond 50 m or further than 4 km away from the coast. Thus, the variability in depth and distance of this study is very low and a gradient of 50 m within 4000 m may not be enough to promote a heterogeneous habitat that warrants specific behavioural changes (Ballance et al., Reference Ballance1992; Sargeant et al., Reference Sargeant, Würsing, Heithaus and Mann2007). Furthermore, these predominantly shallow waters and the high incidence of travelling behaviour supports previous findings that suggest travelling occurs primarily at shallow depths that offer protection from predation and enable the use of the coastline as a navigation tool (Ballance, Reference Ballance1992; Bearzi, Reference Bearzi2005). Despite the lack of support for spatial effects on behaviour in our study, we expect that increased survey area coverage will reveal behavioural patterns that can be linked to depth and distance from shore (Di Sciara et al., Reference Di Sciara, Venturino, Zanardelli, Bearzi, Borsani and Cavalloni1993; Hanson & Defran, Reference Hanson and Defran1993). Nevertheless, the high occurrence (75%) of dolphin groups in very shallow waters corresponds to the coastal distribution of this species’ coastal ecotype described globally (Ballance, Reference Ballance1992; Di Sciara et al., Reference Di Sciara, Venturino, Zanardelli, Bearzi, Borsani and Cavalloni1993).
Additionally, we do not detect any effect of diurnal changes on the behaviour of bottlenose dolphins. With the exception of diel light conditions, daily changes in abiotic factors are small in large marine basins such as the Adriatic Sea (Cushman-Rosin et al., Reference Cushman-Roisin, Gacic, Poulain and Artegiani2013) and are likely insufficient to cause habitat level changes that require behaviour modification in a homoeothermic species such as T. truncatus. Further, anthropogenic activity in the coastal waters of Montenegro does not vary greatly during the day with boat traffic remaining constant from morning to afternoon, as tankers arrive all day long and small fishing vessels leave at dusk and dawn (personal observations). However, splitting the dataset into two, morning and afternoon, may be too coarse a categorization to allow for the detection of a crepuscular pattern identified in previous studies.
Bottlenose dolphins in our study area display a seasonal pattern in behaviour, with sighting rates and behaviours such as surface-feeding and socializing-resting increasing over the colder autumn and winter months. Conservation efforts are directed by the identification of areas ecologically important for foraging and reproduction in bottlenose dolphin populations (Shane et al., Reference Shane, Wells and Würsig1986; Connor et al., Reference Connor, Wells, Mann, Read, Mann, Connor, Tyack and Whitehead2000), which, here, are intrinsically linked to these times of year. Possible explanations for these changes in behaviour include increased productivity in winter and higher prey abundance or availability, favourable environmental conditions and decreased human presence (Hanson & Defran, Reference Hanson and Defran1993; Lusseau & Higham, Reference Lusseau and Higham2004; Lusseau et al., Reference Lusseau, Williams, Wilson, Grellier, Barton, Hammond and Thompson2004). Indeed, Montenegrin waters are considerably warmer in summer months that are characterized by weaker maritime currents, which provide less nutrients and upwelling in the region (CAU, ELARD, ITI, 2014; UNEP, 2015). Moreover, Montenegro's economy relies heavily on tourism, with the peak season taking place in warmer months where boat traffic increases dramatically and high-speed loud boats such as jet skis are encountered on a regular basis (personal observations). Increases in boat traffic have been linked to a decrease in foraging and socializing behaviours in bottlenose dolphins in the Mediterranean (Papale et al., Reference Papale, Azzolin and Giacoma2012; Bas et al., Reference Bas, Christiansen, Öztürk, Öztürk and Watson2017) and elsewhere (Nowacek et al., Reference Nowacek, Wells and Solow2001; Bejder et al., Reference Bejder, Samuels, Whitehead, Gales, Mann, Connor, Heithaus, Watson-Capps, Flaherty and Kruetzen2006). In fact, socializing and resting are the most sensitive behaviours to human disturbance (Lusseau & Higham, Reference Lusseau and Higham2004). The relationship between behaviour and season combined with the increase in sighting rates in autumn and winter suggests that a temporal change in both anthropogenic pressure, comparable to that in other Mediterranean basins (Pennino et al., Reference Pennino, Roda, Pierce and Rotta2016), and environmental conditions is prone to be responsible for the observed patterns in behaviour.
Behavioural choices are responsible for group dynamics in Montenegro's bottlenose dolphin population. Behaviour and group size are fundamentally linked in bottlenose dolphins (Shane et al., Reference Shane, Wells and Würsig1986). Typically, group sizes are smaller in shallow waters where habitats provide relatively predictable food patches in space and time (Campbell et al., Reference Campbell, Bilgre and Defran2002; Gowans et al., Reference Gowans, Würsig and Karczmarski2007). Our results show that choices in behaviour are likely driving group size. Despite the lack of significance in pairwise differences, socializing and foraging show larger group sizes expected from behaviours requiring advanced coordination and the presence of conspecifics (Connor et al., Reference Connor, Wells, Mann, Read, Mann, Connor, Tyack and Whitehead2000; Sargeant & Mann, Reference Sargeant and Mann2009). Populations in Croatia and Greece display larger group sizes associated with foraging behaviour than in neighbouring coastal Montenegro (Politi et al., Reference Politi, Bearzi, Notarbartolo di Sciara, Cussino and Gnone1992; Bearzi et al., Reference Bearzi, Notarbartolo di Sciara and Bonomi1992, Reference Bearzi, Politi and di Sciara1999; Genov et al., Reference Genov, Kotnjek, Lesjak, Hace and Fortuna2008). The smaller average group size found in Montenegrin waters can be linked to the predominance of travelling behaviours recorded in this study (Bearzi et al., Reference Bearzi, Notarbartolo di Sciara and Bonomi1992; Rogers et al., Reference Rogers, Brunnick, Herzing and Baldwin2004). These findings support the idea that T. truncatus are using these waters as travelling grounds for small groups between feeding sites where they form larger assemblages.
Critical habitat delineation, using key biological behaviours, forms the baseline of marine protected area implementation (Hoyt, Reference Hoyt2005; Pennino et al., Reference Pennino, Arcangeli, Fonseca, Campana, Pierce, Rotta and Bellido2017). We suggest that combining our approach with new studies of T. truncatus found offshore and photo-identification of the local population will help to understand both its variation in behaviour and movement patterns. Mark-recapture analysis of bottlenose dolphins in the area can further reveal whether the same individuals are regularly seen travelling along the coast over time and if these are sighted in larger foraging groups in other territorial waters. Such an approach would benefit tremendously from a yet lacking international collaboration of all scientists working in the Adriatic. Additionally, long-term monitoring of the local population will be necessary to identify if the seasonal patterns found here, and their implications, are consistent over years. This effort, combined with specific analysis of behavioural responses to human presence (Lusseau, Reference Lusseau2003) will reveal whether there is a chronic anthropogenic pressure on the present dolphins (Nowacek et al., Reference Nowacek, Wells and Solow2001; Bas et al., Reference Bas, Christiansen, Öztürk, Öztürk and Watson2017), forcing them to reduce ecologically important behaviours and avoid the area over summer months. This is especially important in the face of recorded changes in dolphin behaviours and abundance due to seasonal variations in marine vessel traffic and type across the Mediterranean (La Manna et al., Reference La Manna, Manghi, Pavan, Lo Mascolo and Sarà2013; Pennino et al., Reference Pennino, Roda, Pierce and Rotta2016; Campana et al., Reference Campana, Angeletti, Crosti, Luperini, Ruvolo, Alessandrini and Arcangeli2017).
Our work provides the very first data on South-eastern Adriatic Sea bottlenose dolphin behaviour. We do not find any support for the effect of spatial variables on the observed variation in behaviour, possibly due to the limited geographic range of our sample and the homogeneous habitat found within it. However, we find patterns of seasonality in dolphin behaviour and sightings that suggest coastal Montenegrin waters are more favourable over autumn and winter. Disentangling which effects, natural and/or anthropogenic, are having the greatest impact on T. truncatus behaviour is crucial to designing effective management in these waters. Indeed, elucidating what role Montenegro's territorial waters play in these dolphins’ life history and what effect human disturbance is having on the local population is of paramount importance for the proper identification of threats to the local population. Further effort should focus on collaborative work covering the entire Adriatic basin to better our understanding of local T. truncatus status throughout its range.
Acknowledgements
We would like to thank Nadia Frontier for providing Figures 1 and 2 and Jessica Rayner for her help in preparing the data for analysis. In addition, special thanks go to all the volunteers, part of the Montenegro Dolphin Project, who helped collect the data used in this study. Finally, we would like to thank Becky Rose and Lyndsey Stirling for their help accessing the relevant literature.
Financial support
We extend our gratitude to Rufford Small Grant Foundation for their financial support.









