Introduction
Competition is considered as one of the main drivers of ecological communities leading to niche differentiation (Adler, Harpole, Mutshinda, O’Hara, & Woiwod 2009; HilleRisLambers, Levine, & Mayfield Reference HilleRisLambers, Adler, Harpole, Levine and Mayfield2012; Morin Reference Morin2011). The acoustic competition represents a particular case of competition that might occur between species vocalizing at the same place and at the same time, constituting acoustic communities (Farina & James Reference Farina and James2016; Gasc et al. Reference Gasc, Sueur, Jiguet, Devictor, Grandcolas, Burrow, Depraetere and Pavoine2013). Each species within the acoustic community would compete for the acoustic space and would occupy an acoustic niche (Krause Reference Krause1993). Acoustic niches could lead to acoustic partitioning as has been observed at community level (Aide et al. Reference Aide, Hernández-Serna, Campos-Cerqueira, Acevedo-Charry and Deichmann2017) and species level, mainly in anurans and insects more rarely in birds (Malavasi & Farina Reference Malavasi and Farina2013; Popp, Ficken & Reinartz Reference Popp, Ficken and Reinartz1985) particularly in the tropics (Brumm Reference Brumm2006; Ficken & Hailma Reference Ficken and Hailma1974; Luther Reference Luther2009; Planqué & Slabbekoorn Reference Planqué and Slabbekoorn2008).
The Resplendent Quetzal, Pharomachrus mocinno (De la Llave 1832), is one of the most famous species in the cloud forest of Central America (LaBastille & Allen Reference LaBastille and Allen1969). The species is considered endangered in Guatemala (CONAP 2009) and near threatened according to the red list of endangered species of the International Union for Conservation of Nature (IUCN) (Birdlife International 2016). Pharomachrus mocinno occupies a central place in the Guatemalan culture, being a flagship for conservation of the cloud forest and therefore for the species inhabiting it. In addition, as a seed disperser for at least 32 plant species, it is a keystone species playing a main role in forest regeneration (Avila, Hernández & Verlarde 1996). Pharomachrus mocinno belongs to a bird assemblage cohabitating with species potentially sharing the same ecological resources and predators (Santana & Milligan Reference Santana and Milligan1984). This non-oscine bird produces four types of sounds associated with the following behaviours: territory defence, courtship, alarm and contact between individuals (supplementary Figure 1) (Bolaños-Sittler, Sueur, Fuchs & Aubin Reference Bolaños-Sittler, Sueur, Fuchs and Aubin2019), with median frequencies between 950 and 1550 Hz (Bolaños-Sittler Reference Bolaños-Sittler2019). These sounds can be produced at the same time as those of other local species so that P. mocinno belongs to a specific acoustic community where signals have the potential to overlap between them, in particular for the species occupying the same frequency band.
Here, we aimed at estimating the possible acoustic competition between P. mocinno with other bird species belonging to the same community within the cloud forest. More specifically, we estimated the time and frequency overlaps between P. mocinno vocalizations with the vocalizations of other species during the breeding season. To achieve this, we deployed an acoustic survey over 17 consecutive days at different sites of a cloud forest using autonomous recorders. Species sharing the frequency bandwidth with P. mocinno were identified, and the potential and the observed acoustic overlap between vocalizations of P. mocinno with those of other species were estimated. To know which species in the community compete the most with P. mocinno, it was necessary to take into consideration other ecological and biological characteristics of the species. This was conducted with a multivariate analysis considering not only the acoustic overlap but also the phylogenetic distance and several competition traits.
Materials and methods
Study site and data collection
Field work was conducted at ‘Los Andes’, Suchitepéquez, Guatemala (14°54ʼ N – 91°18ʼ W, 1661 m.a.s.l.) (Figure 1). ‘Los Andes’ is a private reserve, including 607 ha on the southern slope of the Atitlán volcano at an elevation of 840–1830 m.a.s.l. The site is covered by cloud forest on the highest part of the reserve (364 ha) and crops of coffee, tea and rubber on the lowest part (243 ha). The reserve facilitates birdwatching and ecotourism activities, the observation of P. mocinno in its habitat being one of the main attractions for visitors.

Figure 1. Recording sites in Los Andes reserve, on the south slope of the volcano Atitlán, Guatemala.
The acoustic community P. mocinno belongs to was recorded passively at six sites using Wildlife Acoustics® SM2 automated recorders. The recorders were separated from each other by an average distance of 450 m so that their area of recording estimated at around 100 m did not overlap. The recorders were programmed to record continuously from 5:00 to 9:30 and from 15:30 to 18:00, during the dawn and dusk choruses when birds are most active. The recorders worked for 17 days, from the 8th of February to the 25th of February 2016. Recordings were split into 30 min files, so that 778 files were obtained. Recordings were done with a sampling frequency of 44.1 kHz and a 16 bit dynamic (microphone frequency response: 0.02–20 kHz ± 6 dB).
Data analysis
The vocalizations of P. mocinno were first manually detected by listening and visualizing the 778 recordings through a spectrographic representation with the software Raven Pro 1.5 (Center for Conservation Bioacoustics 2014) (short-time Fourier transform was calculated with a time precision of 0.0232 s, a frequency precision of 21.5 Hz, a dynamic of 96 dB). Pharomachrus mocinno vocalizations were detected in 93 recordings among the 778 30 min recordings. All the vocalizations of P. mocinno in the 93 recordings were then manually annotated and classified as territorial, alarm or courtship vocalizations (contact vocalizations were not found). The vocalizations of all other species occurring in the same bandwidth between 0.5 and 2.5 kHz and within 300 s before, during or after a vocalization of P. mocinno were similarly annotated. This time window of 300 s was set to obtain a good temporal representation of the community dynamic. Annotations were added with Raven Pro 1.5 (Center for Conservation Bioacoustics 2014) software directly from on-screen measurement cursors on the spectrogram. Selections were made taking the start (s), end (s), highest and lowest frequencies (Hz), resulting in time x frequency rectangles (Figure 2).
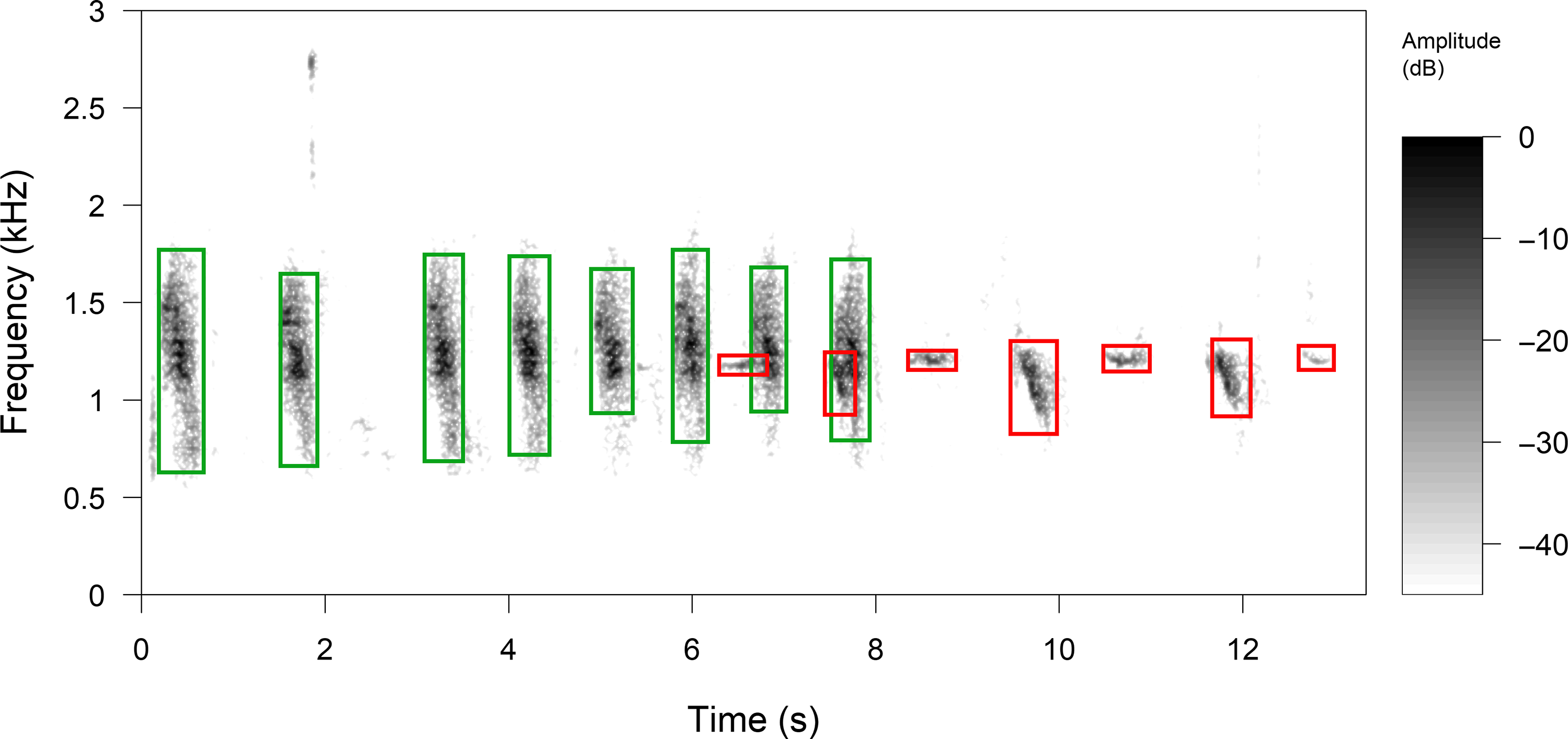
Figure 2. Manual selections of vocalizations were made using the spectrogram display of Raven Pro 1.5 software (time precision = 0.0232 s, frequency precision = 21.5 Hz, dynamic range = 96 dB). All the vocalizations found within 300 s before, during or after a vocalization of P. mocinno were manually selected. Selections were made taking the duration (s) and the highest and lowest frequencies (kHz), between 0.5 and 2.5 kHz, resulting in rectangles with x and y coordinates (respectively time and frequency). Vocalizations with red rectangles are territorial produced by P. mocinno and vocalizations with green rectangles by Aulacorhynchus prasinus. In this example, the first vocalization of P. mocinno overlaps 30% with A. prasinus, the second overlaps 80% and the rest does not overlap. The potential overlap of P. mocinno vocalizations with A. prasinus is 100%, and the observed overlap is an average of all the overlapping areas.
Frequency and time overlap were quantified by estimating the percentage of the time x frequency rectangle of P. mocinno vocalizations that overlapped with the time x frequency rectangle of other species vocalizations. For each species, an observed overlap (overlap rate) was quantified as the sum of all the overlap percentages with P. mocinno, divided by the times that P. mocinno vocalized. According to the frequencies of the vocalizations of each species and P. mocinno, the potential to overlap was different in each case. Thus, for each species, an average of the maximum and minimum frequencies was calculated. Then, the potential overlap or maximum overlap possible between P. mocinno and each other species was calculated when aligning the averaged time x frequency rectangles of P. mocinno with those of the other species.
Then, for each species, a comparison between the observed overlap and the potential overlap was made by subtracting the later value from the former. This calculation made it possible to measure if the species were overlapping more or less (observed overlap) than the expected (potential overlap).
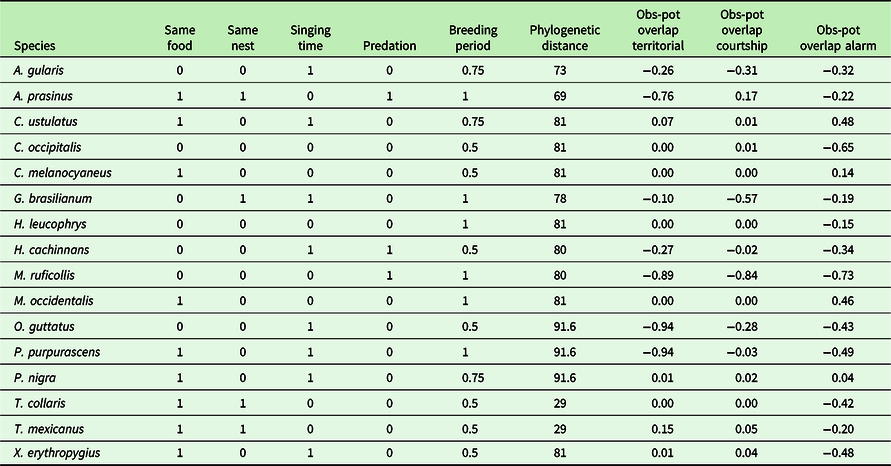
Five other variables were considered: food, nest type, singing period during the day, breeding period during the year and predation. Regarding the food type, the Resplendent Quetzal was considered as essentially frugivorous, and any other regime was considered as different (unless it also includes frugivory). Regarding nest type, the Resplendent Quetzal is a cavity-nesting species, and other nest types were considered as different. The singing day period of the Resplendent Quetzal was mostly at dawn, so that any other period was considered as different. Food, nest type and singing period competition were then encoded as 1 if similar or 0 if different. For the breeding period, the information was estimated as the number of overlapping months and then scaled between 0 and 1, with 0 indicating no overlap in the breeding season and 1 a full overlap. Possible predation of adults, chicks or eggs of P. mocinno was encoded for each species as 1 if it is known to occur regularly and 0 if not. These data were obtained from personal observations (PB, CD) and literature (Avila et al 1996; BirdLife International 2017; Bustamante, Barrios & Juárez Reference Bustamante, Barrios and Juárez2010; Carroll, Kirwan & Boesman Reference Carroll, Kirwan, Boesman, del Hoyo, Elliott, Sargatal, Christie and de Juana2020; Collar Reference Collar, del Hoyo, Elliott, Sargatal, Christie and de Juana2020; Eisermann et al. Reference Eisermann, Komar, Herrera and Brooks2006; Fagan & Komar Reference Fagan and Komar2016; Foster Reference Foster2007; Haverschmidt Reference Haverschmidt1962; Howell & Webb Reference Howell and Webb1995; Johnsgard Reference Johnsgard2000; Mack and Yong Reference Mack, Yong, Poole and Gill2020; Miller, Greeney & Valdez Reference Miller, Greeney and Valdez2010; Motta Reference Motta2007; Santana & Milligan Reference Santana and Milligan1984; Snow Reference Snow, del Hoyo, Elliott, Sargatal, Christie and de Juana2001; Solórzano, Castillo, Valverde & Avila Reference Solórzano, Castillo, Valverde and Avila2000; Specht Mesquita & Santos Reference Specht, Mesquita and Santos2008; Thorstrom Reference Thorstrom2000; Valdez 2010; Wenny 2014; Zimmer & Isler Reference Zimmer, Isler and Editions2003).
In addition, to consider phylogenetic constraints, the median phylogenetic distance between P. mocinno and the other species was retrieved from TimeTree (Kumar, Stecher, Suleski & Hedges Reference Kumar, Stecher, Suleski and Hedges2017). The metric used was the distance to the most recent common ancestor (MRCA) in millions of years. When a species was not present in the tree, the next closest taxon was taken as a reference as suggested by TimeTree methodology.
Statistical analysis
The combination of all competition variables led to a matrix made of 16 rows (species) and nine columns (difference between the observed and the potential overlap with three vocalization types of P. mocinno, five resource competitions and one phylogenetic distance) (Table 1). This competition matrix was treated with a principal component analysis (PCA). Species were used as explained (dependent) variables; the competition for the acoustic space, the competition for ecological resources and phylogenetic distance were included as explanatory (independent) variables. The PCA was conducted (1) to identify inter-correlations between the acoustic overlap and the competition for other ecological resources between P. mocinno and the other species, (2) to analyse possible inter-correlations between acoustic overlap and phylogenetic distance, and (3) to reveal the main competitors of P. mocinno.
Table 1. Competition for resources and phylogenetic distance between P. mocinno and the other species included in the analysis. Resource use comparison between P. mocinno and the other species was encoded as 1 = same use, 0 = different use. Possible predation of P. mocinno was encoded as 1 = known to occur, 0 = not predator or not known to occur. Breeding overlap was encoded between 0 and 1 as a rate of overlapping months with the breeding period of P. mocinno (i.e. 1 = full overlap for the 4 months of the breeding period). Phylogenetic distance was included as the estimated median time in millions of years of the most recent common ancestor (MRCA) (www.timetree.org). Obs-pot overlap: refers to the difference between the observed and the potential overlap respectively for the territorial, courtship and alarm vocalizations of P. mocinno
Results
In total, 7,806 vocalizations belonging to 16 species were identified in a 500–2500 Hz bandwidth with a cumulative duration of 9907 s. The species identified are presented in Table 2.
Table 2. Bird species identified in the recordings
The species that interacted the most with P. mocinno, regardless of their potential acoustic overlap, were C. ustulatus, A. prasinus, T. mexicanus, P. purpurascens, M. occidentalis, P. nigra and M. ruficollis. Among these species, the most acoustically active ones were T. mexicanus, C. ustulatus, M. occidentalis and M. ruficollis (Figure 3).

Figure 3. Raw number of vocalizations detected for each species sharing the same acoustic community than P. mocinno. This number does not consider the overlap with P. mocinno, but only the abundance of vocalizations per species. The number of vocalizations of P. mocinno was 10811. See Table 2 for complete species Latin names.
The species vocalizations with the highest acoustic overlap with the territorial vocalizations of P. mocinno were T. mexicanus, followed by M. ruficollis and C. ustulatus. The species vocalizations with the highest acoustic overlap with the courtship vocalizations of P. mocinno were P. purpurascens, A. prasinus and M. ruficollis. Among the three vocalizations of P. mocinno analysed, the alarm vocalization overlapped the most with the vocalizations of the other species. The species with the highest acoustic overlap with the alarm vocalizations of P. mocinno were C. ustulatus, then A. prasinus, M. occidentalis and T. mexicanus.
According to the averaged frequency bandwidth of the vocalizations of each species studied, the species that had the highest potential to overlap with the territorial vocalization of P. mocinno were M. ruficollis (100%), P. purpurascens (100%) and O. guttatus (94%). The species with the highest potential to overlap with the courtship vocalization of P. mocinno were M. ruficollis (96%), G. brasilianum (57%) and P. purpurascens (42%). The species with highest potential to overlap with the alarm vocalization of P. mocinno were C. occipitalis (96%), M. ruficollis (92%) and A. prasinus (80%).
The species that had the highest difference between the observed overlap and the potential overlap with P. mocinno were T. mexicanus and C. ustulatus for the territorial vocalization (Figure 4); A. prasinus, T. mexicanus and X. erythropygius for the courtship vocalization (Figure 5); and C. ustulatus, M occidentalis, C. melanocyaneus and P. nigra for the alarm vocalization (Figure 6). The other species had an observed acoustic overlap similar to or lower than the potential overlap (a difference between −0.04 and 0.04).
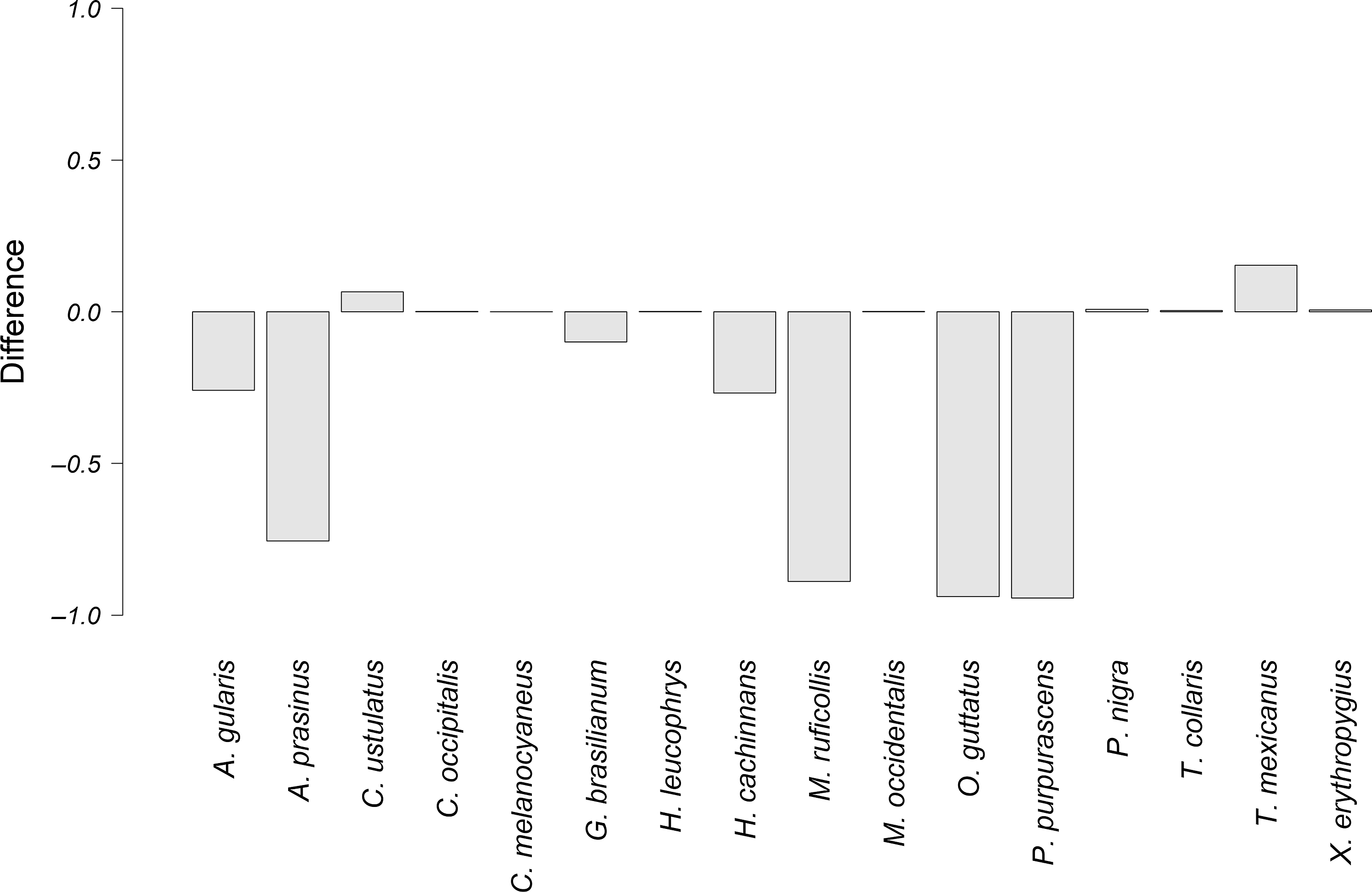
Figure 4. Difference between the observed overlap and the potential overlap of the territorial vocalizations of P. mocinno and vocalizations of other species. A negative number means that the potential overlap is higher than the observed overlap. Conversely, a positive number means that the observed overlap is higher than the potential overlap.
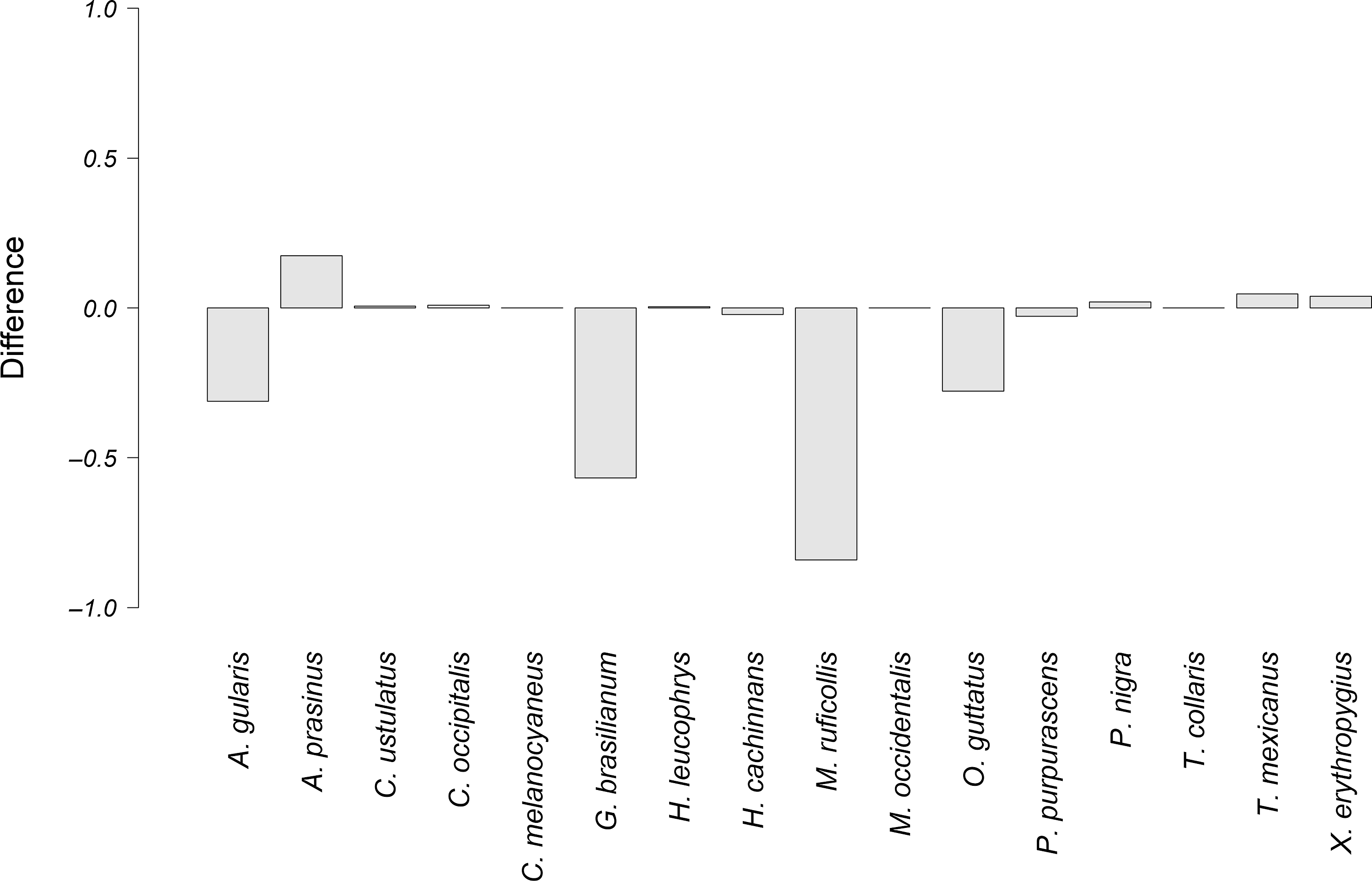
Figure 5. Difference between the observed overlap and the potential overlap of the courtship vocalizations of P. mocinno and vocalizations of other species. A negative number indicates that the potential overlap is higher than the observed overlap. Conversely, a positive number indicates that the observed overlap is higher than the potential overlap.
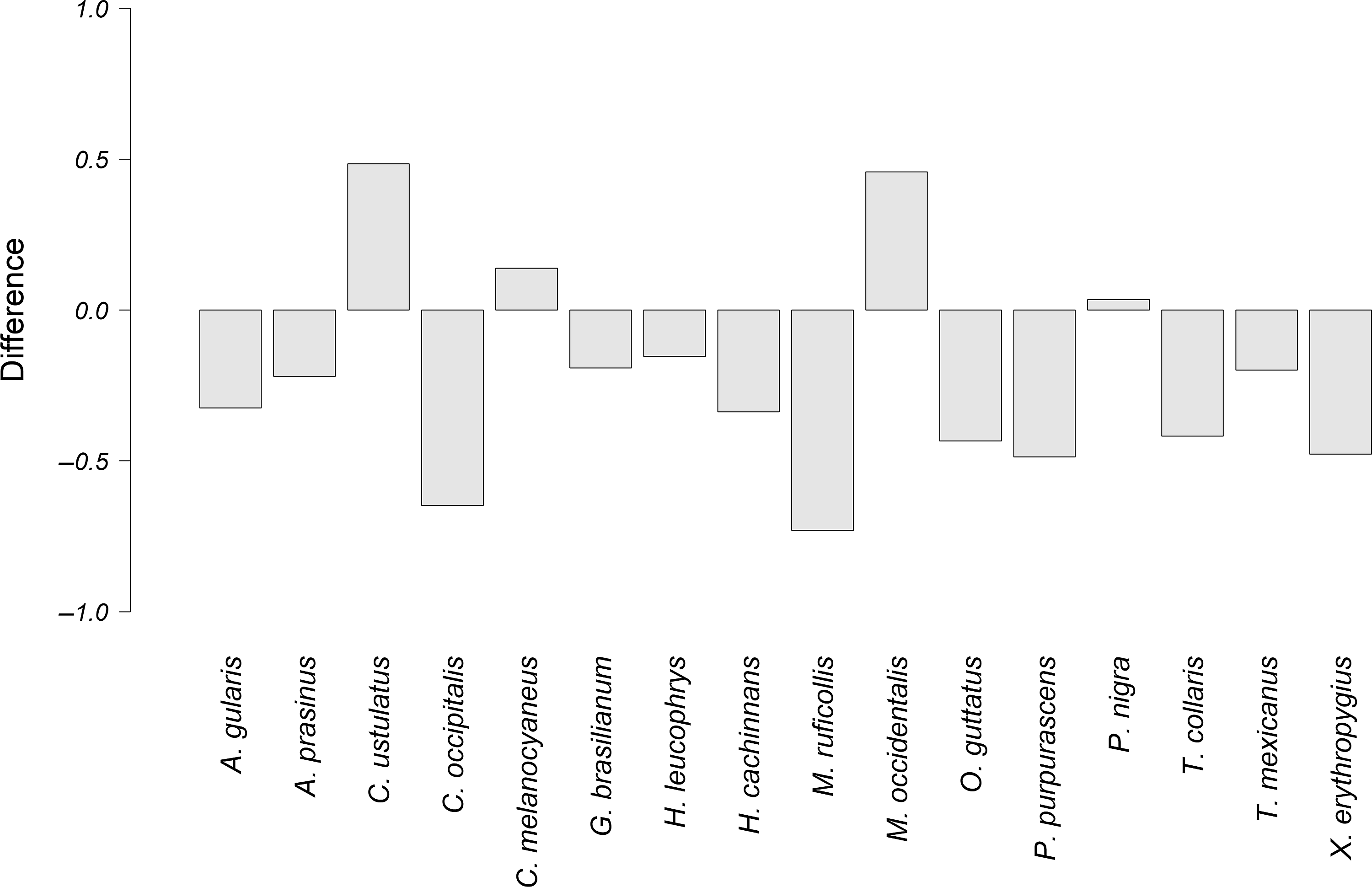
Figure 6. Difference between the observed overlap and the potential overlap, of the alarm vocalizations of P. mocinno and vocalizations of other species. A negative number indicates that the potential overlap is higher than the observed overlap. Conversely, a positive number indicates that the observed overlap is higher than the potential overlap.
The first three axes of the PCA explained 67.94% of the variation for the competition of P. mocinno with other species of the community (Table 3). For the first axis, the most important variables were the difference between the observed overlap and the potential overlap with the territorial vocalization of P. mocinno, then with the courtship vocalization and then the competition for food resources. For the second axis, the most important variables were the competition for the same nest type and the phylogenetic distance (Figure 7).
Table 3. PCA axis coordinates of the different competition for resources. Obs-pot overlap refers to the difference between the potential overlap of one species to acoustically overlap with P. mocinno and the actually observed overlap
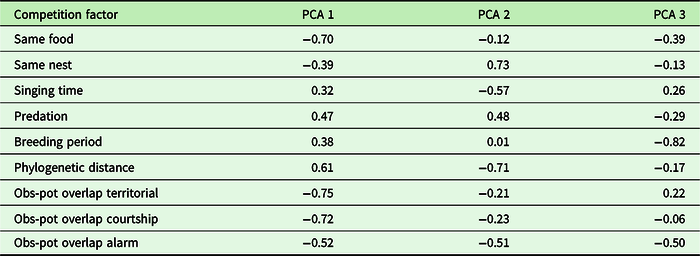
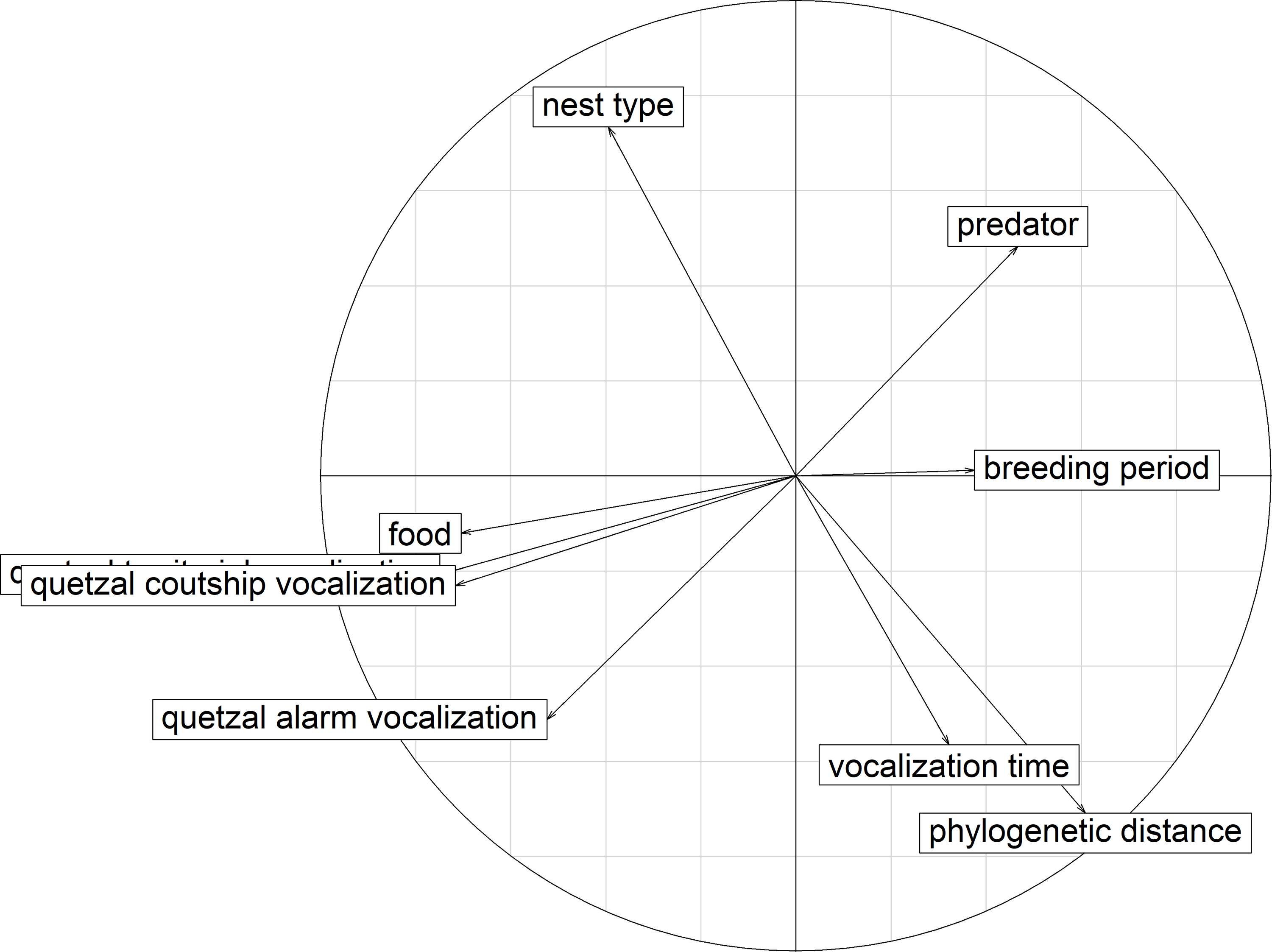
Figure 7. PCA correlation circle for ecological resources. Correlation between the explaining variables according to the first two axes that explained 53.36% of the variation. Quetzal courtship vocalization: difference between the potential acoustic overlap of a species and the observed overlap with the courtship vocalizations of P. mocinno; quetzal territorial vocalization (overlapped in the graph by courtship vocalizations): difference between the potential acoustic overlap of a species and the observed overlap with the territorial vocalizations of P. mocinno; quetzal alarm vocalization: difference between the potential acoustic overlap of a species and the observed overlap with the alarm vocalizations of P. mocinno; vocalization time: same time of vocalizing in the day as P. mocinno; phylogenetic distance: phylogenetic distance of a species with P. mocinno; breeding period: proportion of overlap of the breeding period of a species with the breeding period of P. mocinno; predator: possibility of predation of P. mocinno by a species; nest type: same nest type as P. mocinno.
PCA analysis (Figure 8) revealed the following patterns of competition between the different species and P. mocinno: 1) C. melanocyaneus, C. ustulatus, M. occidentalis, X. erythropygius and P. nigra show a strong correlation between the competition for food resources, and acoustic overlap with P. mocinno vocalizations. Among this group of species, C. ustulatus is migratory (species highlighted in yellow); 2) T. mexicanus and T. collaris are phylogenetically close to P. mocinno, use the same nest type and have some overlap of their vocalizations (species highlighted in red); 3) A. gularis, O. guttatus and P. purpurascens are phylogenetically distant from P. mocinno, vocalize at the same time of day, and their vocalizations have a low overlap with P. mocinno (species highlighted in blue); 4) M. ruficollis is a predator that had a low acoustic overlap with P. mocinno (species highlighted in purple); 5) G. brasilianum, C. occipitalis, H. cachinnans and H. leucophrys had a low acoustic overlap with P. mocinno, with low or well-partitioned ecological niches with it (species highlighted in grey) and 6) A. prasinus is a predator that uses similar nest types to those of P. mocinno. This species was the highest after T. mexicanus (species highlighted in green) to overlap with the alarm vocalization of P.mocinno.
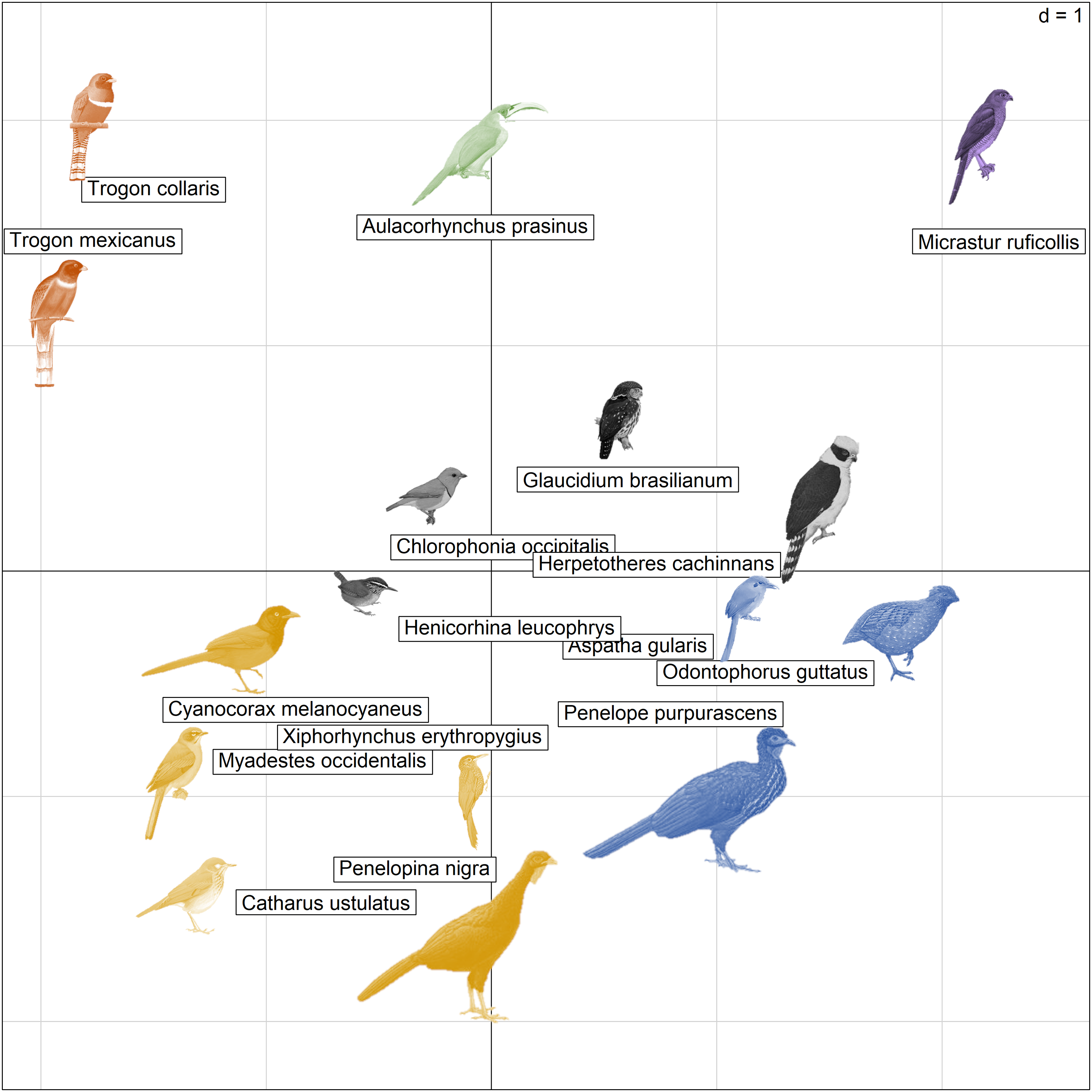
Figure 8. PCA species plot for ecological resources. Individual plot with species (individuals) placed in the PCA space according to the first two axes that explained 53.36% of the variation. Different colours highlight species of the different patterns of ecological competition mentioned in the text.
The patterns suggested by the PCA analysis can be summarized as follows:
-
1. Full or partially frugivorous bird species had a high observed acoustic overlap with P. mocinno.
-
2. Phylogenetically close species with P. mocinno had a high observed acoustic overlap, and distant species had a low observed acoustic overlap with P. mocinno.
-
3. Predator species had a low observed acoustic overlap with P. mocinno.
-
4. Predator species, also competing for fruits and nest types with P. mocinno (A. prasinus), had a low acoustic overlap with territorial and alarm vocalizations, but a high one with courtship vocalization.
Figure 9 shows the general scheme of competition in the acoustic community of P. mocinno.
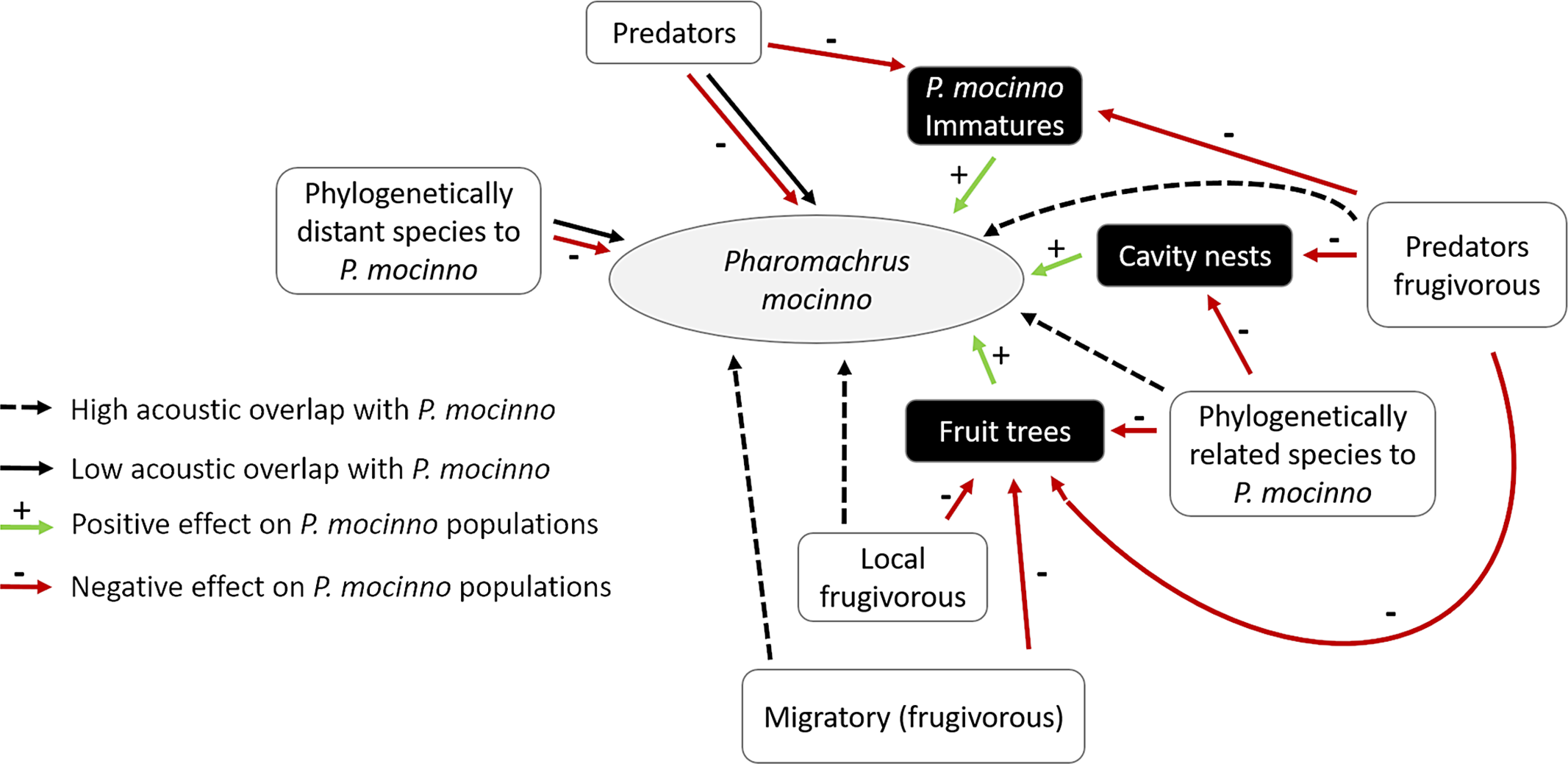
Figure 9. General scheme of competition in the acoustic community of P. mocinno. Specific circumstances may apply. Black squares represent positive resources for P. mocinno, red arrows are negative pressures, and green arrows are positive pressures.
Discussion
An analysis of the bird acoustic community vocalizing within the bandwidth of P. mocinno vocalizations was conducted in a cloud forest in Guatemala. The potential of each species of the community to overlap with the vocalizations of P. mocinno was quantified and compared with the actually observed overlap of their vocalizations. A multivariate analysis was also conducted to assess the relative importance of several ecological traits, including acoustics, for the competition of P. mocinno with other species belonging to the same acoustic community. The potential to overlap acoustically with P. mocinno differed between the species, with a higher overlap when the frequency bandwidths fall in the same range. Nevertheless, the observed overlap was not always related to the potential overlap. This difference could be due to the particular ecological interactions between P. mocinno and the other species and not just to the acoustic characteristics of the vocalizations. According to the degree of correlation between species observed in the multivariate analysis, different categories and patterns were identified.
One pattern observed was a strong correlation between the use of the same food resources and a high acoustic overlap. The bird species in this category are partially or completely frugivorous and are not predators of P. mocinno, so they might compete with P. mocinno for food resources. In addition, species in this category are active mainly in the same forest strata. Thus, the acoustic overlap could be due to competition for food or space in the forest canopy.
Species phylogenetically close to P. mocinno, belonging to the same family Trogonidae, had a high acoustic overlap with P. mocinno. The species Trogon mexicanus and T. collaris compete for nest sites and also for food resources with P. mocinno. The high acoustic overlap observed for the alarm vocalizations of P. mocinno could be due in part to morphological constraints. Defending nest areas and also competing for food might trigger the production of alarm vocalizations and so of acoustic overlap. Despite the low potential of T. mexicanus to overlap with territorial vocalizations of P. mocinno, T. mexicanus was the species with the highest observed overlap with the territorial vocalization of P. mocinno among all the other species of the acoustic community. The production of territorial vocalizations, taking place during immediate competition over resources, is strongly dependent on social and spatial relations of producers (Naguib Reference Naguib2005). Thus, T. mexicanus could be the most important competitor in terms of acoustic space with P. mocinno.
Phylogenetically distant species showed a low acoustic overlap with P. mocinno, even if they vocalized at the same time of day. The species observed with this pattern were A. gularis, O. guttatus and P. purpurascens. The three species have a high potential to overlap acoustically with P. mocinno but the analysis revealed the opposite. Luther (Reference Luther2009) suggested that, in the same community, acoustic partitioning among species singing in a common time interval is expected to be greater than partitioning among species that usually sing in different space and time. This suggests some behavioural plasticity in the time pattern of their vocalization leading to acoustic overlap avoidance. The low acoustic overlap could be another example of temporal avoidance (Planqué & Slabbekoorn, Reference Planqué and Slabbekoorn2008). The interactions of these species with P. mocinno are probably low, and their respective ecological niches could be well differentiated.
The predator species M. ruficollis was the one that acoustically overlapped the less with P. mocinno despite the fact that their vocalizations were in the same frequency band (Fagan & Komar Reference Fagan and Komar2016). This acoustic avoidance could be related to the predation pressure of M. ruficollis over P. mocinno. Thus, to avoid to be localized, this last one does not vocalize when M. ruficollis is in the vicinity, which is a behaviour observed as well in other species, in the presence of predators (Ruxton Reference Ruxton2009).
Aulacorhynchus prasinus is a known predator of eggs and chicks of P. mocinno. In addition, it is a fruit and nest competitor (Skutch Reference Skutch1967) and phylogenetically distant species with P. mocinno. This species shows low acoustic overlap with territorial and alarm vocalizations of P. mocinno, as other predators in the acoustic community (M. ruficollis and H. cachinnans). Aulacorhynchus prasinus is dominant over P. mocinno and sometimes displaces it from its perch (Santana & Milligan Reference Santana and Milligan1984). Physical contact and intense chases have been observed between P. mocinno and A. prasinus (Wheelwright Reference Wheelwright1983). Thus, P. mocinno probably avoids vocalizing when hearing A. prasinus.
Contrary to what was observed with other frugivorous species competing with P. mocinno for food resources, the acoustic overlap was low with A. prasinus. The pressure imposed by the competition for food might be surpassed by the dominance of A. prasinus over P. mocinno. The high potential of these two species to overlap acoustically could be mitigated by a partition of space at foraging areas. Each of the two species has different methods to feed and differences in selectiveness. Aulacorhynchus prasinus looks for fruits with hopping movements on the branch while P. mocinno takes fruits doing short flights (Avila et al. 1996; Santana & Milligan Reference Santana and Milligan1984). Pharomachrus mocinno generally takes only one fruit per flight. Then, the energy obtained from that unique fruit must compensate for the costs of flying for it. In contrast, A. prasinus takes as many fruits it can at the same time. Thus, each species may use different areas in the same tree (Santana & Milligan Reference Santana and Milligan1984). These behavioural differences could influence the low acoustic overlap between them, in comparison to other frugivorous species having a higher acoustic overlap with P. mocinno.
While vocalizations of A. prasinus had a low overlap with the territorial and alarm vocalizations of P. mocinno, they had a high overlap with the courtship vocalization. This is probably due to competition for nesting sites, increasing the interactions between both species and thus increasing the overlap between their vocalizations.
The migratory species Catharus ustulatus had the highest overlap with the alarm vocalizations of P. mocinno and was the second species after T. mexicanus with the highest overlap with the territorial vocalizations. Catharus ustulatus forages on the same canopy stratus than P. mocinno and feeds on insects, fruits and berries (Holmes & Robinson Reference Holmes and Robinson1988). The vocalizations of C. ustulatus were repetitive and the most abundant after T. mexicanus.
Conclusions
In the present study, a detailed quantification of acoustic overlap in time and frequency has been made, and the strongest ecological niche competitors with P. mocinno have been identified. Competitors with P. mocinno have been reported in previous studies. Nevertheless, this is the first quantification of a competition taking into consideration the acoustic space. The results presented here can be useful to conduct other specific studies in P. mocinno community increasing the knowledge of this flagship species.
Acknowledgements
We thank Sébastien Hardy and the CEMCA for their important support to conduct the study in Guatemala. We are grateful to the guides Jesús Lucas and Selvin Xiloj for their useful help during field work and to the Hazard family for the support in Los Andes reserve. We thank Sandrine Pavoine for the valuable comments and her help on the statistics analyses and Claire Dallies for their shared knowledge about the avian community. This work was financed by the National Geographic Society [grant number 9479-6].
Supplementary material
For supplementary material accompanying this paper visit https://doi.org/10.1017/S0266467421000420

















