INTRODUCTION
Synthesis of data from forest inventory plots (Day et al. Reference DAY, BALDAUF, RUTISHAUSER and SUNDERLAND2014, Lewis et al. Reference LEWIS, SONKÉ, SUNDERLAND, BEGNE, VAN DER HEIJDEN, PHILLIPS, AFFUM-BAFFOE, BAKER, BANIN, BASTIN, BEECKMAN, BOECKX, BOGAERT, DE CANNIÈRE, CHEZEAUX, CLARK, COLLINS, DJAGBLETEY, DJUIKOUO, DROISSART, DOUCET, EWANGO, FAUSET, FELDPAUSCH, LLOYD, LOVETT, HART, OJO, HLADIK, MAKANA, MALHI, PEH and VERBEECK2013, Slik et al. Reference SLIK, AIBA, BREARLEY, CANNON, FORSHED, KITAYAMA, NAGAMASU, NILUS, PAYNE and PAOLI2010) and application of remote-sensing techniques (Baccini et al. Reference BACCINI, GOETZ, WALKER, LAPORTE, SUN, SULLA-MENASHE, HACKLER, BECK, DUBAYAH, FRIEDL, SAMANTA and HOUGHTON2012, Saatchi et al. Reference SAATCHI, HARRIS, BROWN, LEFSKY, MITCHARD, SALAS, ZUTTA, BUERMANN, LEWIS and HAGEN2011) have helped us better understand variation in above-ground biomass (AGB) in tropical forests. However, estimates of ABG over wide tropical regions are still fraught with uncertainty (Mitchard et al. Reference MITCHARD, FELDPAUSCH, BRIENEN, LOPEZ-GONZALEZ, MONTEAGUDO, BAKER, LEWIS, LLOYD, QUESADA, GLOOR, HANS TER STEEGE, MEIR, ALVAREZ, ARAUJO-MURAKAMI, ARAGÃO, ARROYO, GERARDO AYMARD, BANKI, BONAL, BROWN, BROWN, CERÓN, MOSCOSO, CHAVE, COMISKEY, MALHI and PHILLIPS2014), in part because environmental variation is rarely accounted for when local AGB estimates are extrapolated at larger scales. Understanding the environmental drivers of AGB variation in tropical forests, in particular the influence of altitude, would allow better estimation.
Owing to the lack of local forest inventories, the relationship between altitude and AGB in tropical montane ecosystems is poorly characterized (Houghton Reference HOUGHTON2005, Spracklen & Righelato Reference SPRACKLEN and RIGHELATO2014). The few existing studies of variation in AGB along altitudinal gradients have yielded variable results, some documenting decreases in AGB with increasing altitude (Girardin et al. Reference GIRARDIN, FARFAN-RIOS, GARCIA, FEELEY, JØRGENSEN, MURAKAMI, CAYOLA PÉREZ, SEIDEL, PANIAGUA, FUENTES CLAROS, MACÍAJ, KILLEEN and MALHI2014, Leuschner et al. Reference LEUSCHNER, MOSER, BERTSCH, RÖDERSTEIN and HERTEL2007, Moser et al. Reference MOSER, HERTEL and LEUSCHNER2007, Reference MOSER, LEUSCHNER, HERTEL, GRAEFE, SOETHE and IOST2011), others showing the opposite pattern (Alves et al. Reference ALVES, VIEIRA, SCARANELLO, CAMARGO, SANTOS, JOLY and MARTINELLI2010) and yet others finding a hump-shaped pattern across the altitudinal gradient (Ensslin et al. Reference ENSSLIN, RUTTEN, POMMER, ZIMMERMANN, HEMP and FISCHER2015) or no significant relationship between biomass and altitude (Culmsee et al. Reference CULMSEE, LEUSCHNER, MOSER and PITOPANG2010, Dossa et al. Reference DOSSA, PAUDEL, FUJINUMA, YU, CHUTIPONG, ZHANG, PAZ and HARRISON2013, Unger et al. Reference UNGER, HOMEIER and LEUSCHNER2012), despite decreased stem density and canopy height with altitude (Culmsee et al. Reference CULMSEE, LEUSCHNER, MOSER and PITOPANG2010). The relationship between ABG and altitude is thus unclear. Moreover, as these examples show, studies have mainly focused on Amazonia and South-East Asia. Except for two recent studies in East Africa (Ensslin et al. Reference ENSSLIN, RUTTEN, POMMER, ZIMMERMANN, HEMP and FISCHER2015, Marshall et al. Reference MARSHALL, WILLCOCK, PLATTS, LOVETT, BALMFORD, BURGESS, LATHAM, MUNISHI, SALTER, SHIRIMA and LEWIS2012), little is known about how ABG varies with altitude in African montane rain forests, especially those of Central Africa.
Several studies have shown that the structure of tropical forests exhibits characteristic changes along altitudinal gradients and that these affect AGB patterns (Girardin et al. Reference GIRARDIN, MALHI, ARAGAO, MAMANI, HUARACA HUASCO, DURAND, FEELEY, RAPP, SILVA-ESPEJO, SILMAN, SALINAS and WHITTAKER2010, Reference GIRARDIN, FARFAN-RIOS, GARCIA, FEELEY, JØRGENSEN, MURAKAMI, CAYOLA PÉREZ, SEIDEL, PANIAGUA, FUENTES CLAROS, MACÍAJ, KILLEEN and MALHI2014; Kitayama & Aiba Reference KITAYAMA and AIBA2002, Leuschner et al. Reference LEUSCHNER, MOSER, BERTSCH, RÖDERSTEIN and HERTEL2007, Moser et al. Reference MOSER, HERTEL and LEUSCHNER2007). Individuals of the same species, for example, might grow to different heights and occur at different densities depending on altitude. However, species composition also varies with altitude (Ashton Reference ASHTON2003, Kessler Reference KESSLER2002). If species characteristic of different altitudes differ in size or wood density, changes in AGB could simply reflect variation in floristic composition (Culmsee et al. Reference CULMSEE, LEUSCHNER, MOSER and PITOPANG2010, Girardin et al. Reference GIRARDIN, FARFAN-RIOS, GARCIA, FEELEY, JØRGENSEN, MURAKAMI, CAYOLA PÉREZ, SEIDEL, PANIAGUA, FUENTES CLAROS, MACÍAJ, KILLEEN and MALHI2014). Studies so far have not tried to estimate the influence of variation in floristic composition on AGB patterns (Culmsee et al. Reference CULMSEE, LEUSCHNER, MOSER and PITOPANG2010, Girardin et al. Reference GIRARDIN, FARFAN-RIOS, GARCIA, FEELEY, JØRGENSEN, MURAKAMI, CAYOLA PÉREZ, SEIDEL, PANIAGUA, FUENTES CLAROS, MACÍAJ, KILLEEN and MALHI2014).
The aim of this study was to understand how the AGB of old-growth forests varies across a short altitudinal gradient, and whether changes in species composition can explain this variation. For this purpose, we used a set of 15 1-ha plots established along an altitudinal gradient ranging from 236 to 900 m asl in the Ngovayang Massif, south-western Cameroon. To analyse the potential role of changes in species composition in AGB variation with altitude, we used two species traits known to affect biomass estimates: wood density and potential maximum height, i.e. the maximum height that trees of a species can attain (Baker et al. Reference BAKER, PHILLIPS, MALHI, ALMEIDA, ARROYO, DI FIORE, ERWIN, KILLEEN, LAURANCE and LAURANCE2004, Gourlet-Fleury et al. Reference GOURLET-FLEURY, ROSSI, RÉJOU-MÉCHAIN, FREYCON, FAYOLLE, SAINT-ANDRE, CORNU, GERARD, SARRAILH, FLORES, BAYA, BILLAND, FAUVET, GALLY, HENRY, HUBERT, PASQUIER and PICARD2011, Pérez-Harguindeguy et al. Reference PÉREZ-HARGUINDEGUY, DÍAZ, GARNIER, LAVOREL, POORTER, JAUREGUIBERRY, BRET-HARTE, CORNWELL, CRAINE, GURVICH, STAVER, AQUINO and CORNELISSEN2013, Slik et al. Reference SLIK, AIBA, BREARLEY, CANNON, FORSHED, KITAYAMA, NAGAMASU, NILUS, PAYNE and PAOLI2010). These two traits were chosen for this analysis for three reasons: (1) they are important functional traits that directly influence biomass (Westoby et al. Reference WESTOBY, FALSTER, MOLES, VESK and WRIGHT2002); (2) they vary considerably both within and among species or plant communities, making them appealing candidates for such analyses (Pérez-Harguindeguy et al. Reference PÉREZ-HARGUINDEGUY, DÍAZ, GARNIER, LAVOREL, POORTER, JAUREGUIBERRY, BRET-HARTE, CORNWELL, CRAINE, GURVICH, STAVER, AQUINO and CORNELISSEN2013, Schamp & Aarssen Reference SCHAMP and AARSSEN2009); (3) data on these traits are widely available for many species in published floras and other databases. We hypothesized that AGB decreases with increasing altitude and that changes in species composition contribute to that pattern.
METHODS
Study area
The Ngovayang Massif is a range of hills located in south-western Cameroon, 80 km east of the Atlantic coast. The massif covers an area of 102000 ha and extends between 3°12′–3°25′N and 10°30′–10°45′E (Figure 1). Altitude varies from 50 m asl in the western part to more than 1000 m asl in the eastern part, on the summits of the main hills. Climate is sub-equatorial, and average annual rainfall is 2000 mm (Waterloo et al. Reference WATERLOO, NTONGA, DOLMAN and AYANGMA2000). Mean annual temperature is 25°C at low altitude (50 m asl), decreasing with increasing altitude to below 18°C at 1000 m (Olivry Reference OLIVRY1986). The main vegetation type is dense evergreen rain forest, with many caesalpinioid legumes in lowland areas (< 600 m asl) and a decrease in their importance with altitude (Gonmadje Reference GONMADJE2012, Gonmadje et al. Reference GONMADJE, DOUMENGE, MCKEY, TCHOUTO, SUNDERLAND, BALINGA and SONKÉ2011, Letouzey Reference LETOUZEY1985). For more details about the area see Gonmadje et al. (Reference GONMADJE, DOUMENGE, MCKEY, TCHOUTO, SUNDERLAND, BALINGA and SONKÉ2011, Reference GONMADJE, DOUMENGE, SUNDERLAND, BALINGA and SONKÉ2012).

Figure 1. Location of the 15 1-ha permanent plots established at altitudinal intervals of about 200 m, from 236 m to 900 m in old-growth terra firme rain forest of the Ngovayang Massif (Cameroon). Within each plot, all trees with a diameter at breast height (dbh) ≥ 10 cm were recorded. The studied sites are indicated by black triangles.
Data collection
Fifteen 1-ha (100 × 100 m) permanent plots were established at altitudinal intervals of about 200 m, from 236 m to 900 m (Figure 1). The plots were chosen to cover old-growth terra firme rain forest. Inventories were carried out from February 2008 to April 2010. Data on altitude were collected with a GPS apparatus (Garmin CsX 60).
Within each plot, all trees with a diameter at breast height (dbh) ≥ 10 cm were recorded. The dbh was measured with a diameter tape at 1.3 m above ground level, avoiding any protrusion on the trunk or lianas growing around it. For buttressed trees, the dbh was measured 30 cm above the buttresses. All trees were permanently marked and labelled with numbered aluminium tags. Details on protocols and measurement methods are described in the RAINFOR field manual (http://www.geog.leeds.ac.uk/projects/rainfor/pages/manuals_eng.html).
For height measurements, 10 trees were randomly selected within each of four diameter classes (10–20, 20–30, 30–50, > 50 cm), and their top height measured using a Nikon Forestry Pro laser rangefinder/hypsometer.
Identification of the most common species was undertaken directly in the field whenever possible. Herbarium specimens of most species and morphospecies were collected, identified and preserved at the National Herbarium of Cameroon (YA). Specifically, 74% of morphospecies were identified to species level, 22% to genus level, 3% to family level and 1% remained undetermined.
For families, we used the classification of the Angiosperm Phylogeny Group (APG III; Bremer et al. Reference BREMER, BREMER, CHASE, FAY, REVEAL, BAILEY, SOLTIS, SOLTIS and STEVENS2009). However, given the specificities of each of the subfamilies of Fabaceae sensu APG III (Caesalpinioideae, Mimosoideae, Faboideae), their ecological importance, particularly that of Caesalpinioideae in the forests of Central Africa (Doucet Reference DOUCET2003, Gonmadje Reference GONMADJE2012, Letouzey Reference LETOUZEY1985), and also for purposes of statistical comparison with previous studies, we considered each of the three subfamilies as a ‘family equivalent level’.
Estimation of above-ground biomass
The choice of the allometric regression model for converting tree structural data into AGB estimates is one of the most important sources of uncertainty in estimating carbon stocks in tropical forests (Molto et al. Reference MOLTO, ROSSI and BLANC2013). As the climate of the study area is tropical, we calculated the above-ground biomass (AGB) of living trees per plot by using the allometric model proposed by Chave et al. (Reference CHAVE, RÉJOU-MÉCHAIN, BÚRQUEZ, CHIDUMAYO, COLGAN, DELITTI, DUQUE, EID, FEARNSIDE, GOODMAN, HENRY, MARTÍNEZ-YRÍZAR, MULLER-LANDAU, PÉLISSIER, PLOTON, SALDARRIAGA and VIEILLEDENT2014) for tropical forests, which is based on dbh, wood density and height:
where AGB is estimated above-ground biomass (kg), D is trunk diameter (cm), H is total tree height (m), and WSG is oven-dry wood specific gravity (g cm−3).
This equation, including data on height, is considered to be valid across sites and climates because it captures potential variation in height-diameter relationship across sites. Therefore stand-specific height–diameter regression models were developed. All trees known to be broken or damaged were excluded from the analysis. We constructed plot-specific height–diameter regression models using Weibull functions of the form:
where ap , bp and cp are plot-specific parameters (ap represents the asymptotic height of trees in plot p) and where H (m) and dbh (cm) represent the height and the diameter of trees within plot p, respectively. Weibull equations have been shown to be well-suited to model height–diameter relationships in tropical trees (Feldpausch et al. Reference FELDPAUSCH, LLOYD, LEWIS, BRIENEN, GLOOR, MONTEAGUDO MENDOZA, LOPEZ-GONZALEZ, BANIN, ABU SALIM, AFFUM-BAFFOE, VÁSQUEZ, VILANOVA, WHITE, WILLCOCK, WOELL and PHILLIPS2012).
The wood specific gravity values used in this study were obtained from the global wood density database (http://datadryad.org/handle/10255/dryad.235; Chave et al. Reference CHAVE, COOMES, JANSEN, LEWIS, SWENSON and ZANNE2009, Zanne et al. Reference ZANNE, WESTOBY, FALSTER, ACKERLY, LOARIE, ARNOLD and COOMES2010). When no wood density data were available for a species, we used the genus-level or family-level average to estimate the species value. When an individual tree could not be identified at least to family level or when no data were available at the family level, we assigned the plot average to those trees.
Stand-weighted mean traits
Because biomass is estimated using a model and is not directly measured, plot-level biomass can be approximately considered as the product of stand-level-weighted mean wood density times basal area times mean height. Basal area and height are measured in the field and their change corresponds to a change in forest structure, whether it results from a change in species composition, or whether it is a change of structure independent of species composition (i.e. tree species do not change but individual tree sizes change). Focusing on tree height variation within a given species would allow us to assess the latter source of biomass variation. However, most of the species were absent from several plots, and among the few species whose abundance was quite balanced across plots, there were not enough tree height measurements to test for tree height changes with altitude. In contrast to actual height, wood density and potential maximum height are relatively invariant species traits (King et al. Reference KING, DAVIES, TAN and NOOR2006, sensu Violle et al. Reference VIOLLE, NAVAS, VILE, KAZAKOU, FORTUNEL, HUMMEL and GARNIER2007). A change in one or both of these traits with altitude would indicate an effect of floristic composition on AGB variation.
We estimated the mean wood specific gravity of each plot (WSGp), as the sum of the wood specific gravity for each tree species present in a plot weighted by the basal area (BA) of the species in the plot:
where ρs is the wood specific gravity of a species s and BAs is the cumulated basal area of all trees belonging to species s in plot p.
Potential maximum height (Hmax
) for each species was obtained from the Cofortrait database (unpublished dataset), which provides trait information on Central African trees based on a compilation of local and regional floras. When information on these traits was missing, we tried as far as possible to find the information in additional floras or databases, such as Jstor and Prota 4U. We were unable to find any relevant information for 269 species out of a total of 583 species. However, these species without information represented less than 12% of individuals in all plots. We then calculated the mean potential maximum height of each plot (Hpmax
) as:
![]() ${{H}\!{p}_{max}} = \ \frac{1}{n}\sum {H_{max}}$
where n is the number of tree individuals in the plot p, the sum is over the trees in plot p and Hmax
is the potential maximum height of the species of each tree.
${{H}\!{p}_{max}} = \ \frac{1}{n}\sum {H_{max}}$
where n is the number of tree individuals in the plot p, the sum is over the trees in plot p and Hmax
is the potential maximum height of the species of each tree.
Details on mean potential maximum height and other stand structural characteristics for each plot are given in Appendix 1.
Statistical analyses
To clarify the contribution of changes in floristic composition to change in biomass with altitude, we investigated the relationship between altitude and above-ground biomass using a simple regression analysis between AGB as dependent variable and altitude as predictor variable. We then performed a principal components analysis (PCA) on altitude, above-ground biomass and its major components: the structural variables (tree density, asymptotic height, i.e. coefficient ap of the height-diameter equation, and basal area) and the functional traits (mean wood density and mean potential maximum height). The use of the mean potential maximum height in this analysis allowed us to disentangle the co-varying effects of floristic composition and tree sizes in AGB variation. Prior to the PCA, we first checked whether the relationship between altitude and mean potential maximum height was linear.
We also assessed the contribution of trees of different sizes to above-ground biomass along the altitudinal gradient. We defined three diameter size classes as follows: a lower stratum with small trees (10 ≤ dbh < 30 cm), a middle stratum with large trees, most of which reach the canopy (30 cm ≤ dbh < 70 cm), and the upper stratum corresponding to the largest trees, which were either in the canopy or emergent, with dbh ≥ 70 cm (Paoli et al. Reference PAOLI, CURRAN and SLIK2008, Slik et al. Reference SLIK, AIBA, BREARLEY, CANNON, FORSHED, KITAYAMA, NAGAMASU, NILUS, PAYNE and PAOLI2010). This allowed us to quantify the contributions of each diameter size class to the biomass variation, and to determine whether the biomass pattern was related to the occurrence of large trees along the altitudinal gradient.
Finally, we used a canonical correspondence analysis (CCA) to directly assess the floristic variation along the altitudinal gradient. The CCA was performed on the site × species matrix giving the basal area (rather than the abundance) of each species at each site, and on the column-vector giving the altitudes of the sites. To quantify variation in floristic composition that may explain AGB variation, basal area was preferred to abundance. Because we only used one explanatory variable in the CCA (i.e. the altitude), the CCA resulted in only one axis, but summarizing in the best possible way the floristic gradient with altitude.
At the family level, variation in the dominance of families along the altitudinal gradient was also analysed by using their basal area.
All statistical analyses were performed with R version 2.15 (http://cran.r-project.org).
RESULTS
Changes in floristic composition as the main driver of AGB variation along an altitudinal gradient
Among the 15 plots, total above-ground biomass varied more than two-fold, between 257 and 665 Mg ha−1, with an average value of 415 ± 140 Mg ha−1. We found a significant decline in above-ground biomass with increasing altitude in the Ngovayang Massif (R2 = −0.56, P = 0.001; Figure 2a). Stem density and basal area also varied greatly with altitude, despite the limited extent and altitudinal range of the massif.

Figure 2. Linear regressions showing the significant effect of altitude respectively on above-ground biomass (a) and potential maximum height i.e. the plot-level average of the maximum height that trees of a species can attain (b) of the 15 1-ha study plots in the Ngovayang Massif (Cameroon).
The PCA analysis of AGB, altitude, floristic composition and stand structural factors revealed two main axes, which explained 75% of the total variance (Figure 3). Axis 1 (55% of total variance) illustrated a strong negative correlation between altitude and AGB (Pearson; R = −0.77, P = 0.0007), and between altitude and basal area (R = −0.77, P = 0.002). Logically, it also illustrated the strong positive correlation between basal area and AGB. Asymptotic height also decreased significantly with increasing altitude (R = −0.7, P = 0.003). In contrast, there was a positive correlation between stem density and altitude (R = 0.57, P = 0.02). Although AGB tended to decrease with increasing stem density, this negative correlation was not significant (R = −0.48, P = 0.08).

Figure 3. Correlation circle of the principal components analysis (PCA; first two axes) of seven variables characterizing 15 1-ha study plots established in the Ngovayang Massif (Cameroon). Variables are altitude (Alt), above-ground biomass (AGB), descriptors of the floristic composition (Hpmax: potential maximum height, i.e. the plot-level average of the maximum height that trees of a species can attain; WSG: mean wood specific gravity), and descriptors of the stand structure (N: stem density; BA: basal area; Height: asymptotic height, i.e. the asymptote coefficient of the height-diameter model fitted to tree data from each plot).
Noticeably, there was no altitudinal trend in mean WSG (R = −0.04, P = 0.8), and the plot of the two variables showed no linear relationship. There was also no significant correlation between mean WSG and AGB (R = 0.2, P = 0.4). However, the potential maximum height (and therefore the abundance of the large-tree species) decreased significantly with increasing altitude (R = −0.57, P = 0.024; Figure 2b).
Contribution of variation in tree size to AGB variation
For all plots taken together, the majority of AGB (76%) was contributed by large stems (≥ 30 cm dbh) in spite of their low abundance (19.5%). Stems ≥ 70 cm dbh encompassed 35% of total AGB but only 2.5% of stems measured, while stems 10–30 cm dbh encompassed only 24% of total AGB but 80.5% of the stems measured.
The biomass distribution by diameter class showed that the biomass stored in each of the two lower strata of trees (10–30 cm and 30–70 cm dbh) was almost constant across the altitudinal gradient (Figure 4). In contrast, the contribution of the large-tree (dbh ≥ 70 cm) stratum to the total biomass decreased with altitude. Indeed, in this stratum, the biomass accumulation was higher in lowland and mid-altitude forests (50% of the total biomass) than in submontane forests (22%). Thus, the proportion of biomass stored in large trees in low- and mid-altitude forests was more than twice that in submontane forests.

Figure 4. Contribution of the three different tree diameter classes (10–30 cm, 30–70 cm, ≥ 70 cm) to the total AGB along the altitudinal gradient in the Ngovayang Massif (Cameroon). The biomass distribution by diameter class from 15 1-ha study plots shows that the biomass stored in each of the two lower strata of trees (10–30 cm and 30–70 cm dbh, red and blue dashed lines respectively) was almost constant across the altitudinal gradient. In contrast, the contribution of the large-tree (dbh ≥ 70 cm, black line) stratum to the total biomass decreased with altitude.
Floristic variation along the altitudinal gradient
The CCA showed that altitude explained 8.4% of the total BA-based variation in floristic composition (Figure 5). The species score along the altitudinal axis was significantly correlated with the potential maximum height (Pearson's R = −0.2, P = 0.0006) but not correlated with the species’ wood density (R = 0.02, P = 0.7), confirming the previous results. This floristic gradient clearly illustrates that large-stature species, such as Pycnanthus angolensis, Tetraberlinia bifoliolata, Guibourtia tessmannii and Staudtia kamerunensis tend to contribute more to the total basal area of the lowland forests than to the higher-altitude forests.

Figure 5. Floristic variation along the altitudinal gradient. The canonical correspondence analysis (CCA) showed that altitude explains 8.4% of the total basal area-based variation in floristic composition. The x axis represents the score of the species along the first axis of a CCA performed on the site × species matrix of basal areas and on the vector column of the altitudes of the sites. This axis thus summarizes in the best possible way the altitudinal gradient, with negative and positive scores representing low and high altitude respectively. The y axis represents the potential maximum height of the species. Size of the points is proportional to the total basal area of the species in the study area. The dotted line represents the result of a standard major axis regression. The species scores along the altitudinal axis are significantly correlated with the potential maximum height (R = −0.2, P = 0.0006). Species having the 2% highest contribution to basal area are shown: Staukame: Staudtia kamerunensis, Guibtess: Guibourtia tessmannii, Trecobov: Treculia obovoidea, Pycnango: Pycnanthus angolensis, Tetrbifo: Tetraberlinia bifoliolata, Coelpreu: Coelocaryon preussi, Santtrim: Santiria trimera, Couledul: Coula edulis, Plagafri: Plagiostyles africana, Allaflor: Allanblackia floribunda, Scypmann: Scyphocephalium mannii, Braccyno: Brachystegia cynometroides.
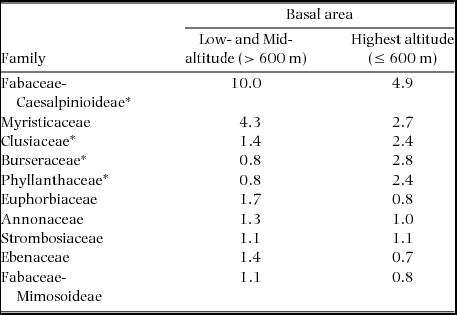
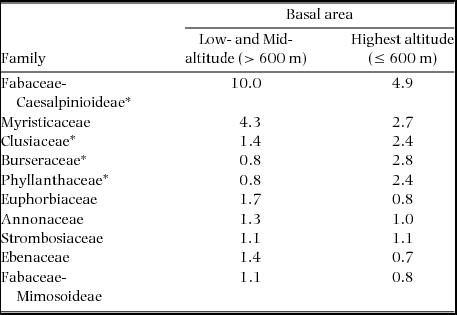
Furthermore, at family level, there was a change in floristic composition and dominance patterns along the altitudinal gradient. Among the 10 most dominant families in terms of basal area, there was a decrease of Fabaceae subfamily Caesalpinioideae, while Clusiaceae, Phyllanthaceae and Burseraceae, all characteristic of submontane forests, increased (Table 1).
Table 1. Altitudinal trends of the 10 top dominant families in terms of basal area in the Ngovayang Massif. Families whose dominance was significantly affected by altitude are indicated with an asterisk. The Fabaceae subfamily Caesalpinioideae are characteristic of low- and mid-altitude forest, while Clusiaceae, Phyllanthaceae and Burseraceae, are characteristic of submontane forests.
DISCUSSION
Our study provides two important advances to understanding spatial patterns of AGB in an old-growth tropical submontane forest of Atlantic Central Africa. First, we present clear evidence of contrasting patterns of AGB along a short altitudinal gradient. Second, we underline the contribution of floristic composition to this variation in AGB.
Altitudinal filtering of large-tree species as a driver of variation in AGB
The mean AGB value of Ngovayang's forests (415 Mg ha−1) is close to the mean AGB estimates observed for African tropical forests and Central African lowland moist forests, 404 and 429 Mg ha−1 respectively (Lewis et al. Reference LEWIS, LOPEZ-GONZALEZ, SONKÉ, AFFUM-BAFFOE, BAKER, OJO, PHILLIPS, REITSMA, WHITE, COMISKEY, DJUIKOUO, EWANGO, FELDPAUSCH, HAMILTON, GLOOR, HART, HLADIK, LLOYD, LOVETT, MAKANA, MALHI, MBAGO, HENRY, NDANGALASI, PEACOCK, PEH, SHEIL, SUNDERLAND, SWAINE, TAPLIN, TAYLOR, THOMAS, VOTERE and WÖLL2009, Reference LEWIS, SONKÉ, SUNDERLAND, BEGNE, VAN DER HEIJDEN, PHILLIPS, AFFUM-BAFFOE, BAKER, BANIN, BASTIN, BEECKMAN, BOECKX, BOGAERT, DE CANNIÈRE, CHEZEAUX, CLARK, COLLINS, DJAGBLETEY, DJUIKOUO, DROISSART, DOUCET, EWANGO, FAUSET, FELDPAUSCH, LLOYD, LOVETT, HART, OJO, HLADIK, MAKANA, MALHI, PEH and VERBEECK2013; Slik et al. Reference SLIK, PAOLI, MCGUIRE, AMARAL, BARROSO, BASTIAN, BLANC, BONGERS, BOUNDJA, CLARK, COLLINS, DAUBY, DING, DOUCET, ELER, FERREIRA, FORSHED, FREDRIKSSON, GILLET, HARRIS, LEAL, LAUMONIER, MALHI, MANSOR, MARTIN, KAZUKI MIYAMOTO, NAGAMASU, POORTER, POULSEN, REITSMA, SHEIL, TER STEEGE, SUNDERLAND, SUZUKI, WICH, WÖLL, YONE DA, ZANG, ZHANG and ZWEIFEL2013). The AGB of forests on Ngovayang Massif was significantly lower at higher altitudes than in the lowlands. The finding of decreased AGB with increasing altitude is supported by previous studies conducted in other areas (Girardin et al. Reference GIRARDIN, MALHI, ARAGAO, MAMANI, HUARACA HUASCO, DURAND, FEELEY, RAPP, SILVA-ESPEJO, SILMAN, SALINAS and WHITTAKER2010, Reference GIRARDIN, FARFAN-RIOS, GARCIA, FEELEY, JØRGENSEN, MURAKAMI, CAYOLA PÉREZ, SEIDEL, PANIAGUA, FUENTES CLAROS, MACÍAJ, KILLEEN and MALHI2014; Kitayama & Aiba Reference KITAYAMA and AIBA2002, Leuschner et al. Reference LEUSCHNER, MOSER, BERTSCH, RÖDERSTEIN and HERTEL2007, Moser et al. Reference MOSER, HERTEL and LEUSCHNER2007). In the cited studies, soil nutrient supply became progressively more limiting relative to plant demands at higher, cooler sites, thereby limiting forest productivity and biomass accumulation. In general, slower mineralization of dead organic matter and decreasing availability of nutrients (due to lower decomposition in cooler soils at higher altitude), especially nitrogen, has been suggested to limit tree height and biomass stocks with increasing altitude (Girardin et al. Reference GIRARDIN, FARFAN-RIOS, GARCIA, FEELEY, JØRGENSEN, MURAKAMI, CAYOLA PÉREZ, SEIDEL, PANIAGUA, FUENTES CLAROS, MACÍAJ, KILLEEN and MALHI2014, Tanner et al. Reference TANNER, VITOUSEK and CUEVAS1998). This could simultaneously result in greater carbon storage in the soil at higher altitudes (Girardin et al. Reference GIRARDIN, MALHI, ARAGAO, MAMANI, HUARACA HUASCO, DURAND, FEELEY, RAPP, SILVA-ESPEJO, SILMAN, SALINAS and WHITTAKER2010). However, some authors in other tropical forests have found the opposite pattern, i.e. increasing density of large trees and higher AGB with altitude (Alves et al. Reference ALVES, VIEIRA, SCARANELLO, CAMARGO, SANTOS, JOLY and MARTINELLI2010, Marshall et al. Reference MARSHALL, WILLCOCK, PLATTS, LOVETT, BALMFORD, BURGESS, LATHAM, MUNISHI, SALTER, SHIRIMA and LEWIS2012, Rai & Proctor Reference RAI and PROCTOR1986). Indeed, in these studies, local topographic factors control the abundance of large trees. Yet other studies found that AGB remained invariant along altitudinal gradients (Culmsee et al. Reference CULMSEE, LEUSCHNER, MOSER and PITOPANG2010, Unger et al. Reference UNGER, HOMEIER and LEUSCHNER2012), despite large changes in tree size and species composition (Culmsee et al. Reference CULMSEE, LEUSCHNER, MOSER and PITOPANG2010).
Two species traits, potential maximum height and wood specific gravity, were selected in our study to assess whether or not the AGB pattern was related to changes in floristic composition along the altitudinal gradient. Wood density in Ngovayang showed no trend with altitude. This finding is consistent with some previous findings in Amazonia (Moser et al. Reference MOSER, RÖDERSTEIN, SOETHE, HERTEL, LEUSCHNER, Beck, Bendix, Kottke, Makeschin and Mosandl2008). In contrast, other authors found a positive correlation, due to the increasing dominance of species with dense wood at higher altitude in South-East Asian forests (Culmsee et al. Reference CULMSEE, LEUSCHNER, MOSER and PITOPANG2010, Slik et al. Reference SLIK, AIBA, BREARLEY, CANNON, FORSHED, KITAYAMA, NAGAMASU, NILUS, PAYNE and PAOLI2010). Patterns in community-wide wood density have mainly been linked to forest disturbance regimes and productivity (Baker et al. Reference BAKER, PHILLIPS, MALHI, ALMEIDA, ARROYO, DI FIORE, ERWIN, KILLEEN, LAURANCE and LAURANCE2004, Malhi et al. Reference MALHI, BAKER, PHILLIPS, ALMEIDA, ALVAREZ, ARROYO, CHAVE, CZIMCZIK, FIORE, HIGUCHI, LEZAMA, MARTÍNEZ, TERBORGH, VINCETI and LLOYD2004, Réjou-Méchain et al. Reference RÉJOU-MÉCHAIN, FLORES, PÉLISSIER, FAYOLLE, FAUVET and GOURLET-FLEURY2014, Slik et al. Reference SLIK, BERNARD, BREMAN, VAN BEEK, SALIM and SHEIL2008), but also to annual rainfall gradients, rainfall seasonality and droughts (Baker et al. Reference BAKER, PHILLIPS, MALHI, ALMEIDA, ARROYO, DI FIORE, ERWIN, KILLEEN, LAURANCE and LAURANCE2004, Slik Reference SLIK2004, Van Nieuwstadt & Sheil Reference VAN NIEUWSTADT and SHEIL2005). In the Ngovayang Massif, all plots were established in old-growth forest. Furthermore, there was no significant gradient in either annual rainfall or rainfall seasonality along the altitudinal gradient. This might explain why wood specific gravity remained constant along the altitudinal gradient.
However, our study showed a decrease of Hpmax with altitude, which reflected a direct influence of changes in species composition on biomass variation. This finding underlines our contention that changes in AGB along the altitudinal gradient were at least partly due to changes in floristic composition. Thus, our results indicate that species with large stature are filtered out at higher altitude, contributing to the decline in biomass along the altitudinal gradient. Indeed, the lowland Atlantic forests of the Ngovayang Massif, rich in Fabaceae-Caesalpinioideae, are characterized by a high canopy and by the abundance of individuals of large diameter. Species of this family decline in frequency at higher altitude. At higher altitude, families such as Burseraceae, Clusiaceae and Phyllanthaceae, characteristic of submontane forests, become more dominant. Potential maximum height of species of these families is much lower. These submontane forests are thus characterized by a lower canopy and a greater representation of individuals of small diameter (dbh between 10–30 cm) (Gonmadje Reference GONMADJE2012, Letouzey Reference LETOUZEY1985). Thus, changes in abundance of large trees along the altitudinal gradient were also attributable to changes in floristic composition. This finding is consistent with those of some previous studies in the tropics (Culmsee et al. Reference CULMSEE, LEUSCHNER, MOSER and PITOPANG2010, Girardin et al. Reference GIRARDIN, FARFAN-RIOS, GARCIA, FEELEY, JØRGENSEN, MURAKAMI, CAYOLA PÉREZ, SEIDEL, PANIAGUA, FUENTES CLAROS, MACÍAJ, KILLEEN and MALHI2014). Therefore, our study highlighted the contribution of a shift in species composition through the potential maximum height of species to AGB variation along the altitudinal gradient.
Moreover, we showed the consistently high contribution of large trees (≥70 cm dbh) to biomass in the Ngovayang Massif and their major role in the altitudinal AGB gradient. This corroborates results obtained in other tropical forests, where individual trees ≥70 cm in diameter are usually reported to contribute more than 30% of the AGB (Bastin et al. Reference BASTIN, BARBIER, RÉJOU-MÉCHAIN, FAYOLLE, GOURLET-FLEURY, MANIATIS, DE HAULLEVILLE, BAYA, BEECKMAN, BEINA, COUTERON, CHUYONG, DAUBY, DOUCET, DROISSART, DUFRÊNE, EWANGO, GILLET, GONMADJE, HART, KAVALI, KENFACK, LIBALAH, MALHI, MAKANA, PÉLISSIER, DE CANNIÈRE and BOGAERT2015, Paoli et al. Reference PAOLI, CURRAN and SLIK2008, Sist et al. Reference SIST, MAZZEI, BLANC and RUTISHAUSER2014, Slik et al. Reference SLIK, AIBA, BREARLEY, CANNON, FORSHED, KITAYAMA, NAGAMASU, NILUS, PAYNE and PAOLI2010, Reference SLIK, PAOLI, MCGUIRE, AMARAL, BARROSO, BASTIAN, BLANC, BONGERS, BOUNDJA, CLARK, COLLINS, DAUBY, DING, DOUCET, ELER, FERREIRA, FORSHED, FREDRIKSSON, GILLET, HARRIS, LEAL, LAUMONIER, MALHI, MANSOR, MARTIN, KAZUKI MIYAMOTO, NAGAMASU, POORTER, POULSEN, REITSMA, SHEIL, TER STEEGE, SUNDERLAND, SUZUKI, WICH, WÖLL, YONE DA, ZANG, ZHANG and ZWEIFEL2013). Large trees store large quantities of carbon due to their high wood volumes (Slik et al. Reference SLIK, PAOLI, MCGUIRE, AMARAL, BARROSO, BASTIAN, BLANC, BONGERS, BOUNDJA, CLARK, COLLINS, DAUBY, DING, DOUCET, ELER, FERREIRA, FORSHED, FREDRIKSSON, GILLET, HARRIS, LEAL, LAUMONIER, MALHI, MANSOR, MARTIN, KAZUKI MIYAMOTO, NAGAMASU, POORTER, POULSEN, REITSMA, SHEIL, TER STEEGE, SUNDERLAND, SUZUKI, WICH, WÖLL, YONE DA, ZANG, ZHANG and ZWEIFEL2013). Our data suggest that variation in tree biomass along the altitudinal gradient is closely linked to variation in the abundance of large trees. Such trees could thus be responsible for a large portion of local variation in AGB (Slik et al. Reference SLIK, PAOLI, MCGUIRE, AMARAL, BARROSO, BASTIAN, BLANC, BONGERS, BOUNDJA, CLARK, COLLINS, DAUBY, DING, DOUCET, ELER, FERREIRA, FORSHED, FREDRIKSSON, GILLET, HARRIS, LEAL, LAUMONIER, MALHI, MANSOR, MARTIN, KAZUKI MIYAMOTO, NAGAMASU, POORTER, POULSEN, REITSMA, SHEIL, TER STEEGE, SUNDERLAND, SUZUKI, WICH, WÖLL, YONE DA, ZANG, ZHANG and ZWEIFEL2013). Moreover, there was a negative relationship between altitude and both asymptotic height and basal area, and this could explain the decrease of AGB along the altitudinal gradient. Thus, one of the key factors responsible for the altitudinal change in AGB in the Ngovayang forest is a substantial decrease with altitude in the contribution of large stems to the total AGB. However, the variation in the abundance of these large-tree species was strongly associated with changes in floristic composition along the altitudinal gradient, thus indicating a strong role of altitudinal filtering in the AGB gradient.
Forest AGB in relation to altitudinal trends in tree sizes
We showed that along the short altitudinal gradient of the Ngovayang Massif, there was a continuous decrease with altitude of the asymptotic height, while stem density increased. Our observations are consistent with previous results found across the tropics (Culmsee et al. Reference CULMSEE, LEUSCHNER, MOSER and PITOPANG2010, Girardin et al. Reference GIRARDIN, FARFAN-RIOS, GARCIA, FEELEY, JØRGENSEN, MURAKAMI, CAYOLA PÉREZ, SEIDEL, PANIAGUA, FUENTES CLAROS, MACÍAJ, KILLEEN and MALHI2014, Slik et al. Reference SLIK, AIBA, BREARLEY, CANNON, FORSHED, KITAYAMA, NAGAMASU, NILUS, PAYNE and PAOLI2010, Takyu et al. Reference TAKYU, KUBOTA, AIBA, SEINO and NISHIMURA2005). These previous studies interpreted these trends as a consequence of cooler temperatures, fog, reduced light incidence, higher relative humidity and lower energy inputs at higher elevations. Winds are also stronger and more frequent on the summits of the Ngovayang Massif. Strong winds partly explain the short stature of the canopy of tropical montane forests (Lieberman et al. Reference LIEBERMAN, LIEBERMAN, PERALTA and HARTSHORN1996). Published studies on the below-ground carbon allocation demonstrated that the decrease of tree height and therefore the decrease of AGB along the altitudinal gradient are related to a progressive shift in carbon allocation from above-ground to below-ground tree organs. The decrease in AGB thus does not necessarily translate into a loss of total ecosystem biomass (Girardin et al. Reference GIRARDIN, MALHI, ARAGAO, MAMANI, HUARACA HUASCO, DURAND, FEELEY, RAPP, SILVA-ESPEJO, SILMAN, SALINAS and WHITTAKER2010, Reference GIRARDIN, FARFAN-RIOS, GARCIA, FEELEY, JØRGENSEN, MURAKAMI, CAYOLA PÉREZ, SEIDEL, PANIAGUA, FUENTES CLAROS, MACÍAJ, KILLEEN and MALHI2014; Leuschner et al. Reference LEUSCHNER, MOSER, BERTSCH, RÖDERSTEIN and HERTEL2007, Moser et al. Reference MOSER, RÖDERSTEIN, SOETHE, HERTEL, LEUSCHNER, Beck, Bendix, Kottke, Makeschin and Mosandl2008).
As in most other studies (Baraloto et al. Reference BARALOTO, RABAUD, MOLTO, BLANC, FORTUNEL, HERAULT, DAVILA, MESONES, RIOS and VALDERRAMA2011, Slik et al. Reference SLIK, AIBA, BREARLEY, CANNON, FORSHED, KITAYAMA, NAGAMASU, NILUS, PAYNE and PAOLI2010), basal area in the Ngovayang Massif decreased with increasing altitude, and this was associated with a decrease in density of large trees. However, other studies in the tropics found that basal area increased with altitude (Culmsee et al. Reference CULMSEE, LEUSCHNER, MOSER and PITOPANG2010) or remained constant (Girardin et al. Reference GIRARDIN, FARFAN-RIOS, GARCIA, FEELEY, JØRGENSEN, MURAKAMI, CAYOLA PÉREZ, SEIDEL, PANIAGUA, FUENTES CLAROS, MACÍAJ, KILLEEN and MALHI2014). The positive relationship that Culmsee et al. (Reference CULMSEE, LEUSCHNER, MOSER and PITOPANG2010) observed between basal area and altitude was mostly due to a relatively high density of large trees of the Fagaceae, which was dominant at higher altitude in the Indonesian forests that they studied. Therefore, it appears that the variation in AGB variation along the altitudinal gradient is strongly related to the abundance of large trees. Thus, one of the key factors for understanding the altitudinal change in AGB is knowledge of the patterns of variation in the abundance of large-tree species, as we saw above. This result is not surprising because diameter and height are used to estimate tree AGB, as illustrated by the strong linear relation between AGB and basal area, and between AGB and asymptotic height.
However, altitude does not directly control variation in above-ground biomass (Körner Reference KÖRNER1998). Indeed, any significant relationship observed between forest ecosystem characteristics and altitude could be due to variation in the pool of species available regionally to colonize highland habitats (Culmsee et al. Reference CULMSEE, LEUSCHNER, MOSER and PITOPANG2010), and also to variation in many environmental conditions along the altitudinal gradient, including temperature, humidity, rainfall, cloud cover, incoming solar radiation, wind speed and nutrient availability (de Castilho et al. Reference DE CASTILHO, MAGNUSSON, DE ARAÚJO, LUIZAO, LUIZAO, LIMA and HIGUCHI2006, Fisher et al. Reference FISHER, MALHI, TORRES, METCALFE, VAN DE WEG, MEIR, SILVA-ESPEJO and HUASCO2013, Girardin et al. Reference GIRARDIN, FARFAN-RIOS, GARCIA, FEELEY, JØRGENSEN, MURAKAMI, CAYOLA PÉREZ, SEIDEL, PANIAGUA, FUENTES CLAROS, MACÍAJ, KILLEEN and MALHI2014, Körner Reference KÖRNER1998, Takyu et al. Reference TAKYU, KUBOTA, AIBA, SEINO and NISHIMURA2005, Unger et al. Reference UNGER, HOMEIER and LEUSCHNER2012).
Diminution of tree size with increasing altitude can have two sources. We examined one of these, the filtering-out with increasing altitude of tree species capable of attaining large size. However, the same environmental factors ultimately responsible for changes in species composition can also have direct effects on the growth, the mortality, or both, of individual trees. Our study did not address the contribution of this second source of variation, diminution with increasing altitude in the size actually attained by individuals of a given tree species. Thus, while we have demonstrated an effect of variation in species composition on AGB variation, its importance relative to intraspecific variation in tree size over the gradient must still be determined.
CONCLUSION
Our study has strong implications for understanding the factors explaining variation in AGB over a short altitudinal gradient (1000 m) in the Ngovayang Massif. We analysed the relative influence of floristic composition and tree sizes in AGB variation, by using species traits, especially the potential maximum height. Indeed, AGB variation was due to a large degree to shift in species composition, especially the filtering out of large-tree species along the altitudinal gradient in the Ngovayang Massif. Our results also demonstrate that the contribution of the large trees (dbh ≥ 70 cm) stratum to the total biomass decreased with altitude. Thus, variation in tree biomass along the altitudinal gradient was strongly related to the variation in the abundance of large trees. This variation over the gradient in species composition in turn leads to changes in forest structure. Any impacts on large trees, either by global change or other disturbances that affect their abundance and persistence, are therefore likely to have a major impact on forest AGB (Slik et al. Reference SLIK, PAOLI, MCGUIRE, AMARAL, BARROSO, BASTIAN, BLANC, BONGERS, BOUNDJA, CLARK, COLLINS, DAUBY, DING, DOUCET, ELER, FERREIRA, FORSHED, FREDRIKSSON, GILLET, HARRIS, LEAL, LAUMONIER, MALHI, MANSOR, MARTIN, KAZUKI MIYAMOTO, NAGAMASU, POORTER, POULSEN, REITSMA, SHEIL, TER STEEGE, SUNDERLAND, SUZUKI, WICH, WÖLL, YONE DA, ZANG, ZHANG and ZWEIFEL2013). This finding stresses the importance of including shifts in species composition as explanations of AGB variation along altitudinal gradients in tropical forests (Culmsee et al. Reference CULMSEE, LEUSCHNER, MOSER and PITOPANG2010, Slik et al. Reference SLIK, AIBA, BREARLEY, CANNON, FORSHED, KITAYAMA, NAGAMASU, NILUS, PAYNE and PAOLI2010).
In terms of carbon storage, what is pertinent is overall carbon storage in the ecosystem. Thus, a key question is to know whether the greater storage of carbon in soils at higher altitudes (Girardin et al. Reference GIRARDIN, MALHI, ARAGAO, MAMANI, HUARACA HUASCO, DURAND, FEELEY, RAPP, SILVA-ESPEJO, SILMAN, SALINAS and WHITTAKER2010, Leuschner et al. Reference LEUSCHNER, MOSER, BERTSCH, RÖDERSTEIN and HERTEL2007, Moser et al. Reference MOSER, LEUSCHNER, HERTEL, GRAEFE, SOETHE and IOST2011) compensates, at least partially, the lower AGB at higher altitudes in tropical montane forests. Further investigations on the below-ground biomass component are needed to better estimate total carbon stock along the altitudinal gradient in Central African forests. Such investigations will allow us to improve our understanding of AGB storage in tropical montane forests. Understanding the key factors that drive biomass variation in montane forests could also improve our ability to predict the future changes resulting from increasing temperatures in the tropics under global change (Clark Reference CLARK2004, Malhi & Phillips Reference MALHI and PHILLIPS2004).
ACKNOWLEDGEMENTS
We thank ‘Sud Expert Plantes’ (SEP), a programme funded by the French Ministry of Foreign Affairs, the Centre de Cooperation Internationale en Recherche Agronomique pour le Developpement (CIRAD), and the Service de Cooperation et d'Action Culturelle (SCAC) of the French Embassy in Cameroon for financial assistance through research grants that made this study possible. We are grateful to N. Fauvet for his technical assistance in GIS and the preparation of the maps, and to P. Deleporte, D. Ouédroaogo and Y. Laumonier for valuable comments on earlier drafts of this article. Special thanks are extended to M. Sainge, P. Mezili and J.-M. Onana for their technical expertise on specimen identification in the field and at the National Herbarium of Cameroon. We thank the people of Ngovayang for allowing us to work on their land and for assisting us in fieldwork. Finally, a reviewer's constructive comments improved the paper.
Appendix 1. Altitude and stand structural characteristics of the 15 1-ha permanent plots established in old-growth terra firme rain forest of the Ngovayang Massif (Cameroon). Within each plot, all trees with a diameter at breast height ≥ 10 cm were recorded. BA, basal area; AGB, above-ground biomass; Hpmax , potential maximum height i.e. the plot-level average of the maximum height that trees of a species can attain.