Introduction
Gagaku is the Japanese ancient imperial court music from the 7th century and was added in 2009 to the UNESCO list of masterpieces of the Intangible Cultural Heritage of Humanity [1]. Hichiriki, shown in Figure 1, is a Japanese double-reed woodwind instrument, or flageolet, which usually performs the main melody of a gagaku piece [2]. (Listen to the hichiriki online in the digital edition of this issue at this web address: http://dx.doi.org/10.1017/S1551929516000110). A reed is a thin strip of material that vibrates to produce a sound by setting oscillation in the air column inside the tube of a wind instrument, such as clarinet, saxophone, oboe, bassoon, and hichiriki. The reed plant Phragmites australis (P. australis, common reed) commonly grows in wetlands in wide regions of the world. Although it can be found in reed beds along many rivers and lakesides in Japan, for more than 1,200 years the best reeds for the hichiriki have been made only out of canes of P. australis harvested from Udono (pronouced as [udono]), which is a limited reed bed of riverbanks located south of the border between Kyoto and Osaka along the Yodo River (Figure 2). This situation resembles that in which the best reeds for clarinet, oboe, or bassoon are manufactured from a cane of Arundo donax (A. donax, giant reed) that grows only in a few areas in the Var in southeastern France where the Mediterranean climate is very mild. Now, environmental disruption in Udono endangers the P. australis for hichiriki reeds and may bring a catastrophic crisis to gagaku. The International Double Reed Society, with members in more than 50 countries, expressed support for the call to protect and preserve the riverbanks of Udono, where the soul of hichiriki resides [Reference Schuring3].

Figure 1 (a) Playing hichiriki (photograph taken by Katsuhiko Tabuchi). (b) Hichiriki (photogragh taken by Hitomi Nakamura). The length of the hichiriki is about 180 mm. The reed used in hichiriki is called rozetsu in Japanese. An adjuster to control the space between two blades is called seme.

Figure 2 (a) Udono, a reed bed of the riverbanks near Kyoto along the Yodo River, which is seen upper right. (b) Young grasses of Phragmites australis. Photographs were taken by Makoto Shiojiri in June 2015.
Plant anatomy and physical properties of A. donax related to the musical performance of the reeds of wind instruments such as clarinet, oboe, and bassoon have been studied by many researchers [Reference Backus4–Reference Spatz12]. However, there are few papers regarding the plant anatomy and biomechanics of P. australis or hichiriki reeds (rozetsu in Japanese).
In this article, we review our recent investigation of plant anatomy and morphology for selected canes of P. australis grown in different reed beds in Japan. Moreover, the local indentation hardness and Young’s modulus were measured for different tissues on cross sections of representative hichiriki reeds, rozetsu [Reference Kawasaki13]. We were searching for the reason the P. australis canes from Udono along the Yodo River are the best materials for rozetsu. A geographical answer may also be necessary, considering envelopments for the growth of P. australis, such as soil, quantity and quality of the river water, and climate, as well as social priorities, such as traditional monopolies for the cultivation and distribution of reeds generated from Kyoto, the Imperial Capital of Japan for over 1,000 years. However, the first step toward the answer is to examine the differences in the structure of the P. australis grown in Udono compared with those grown in other areas. We develop this discussion in a later section of this article with the results from our new investigation on the plant anatomy and biomechanics of clarinet reeds.
Materials and Methods
Various canes of P. australis were sampled from five reed beds along different Japanese rivers: the Kitakami River, the Watarase River, the Turumi River, the Uji River, and the Yodo River. A reed bed along the Yodo River is particularly called Udono. Those canes were examined for their utility in producing rozetsu and were used for the present plant anatomy observations [Reference Kawasaki13]. We selected an appropriate internode (about 11 mm in outer diameter) from each cane in the same way that a reed producer would choose it as material for rozetsu. Specimens for light microscopy were prepared first by cutting the selected internodes to transverse sections of about 30 μm in thickness with a sliding microtome, followed by conventional double staining with safranin and fast green FCF. Then the sections were mounted with Canada balsam as specimens for observation.
For biomechanical measurements, we obtained three pieces of rozetsu that were made of the canes carefully harvested from Udono and one from a reed bed of Kitakami River. Each was crafted and examined for musical performance by a hichiriki player (who routinely handcrafts rozetsu [14]). Hardness and Young’s modulus were measured for each specimen, prepared by cutting a section out of the bottom part of rozetsu in the transverse plane for observation of the whole cross section, followed by embedding the cut piece in the epoxy resin and polishing it successively with a series of wet sandpaper sheets of finer grades (#320, #400, and #600) on a glass plate to create a mirror-like surface. An indenter tip was placed at different locations on the polished surface for the measurements using a nanoindentation system (Fischerscope, H100C-XYp) at room temperature. The load for the indentation with a Vickers indenter was 5 mN.
Results
Plant anatomy
Hichiriki reeds.
Figure 3 shows light micrographs of the canes collected from the five reed beds mentioned above. The images in (a), (b), (c), and (d) are of specimens from the Kitakami River near Sendai (Spe. 1), the Watarase River near Nikko (Spe. 2), the Turumi River in Tokyo (Spe. 3), the Uji Rever in Kyoto (Spe. 4), respectively, and the images in (e) and (f) are from Udono along the Yodo River (Spe. 5-1 and 5-2). As seen in Figure 3(e), the outermost part of the cane is enclosed by epidermis (Ep) comprising monolayer of cells, and the inside of Ep is cortex (Cor) composed of several layers of cells. The cells in Ep and Cor are small in diameter and have thick cell walls. The central cylinder (Cc) may be divided into three zones from Cor to pith cavity (Pc). They are composed of cells with thin cell walls (Ct), sclerenchymatous cells (Sc) with thick walls, and parenchyma cells (Pa) with larger diameters compared to Ct and Sc. The size of Pa decreases as the cell gets closer to Pc. Vascular bundles (Vb) are distributed in Cc. Each Vb is enclosed by cells with thick walls, which form a vascular bundle sheath (Bs). The Bs is called a fiber ring from the shape of its transverse cross section [Reference Kolesil10]. The plant P. australis almost resembles A. donax in plant anatomical characteristics as previously reported [Reference Kolesil10–Reference Spatz12], but is different not only in scale of outer shape but also size of cells, as their English names of ‘common reed’ for P. australis (see Figure 2(b)) and ‘giant reed’ for A. donax imply. This is a reason why A. donax cannot be used for rozetsu.
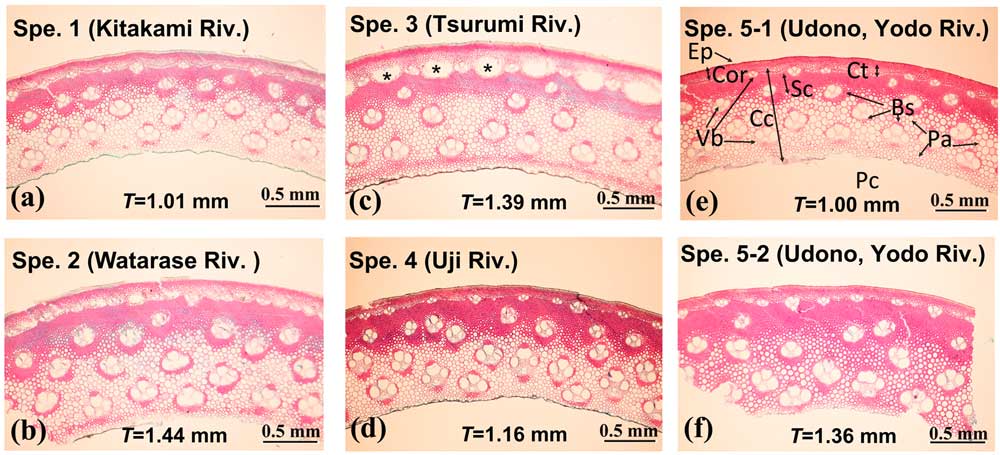
Figure 3 Light micrographs of P. australis canes from different reed beds. (a) Specimen 1 is from the Kitakami River near Sendai; (b) Specimen 2 is from the Watarase River near Nikko; (c) Specimen 3 is from Turumi River in Tokyo; (d) Specimen 4 is from the Uji Rever in Kyoto; and (e–f) Specimens 5-1 and 5-2 are from Udono. Ep: epidermis. Cor: cortex. Vb: vascular bundle. Bs: vascular bundle sheath. Cc: central cylinder. Pa: parenchyma cell. Ct: cells with thin cell wall. Sc: sclerenchymatous cells. Pc: pith cavity. The diameter of a cane is ~11 mm, making a circumference of ~34 mm so that the observed area is about one-tenth of a whole cross section.
Specimen 5-2 harvested from Udono shown in Figure 3f is a reference for an undesirable cane that the hichiriki player didn’t choose after evaluating the cane because it exhibited “a bad complexion and over thickness.” From the images in Figure 3, the wall thicknesses T were estimated to be 1.01, 1.44, 1.39, 1.16, 1.00, and 1.36 mm for Spe. 1, Spe. 2, Spe. 3, Spe. 4, Spe. 5-1, and Spe. 5-2, respectively. All the samples have a similar structure of cellular tissue indicated in Figure 3(e), although Spe. 2 (Watarase), Spe. 3 (Turumi), and Spe. 5-2 (Udono) have a wall too thick to make a rozetsu. The appropriate wall thickness of canes for a rozetsu is T = ~1 mm according to the preference that the hichiriki player usually chooses.
Specimen 5-1 (Udono) was the best of the materials for rozetsu evaluated by the hichiriki player, and we compared it with the other samples. It was found that i) the thickness of the cell wall of Pa in Cc in Spe. 5-1 is almost the same as that of Spe. 3 (Tsurumi) but thicker than those of Spe. 1 (Kitakami), Spe. 2 (Watarase), Spe. 4 (Uji), and Spe. 5-2; ii) the thickness of the Ct zone in Spe.5-1 is almost the same as that of Spe. 4 but thicker than those of the other samples; iii) Bs is not as developed in Spe. 5-1 compared with the other samples; iv) the density of Vb (numbers/unit area) is larger in Spe. 5-1 than Spe. 3 but smaller than the other samples; v) the Pa zone in the innermost layer becomes thicker in Spe. 5-1 as well as Spe. 2 and Spe. 3; vi) the Sc zone in Spe. 5-1 is thin compared with those in the other samples, consisting of a thin wall of T = 1.0 mm for the good hichiriki reed. From these observations it is revealed that Sc cells are not as developed in Spe. 5-1; both the walls of Pa cells enclosing Bs and the walls of Ct cells in the inside of Cor are thicker. Rozetsu (shown in Figure 4(a) and (b) as well as Figure 1(b)) is shaped so as to have double-thin blades where the outer part from the Ep layer to the Sc zone are shaved off [14]. The music performance or the acoustic vibration of the double reed may depend on the structure of the blades constructed with Pa, Vb, and Bs in the inner part of the cane of its thickness around 1 mm, accordingly. In the Udono cane (Spe. 5-1), there are hard tissues such as Ep, Cor, Sc, and Bs and soft tissues such as Pa and Vb with thicker cell walls uniformly distributed. By such particular growth, the reed has more uniform hardness in the whole blade because the thicker cell walls can be as hard. This will be discussed later with the results of hardness measurements done at those locations on the specimens. Hence, we concluded that the homogeneous structure over the hard and soft tissues would be an important condition for an excellent rozetsu.
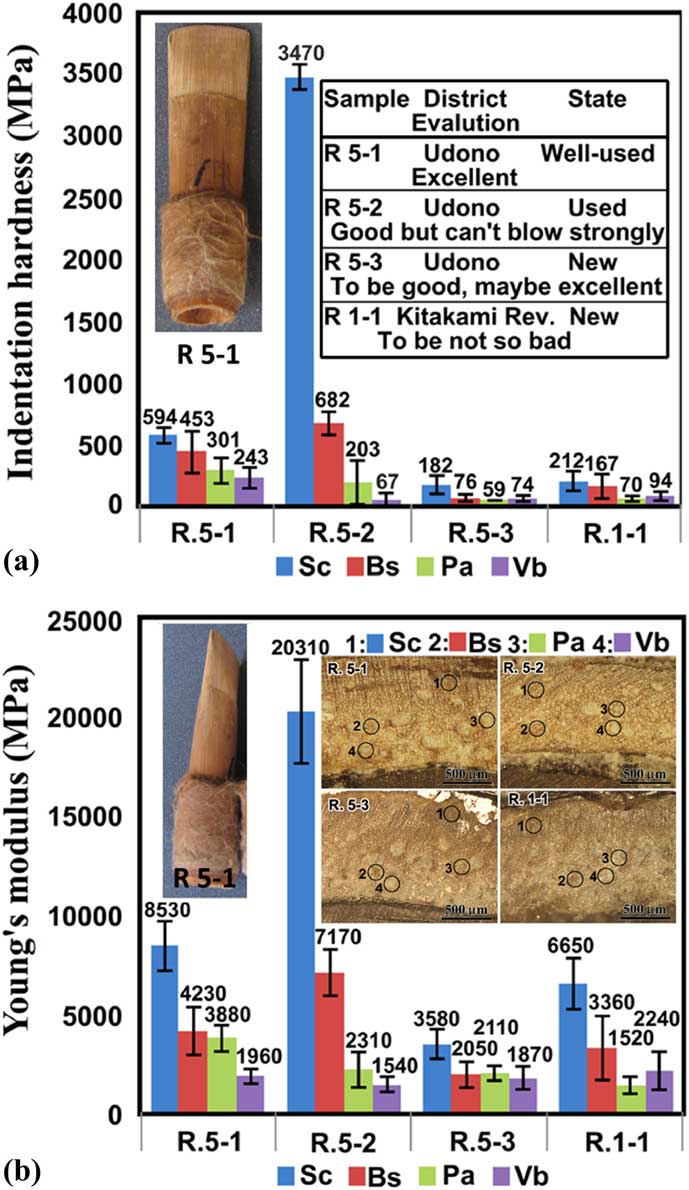
Figure 4 (a) Indentation hardness. (b) Indention Young’s modulus. Measurements were performed on different tissue features (shown in the inset in (b)) of different rozetsu (indicated in the inset in (a)). Photographs of rozetsu R 5-1 are shown in (a) and (b).
Clarinet reeds
We also observed clarinet reeds [15] and found that the acoustic quality of a clarinet reed is mainly ascribed to the shape and configuration of the Vb bundles with Bs, as well as the networks of the surrounding Pa cells. Each Bs tube enclosing Vb bundle works like a string for vibration, running along the reed. A reed with good musical performance has homogeneously distributed Vb bundles that provide the balance or symmetry of the local rigidity. This is necessary for good vibration, in particular on the blade tip of the reed. With the good acoustic double-reed rozetsu, each blade might vibrate smoothly and resonate as one body along their surfaces. So a homogeneous structure is required for resonance of the rozetsu blades. The quality is very different among canes even grown in the same place and among parts of the same cane. A good cane for rozetsu should have an outer diameter of about 11 mm to fit it in the hichiriki tube, a wall thickness of about 1 mm to make its double blades, and a homogeneous structure to transmit good vibration. Hichiriki players say that it has become harder and harder to get good canes for reed material, similar to the situation of clarinet and oboe players using Arundo donax reeds. However, it is certain that good canes are still cultivated in Udono but may be scarcely found in other reed beds.
Biomechanics
Four pieces of practical rozetsu, handcrafted and provided as typical samples by the hichiriki player, were used for biomechanical measurements (Figure 4). Samples R 5-1, R 5-2, and R 5-3 were made out of canes grown at Udono, and sample R 1-1 was from a cane grown at the reed bed along the Kitakami River.
Reed playability.
The player’s comments about the performance of the reeds that were used to prepare the samples are as follows: R 5-1, a well-used reed (shown in Figure 4a), felt as if it were of light material. It exhibited good musical performance (excellent); R 5-2, a used reed made of a part of cane away from a node, felt a little soft. It could make a sound but could not be blown strongly (good); R 5-3, an unused, slightly thin reed made from harder cane near a node, would make a good sound while being used (excellent); R 1-1, an unused reed made on trial, was a little salty and smelled like grass. The material felt thin and hard, and the sound was clear but also thin and hard. Contrary to expectations, R 1-1 may not be so bad in the performance. Those comments and feelings for the unused reeds, R 5-3 and R 1-1, were based on her trial of blowing on them and her long-standing experience.
Microstructure and mechanical tests.
Light micrographs of the cross sections of the bottoms of these samples are shown in Figure 4b. Although these images are of only modest quality, as compared with the micrographs of the specimens cut with the microtome shown in Figure 3, we can still identify the tissue features. The local indentation hardness H IT and Young’s modulus E IT were measured at different tissue features marked 1 to 4 corresponding to Sc, Bs, Pa, and Vb, respectively, and the results are shown in Figures 4a and 4b. The tests revealed the following: i) In all the samples, Sc and Bs exhibit higher H IT and E IT than Pa and Vb, which is natural because Sc and Bs are known to be harder features, and Pa and Vb are softer features. Accordingly, the indentation measurement can be qualitatively used for these materials. ii) H IT and E IT of the used reeds (R 5-1 and R 5-2) are higher than those of the unused reed (R 5-3 and R 1-1), indicating that the tissues in rozetsu become harder and tighter as the reeds are played during the musical performances. The raw materials for rozetsu would be expected to have hardness and rigidity as low as the values for R 5-3 or R 1-1. Players say that rozetsu becomes softer as it is used. The term “soft” might be different in meaning between the physical property and the musical performance. iii) In comparison with R 5-2 (the well-used, good reed of Udono), R 5-1 (the well-used, excellent reed of Udono) has softer Bs and harder Pa and Vb; that is, the difference of H IT (and E IT) between the hard materials (Bs) and soft materials (Pa and Vb) is not so large. H IT and E IT for Sc at measured point 1 in R.5-2 are abnormally large. However, they do not need to be considered when we discuss the acoustic performance of the reed because the Sc zones are not included in the blades. This is similar for R 5-3 (Udono reed) as compared with R1-1 (Kitakami reed). In fact, the assessment of the player was a little different; she found the former to be excellent and the latter to be not so bad.
Discussion
The biomechanical measurements are consistent with the conclusion from the plant anatomy—that homogeneity of the structure over the harder and softer materials is higher for an excellent rozetsu. The vibration is mainly influenced by the fiber tubes Bs passing through from the bottom to the top of the reed, enclosed by Pa cells. It is natural that the homogeneous distribution of Vb with Bs and the homogeneity of hard and soft tissues are favorable for the vibration of the rozetsu blades. It may be noted that we cannot say what hardness and rigidity are best for rozetsu because they depend on the player, the type of music, and other factors.
Conclusion
To understand why the best canes of P. australis for hichiriki reeds, rozetsu, have been provided only from Udono for more than 1,200 years, plant anatomy and morphology were examined for selected canes of P. australis that were harvested from the different reed beds in Japan, and the local indentation hardness and Young’s modulus of tissues on the cross-sections of some representative rozetsu were measured. It is concluded that the good canes for rozetsu have an outer diameter of about 11 mm to fit the rozetsu into the hichiriki tube, a wall thickness of about 1 mm to form the blades for the reed, and comparatively homogeneous structure where harder materials such as sclerenchymatous cells (Sc) and vascular bundle sheaths (Bs) with hard cell walls are orderly deployed with softer materials such as parenchyma cells (Pa) and vascular bundles (Vb), in particular. This structure exhibits smaller differences in hardness and in Young’s modulus between the hard and soft tissues, allowing the rozetsu blades to vibrate smoothly and provide the best musical performance. Good P. australis canes for rozetsu can be still harvested in Udono but are difficult to find in other reed beds. It is not so easy to describe quantitatively or objectively the relation between the structure of musical instruments and the musical tones or sounds because the latter is evaluated subjectively by the feeling and sensitivity of the players. The present paper is the first step in physically characterizing the hichiriki.
Acknowledgements
The authors deeply thank Ms. Hitomi Nakamura, a hichiriki player in the Reigakusha Gagaku Ensemble, for providing all the samples used in this investigation, the photographs in Figure 1, and valuable comments from a view of musical performance. The authors also thank Mr. Yuta Nakabuse for assistance in biomechanical measurements. Special thanks are due to Prof. Charles Lyman for carefully reading the manuscript and providing invaluable advice.








