Article contents
Transmission electron microscopy study of the epitaxial association of hedenbergite whiskers with babingtonite
Published online by Cambridge University Press: 28 February 2018
Abstract
Overgrowths of whiskers of hedenbergite (Ca(Fe2+,Mg)Si2O6) on the hydrous pyroxenoid babingtonite (Ca2Fe2+Fe3+[Si5O14(OH)]) have been observed at Arvigo in Switzerland and Kreimbach/Kaulbach in Germany, and we have studied them with transmission electron microscopy in order to understand their structural relationships and formation. The boundaries between babingtonite and hedenbergite are sharply defined, and the two minerals are in direct contact with no additional phases present. The relationships of babingtonite (Bab) and hedenbergite (Hd) were determined as Bab[100]//Hd[112] in the Arvigo specimen and Bab[ $\bar 1$00]//Hd[1
$\bar 1$00]//Hd[1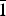 $\bar 1$2] in the Kreimbach/Kaulbach specimen. Diffraction derived from Bab(031) and Hd(02
$\bar 1$2] in the Kreimbach/Kaulbach specimen. Diffraction derived from Bab(031) and Hd(02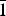 $\bar 1$) in the Arvigo samples and Bab(031) and Hd(021) in the Kreimbach/Kaulbach samples were observed in identical positions. The reciprocity between the babingtonite and hedenbergite structures is governed by the direction of the SiO4-tetrahedral chains, and the related configuration of octahedra. Thus, hedenbergite is apparently an epitaxial phase grown on a base of {010} plates of babingtonite. The defined orientation relationship is also consistent with that shown in topotaxial intergrowths of other clinopyroxenes and pyroxenoids. The topotaxial intergrowths may result from diffusion-controlled solid-state reactions, whereas rapid whisker growth is characteristic of supersaturated solutions or a vapour medium. The epitaxial growth of hedenbergite whiskers on babingtonite with an abrupt but coherent change of structure at the interface represents an ideal example where the similar chemical compositions of host and guest contribute strongly to the close structural relationship.
$\bar 1$) in the Arvigo samples and Bab(031) and Hd(021) in the Kreimbach/Kaulbach samples were observed in identical positions. The reciprocity between the babingtonite and hedenbergite structures is governed by the direction of the SiO4-tetrahedral chains, and the related configuration of octahedra. Thus, hedenbergite is apparently an epitaxial phase grown on a base of {010} plates of babingtonite. The defined orientation relationship is also consistent with that shown in topotaxial intergrowths of other clinopyroxenes and pyroxenoids. The topotaxial intergrowths may result from diffusion-controlled solid-state reactions, whereas rapid whisker growth is characteristic of supersaturated solutions or a vapour medium. The epitaxial growth of hedenbergite whiskers on babingtonite with an abrupt but coherent change of structure at the interface represents an ideal example where the similar chemical compositions of host and guest contribute strongly to the close structural relationship.
- Type
- Article
- Information
- Copyright
- Copyright © Mineralogical Society of Great Britain and Ireland 2018
Footnotes
Associate Editor: Anthony Kampf
References
- 1
- Cited by




