Article contents
Density Functional Theory study of Cu doped {0001} and {01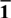 ${{\rm{\bar{1}}}}$2} surfaces of hematite for water splitting
${{\rm{\bar{1}}}}$2} surfaces of hematite for water splitting
Published online by Cambridge University Press: 01 March 2018
Abstract
Density Functional Theory (DFT) calculations study of Cu doped {0001} and {01-12} surfaces of hematite for enhanced water splitting have been carried out. The doping was restricted to planes in the vicinity of the surface, specifically from the top most layers to the third inner layer of Fe atoms. Thermodynamic stabilities were evaluated based on surface energies and formation energies. The evaluation of thermodynamic stabilities (negative formation energy values) shows that the systems are thermodynamically stable which suggest that they can be synthesized in the laboratory under favorable conditions. Doping on the top most layer yields the energetically most favorable structure. The calculated charge density difference plots showed the concentration of charge mainly at the top of the surface (termination region), and this charge depleted from the Cu atom to the surrounding Fe and O atoms. This phenomenon (concentration of charge at the top of the surface) is likely to reduce the distance moved by the charge carriers, decrease in charge recombination leading to facile transfer of charge to the adsorbate and, suggesting improved photoelectrochemical water oxidation activity of hematite. The analysis of electron electronic structure reveals that Cu doped surface systems does not only decrease the band gap but also leads to the correct conduction band alignment for direct water splitting without external bias voltage.
Keywords
- Type
- Articles
- Information
- Creative Commons
- This is an Open Access article, distributed under the terms of the Creative Commons Attribution licence (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted re-use, distribution, and reproduction in any medium, provided the original work is properly cited.
- Copyright
- Copyright © Materials Research Society 2018
References
REFERENCES:
- 12
- Cited by




