Article contents
A cross-talk EGFR/VEGFR-targeted bispecific nanoprobe for magnetic resonance/near-infrared fluorescence imaging of colorectal cancer
Published online by Cambridge University Press: 18 July 2018
Abstract
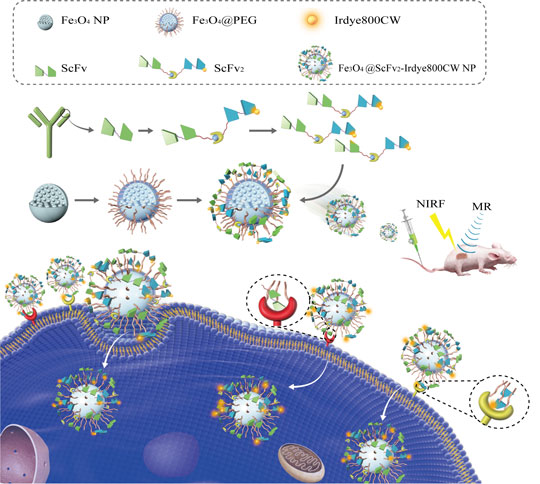
Due to the lack of an effective and noninvasive screening tool, the early diagnosis of colorectal cancer (CRC) is currently difficult. For the early diagnosis of CRC, we have developed Fe3O4-Dye800-single chain fragment variable (ScFv)egfr/vegfr nanoprobes. ScFvegfr/vegfr (ScFv2) conjugated onto Fe3O4 nanoprobes efficiently recognized CRC tumors in vitro and in vivo. Near-infrared fluorescence imaging modalities such as Dye800 were utilized simultaneously with magnetic resonance to enhance detection efficiency. Fe3O4-Dye800-ScFv2 successfully detected tiny CRC tumors; the synergistic ScFv2 successfully enhanced CRC targeting. Thus, Fe3O4-Dye800-ScFv2 nanoprobes may represent a new molecular imaging strategy for the early detection of CRC.
- Type
- Research Letters
- Information
- Copyright
- Copyright © Materials Research Society 2018
References
- 3
- Cited by





