Article contents
Economical Fe-doped Ta2O5 electrocatalyst toward efficient oxygen evolution: a combined experimental and first-principles study
Published online by Cambridge University Press: 03 August 2017
Abstract
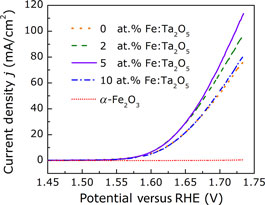
A non-precious metal catalytic system of Fe-doped Ta2O5 is developed by pulsed laser deposition toward efficient oxygen evolution reaction (OER). The optimal Fe concentration is determined to be 5 at.% for optimized OER activity via a series of electrochemical characterizations. The 5 at.% Fe-doped Ta2O5 nanolayer possesses a low onset overpotential of 0.22 V, an overpotential of 0.38 V at 10 mA/cm2 and a Tafel slope of 54 mV/dec. Comprehensive first-principles calculations attribute the enhanced OER activity to the substitutional FeTa dopants, which generate a new active OER site on surface and simultaneously accelerate electron transfer over oxygens.
Information
- Type
- Research Letters
- Information
- Copyright
- Copyright © Materials Research Society 2017
References
1.Dresselhaus, M.S. and Thomas, I.L.: Alternative energy technologies. Nature 414, 332 (2001).Google Scholar
2.Montoya, J.H., Seitz, L.C., Chakthranont, P., Vojvodic, A., Jaramillo, T.F., and Norskov, J.K.: Materials for solar fuels and chemicals. Nat. Mater. 16, 70 (2017).Google Scholar
3.Hoang, S. and Gao, P.-X.: Nanowire array structures for photocatalytic energy conversion and utilization: a review of design concepts, assembly and integration, and function enabling. Adv. Energy Mater. 6, 1600683 (2016).Google Scholar
4.Liu, C., Roder, R., Zhang, L., Ren, Z., Chen, H., Zhang, Z., Ronning, C., and Gao, P.-X.: Highly efficient visible-light driven photocatalysts: a case of zinc stannate based nanocrystal assemblies. J. Mater. Chem. A 2, 4157 (2014).Google Scholar
5.Weng, B., Xu, F., Wang, C., Meng, W., Grice, C.R., and Yan, Y.: A layered Na1−xNiyFe1−yO2 double oxide oxygen evolution reaction electrocatalyst for highly efficient water-splitting. Energy Environ. Sci. 10, 121 (2017).Google Scholar
6.Xu, X., Song, F., and Hu, X.: A nickel iron diselenide-derived efficient oxygen-evolution catalyst. Nat. Commun. 7, 12324 (2016).Google Scholar
7.Qiu, Y., Xin, L., and Li, W.: Electrocatalytic oxygen evolution over supported small amorphous Ni–Fe nanoparticles in alkaline electrolyte. Langmuir 30, 7893 (2014).Google Scholar
8.Song, W., Ren, Z., Chen, S.-Y., Meng, Y., Biswas, S., Nandi, P., Elsen, H.A., Gao, P.-X., and Suib, S.L.: Ni- and Mn-promoted mesoporous Co3O4: a stable bifunctional catalyst with surface-structure-dependent activity for oxygen reduction reaction and oxygen evolution reaction. ACS Appl. Mater. Interfaces 8, 20802 (2016).Google Scholar
9.Gao, D., Zhang, J., Wang, T., Xiao, W., Tao, K., Xue, D., and Ding, J.: Metallic Ni3N nanosheets with exposed active surface sites for efficient hydrogen evolution. J. Mater. Chem. A 4, 17363 (2016).Google Scholar
10.Huang, X., Leng, M., Xiao, W., Li, M., Ding, J., Tan, T.L., Lee, W.S.V., and Xue, J.: Activating basal planes and S-terminated edges of MoS2 toward more efficient hydrogen evolution. Adv. Funct. Mater. 27, 1604943 (2017).Google Scholar
11.Xiao, W., Liu, P., Zhang, J., Song, W., Feng, Y.P., Gao, D., and Ding, J.: Dual-functional N dopants in edges and basal plane of MoS2 nanosheets toward efficient and durable hydrogen evolution. Adv. Energy Mater. 7, 1602086 (2017).Google Scholar
12.Xiao, W., Huang, X., Song, W., Yang, Y., Herng, T.S., Xue, J.M., Feng, Y.P., and Ding, J.: High catalytic activity of oxygen-induced (200) surface of Ta2O5 nanolayer towards durable oxygen evolution reaction. Nano Energy 25, 60 (2016).Google Scholar
13.Wang, L., Huang, X., and Xue, J.: Graphitic mesoporous carbon loaded with iron–nickel hydroxide for superior oxygen evolution reactivity. ChemSusChem 9, 1835 (2016).Google Scholar
14.Li, Y., Gong, M., Liang, Y., Feng, J., Kim, J.-E., Wang, H., Hong, G., Zhang, B., and Dai, H.: Advanced zinc-air batteries based on high-performance hybrid electrocatalysts. Nat. Commun. 4, 1805 (2013).Google Scholar
15.Dresp, S., Luo, F., Schmack, R., Kuhl, S., Gliech, M., and Strasser, P.: An efficient bifunctional two-component catalyst for oxygen reduction and oxygen evolution in reversible fuel cells, electrolyzers and rechargeable air electrodes. Energy Environ. Sci. 9, 2020 (2016).Google Scholar
16.Trasatti, S.: Electrocatalysis in the anodic evolution of oxygen and chlorine. Electrochim. Acta 29, 1503 (1984).Google Scholar
17.Cherevko, S., Reier, T., Zeradjanin, A.R., Pawolek, Z., Strasser, P., and Mayrhofer, K.J.J.: Stability of nanostructured iridium oxide electrocatalysts during oxygen evolution reaction in acidic environment. Electrochem. Commun. 48, 81 (2014).Google Scholar
18.Yamashita, Y., Tada, M., Kakihana, M., Osada, M., and Yoshida, K.: Synthesis of RuO2-loaded BaTinO2n+1 (n = 1, 2 and 5) using a polymerizable complex method and its photocatalytic activity for the decomposition of water. J. Mater. Chem. 12, 1782 (2002).Google Scholar
19.Morales, S. and Fernandez, A.: Unsupported PtxRuyIrz and PtxIry as bi-functional catalyst for oxygen reduction and oxygen evolution reactions in acid media, for unitized regenerative fuel cell. Int. J. Electrochem. Sci. 8, 12692 (2013).Google Scholar
20.Burke, M.S., Enman, L.J., Batchellor, A.S., Zou, S., and Boettcher, S.W.: Oxygen evolution reaction electrocatalysis on transition metal oxides and (oxy)hydroxides: activity trends and design principles. Chem. Mater. 27, 7549 (2015).Google Scholar
21.Subbaraman, R., Tripkovic, D., Chang, K.-C., Strmcnik, D., Paulikas, A.P., Hirunsit, P., Chan, M., Greeley, J., Stamenkovic, V., and Markovic, N.M.: Trends in activity for the water electrolyser reactions on 3d M(Ni,Co,Fe,Mn) hydr(oxy)oxide catalysts. Nat. Mater. 11, 550 (2012).Google Scholar
22.McCrory, C.C.L., Jung, S., Peters, J.C., and Jaramillo, T.F.: Benchmarking heterogeneous electrocatalysts for the oxygen evolution reaction. J. Am. Chem. Soc. 135, 16977 (2013).Google Scholar
23.Burke, M.S., Kast, M.G., Trotochaud, L., Smith, A.M., and Boettcher, S.W.: Cobalt–iron (oxy)hydroxide oxygen evolution electrocatalysts: the role of structure and composition on activity, stability, and mechanism. J. Am. Chem. Soc. 137, 3638 (2015).Google Scholar
24.Friebel, D., Louie, M.W., Bajdich, M., Sanwald, K.E., Cai, Y., Wise, A.M., Cheng, M.-J., Sokaras, D., Weng, T.-C., Alonso-Mori, R., Davis, R.C., Bargar, J.R., Nørskov, J.K., Nilsson, A., and Bell, A.T.: Identification of highly active Fe sites in (Ni,Fe)OOH for electrocatalytic water splitting. J. Am. Chem. Soc. 137, 1305 (2015).Google Scholar
25.Görlin, M., Chernev, P., Ferreira de Araújo, J., Reier, T., Dresp, S., Paul, B., Krähnert, R., Dau, H., and Strasser, P.: Oxygen evolution reaction dynamics, Faradaic charge efficiency, and the active metal redox states of Ni–Fe oxide water splitting electrocatalysts. J. Am. Chem. Soc. 138, 5603 (2016).Google Scholar
26.Zou, S., Burke, M.S., Kast, M.G., Fan, J., Danilovic, N., and Boettcher, S.W.: Fe (oxy)hydroxide oxygen evolution reaction electrocatalysis: intrinsic activity and the roles of electrical conductivity, substrate, and dissolution. Chem. Mater. 27, 8011 (2015).Google Scholar
27.Atanassova, E.: Thin RF sputtered and thermal Ta2O5 on Si for high density DRAM application. Microelectron. Reliab. 39, 1185 (1999).Google Scholar
28.Fujii, T., de Groot, F.M.F., Sawatzky, G.A., Voogt, F.C., Hibma, T., and Okada, K.: In situ XPS analysis of various iron oxide films grown by NO2-assisted molecular-beam epitaxy. Phys. Rev. B 59, 3195 (1999).Google Scholar
29.Lee, S.-H., Kim, J., Kim, S.-J., Kim, S., and Park, G.-S.: Hidden structural order in orthorhombic Ta2O5. Phys. Rev. Lett. 110, 235502 (2013).Google Scholar
30.Man, I.C., Su, H.-Y., Calle-Vallejo, F., Hansen, H.A., Martínez, J.I., Inoglu, N.G., Kitchin, J., Jaramillo, T.F., Nørskov, J.K., and Rossmeisl, J.: Universality in oxygen evolution electrocatalysis on oxide surfaces. ChemCatChem 3, 1159 (2011).Google Scholar
- 7
- Cited by

