Article contents
Fabrication of n-type flexible films with a double-layer structure by hybridizing Bi2Se3 and poly(vinyl alcohol)
Published online by Cambridge University Press: 18 July 2018
Abstract
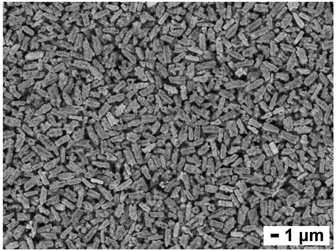
Here we report a new type of n-type flexible film with a double-layer structure fabricated by hybridizing an n-type inorganic thermoelectric material, bismuth selenide (Bi2Se3), and an ordinary insulating polymer, poly(vinyl alcohol) (PVA). Flake-shaped Bi2Se3 nanoparticles (Bi2Se3 nanoflakes) modified with/without gold (Au) nanoparticles were distributed in the one side of PVA film with the particular arrangement, and the hybrid film showed a high Seebeck coefficient (−91 µV/K at room temperature) as an n-type flexible material. Our method is expected to be used for the design of flexible functional devices such as flexible thermoelectric modules.
- Type
- Research Letters
- Information
- Copyright
- Copyright © Materials Research Society 2018
References
- 4
- Cited by





