Article contents
Improvement of the electrochemical performance of LiFePO4 cathode by Y-doping
Published online by Cambridge University Press: 17 August 2017
Abstract
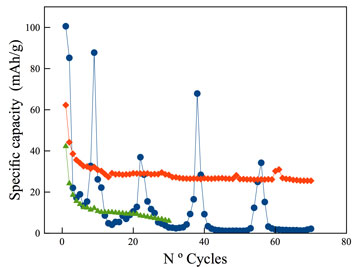
LiFe1−xYxPO4 doped (d-LFP) with amounts of yttrium (0.01% < x < 5% w/w) show a remarkable effect on the electrochemical behavior. The d-LPF samples were investigated on the Li extraction/insertion performance through charge/discharge and capacity–voltage curves. The best performance was attained with Y content of x = 1%. The materials were synthesized by a hydrothermal method and characterized by x-ray diffraction (XRD) and scanning electron microscopy–energy dispersive x-ray spectroscopy (SEM–EDX). The XRD studies showed that d-LPF had the same monoclinic structure as the undoped material. The achieved electrode performance has been attributed to the addition of Y3+ ion by stabilizing the orthorhomic structure. The electrode resistance decreases through the Y-doping.
- Type
- Prospective Articles
- Information
- Copyright
- Copyright © Materials Research Society 2017
References
- 6
- Cited by




