Article contents
Aqueous and Surface Chemistry of Calcium - Metal Hydroxides in High pH Environments
Published online by Cambridge University Press: 10 February 2011
Abstract
Mixed hydroxides of calcium, zinc, cadmium and lead have been recently identified in the high pH environments of hydrating cement. FTIR, XRD, SEM, and SPM have been used to characterize these systems. A chemical equilibrium model of the early hydration of a zinc-doped cement/water system, Na-K-Ca-Zn-H-SO4-OH-Zn(OH)2-Zn(OH)3-Zn(OH)4-H2O, has been developed to better understand the mechanism of the surface formation of calcium hydroxyzincate (CHZ). The model is based on Pitzer's semi-empirical method for calculation of ion-activity coefficients at high ionic strength. The Pitzer parameters for Na+-Zn2+,Na+-Zn2+-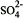 and
and 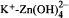 have been evaluated, and the results successfully predict the equilibria (solubilities) of Na2SO4-ZnSO4-H2O, NaOH-Zn(OH)2-H2O and KOH-ZnO-H2O systems. The chemical model clearly demonstrates that the formation of CHZ on the calcium-silicahydrate (C-S-H) surface is critically controlled by the Ca2+ ion concentration as well as pH of the pore water system. The results of this study suggest that the growth of CHZ is preceded by surface complex formation. Sequential charge control and sequential structure development have been used to discuss the surface selectivity of these compounds and their control of cement hydration.
have been evaluated, and the results successfully predict the equilibria (solubilities) of Na2SO4-ZnSO4-H2O, NaOH-Zn(OH)2-H2O and KOH-ZnO-H2O systems. The chemical model clearly demonstrates that the formation of CHZ on the calcium-silicahydrate (C-S-H) surface is critically controlled by the Ca2+ ion concentration as well as pH of the pore water system. The results of this study suggest that the growth of CHZ is preceded by surface complex formation. Sequential charge control and sequential structure development have been used to discuss the surface selectivity of these compounds and their control of cement hydration.
- Type
- Research Article
- Information
- Copyright
- Copyright © Materials Research Society 1997
References
- 2
- Cited by




