Article contents
From Ceramics to Superconductors: Rapid Materials Synthesis by Solid-State Metathesis Reactions
Published online by Cambridge University Press: 25 February 2011
Abstract
The preparation of materials from gas and liquid phase precursor reactions is well documented. However, solid-state precursor routes have remained largely unexplored. This synthetic void led us to develop rapid (< 2 s), solid-state metathesis (exchange) reactions with a very broad range of synthetic applications. An example is the self-propagating reaction:
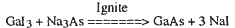
where the Nal is simply washed away with water. Analogous reactions allow the preparation of carbides, suicides, chalcogenides (O, S, Se, and Te), and pnictides (N, P, As, and Sb), of main-group, transition-, and rare-earth metals. These methods can also be exploited to produce mixed-metal and mixed-nonmetal solid solutions.
To gain insight into some of the mechanistic factors involved in these reactions, such as initiation, nucleation, and propagation, we have employed a variety of physical characterization methods. This paper will review the range of materials accessible by our synthetic approaches, as well as the control of product crystallinity, phase, and homogeneity through the optimization of reaction conditions.
- Type
- Research Article
- Information
- Copyright
- Copyright © Materials Research Society 1992
References
REFERENCES
- 5
- Cited by




