Introduction
Marine sediments of Rhaetian age (Late Triassic, c.208.5–201.3 Ma) are well-known from British localities, especially around Cardiff and Bristol, and are known for their fossil content of marine vertebrates. So far, such fossils have not been reported from the Netherlands. Since the 1930s, light-greyish micritic limestone of Middle Triassic (Vossenveld Formation, Anisian, c.247.2–242 Ma) age has been commercially exploited in a quarry near Winterswijk, Gelderland province, eastern Netherlands. The >30 m thick sediments appear overlain by a c.5 m thick layer of Late Triassic (Rhaetian) argillaceous sediment rich in pyrite, a thin layer of Cenozoic (Rupelian) clay and a Late Pleistocene boulder clay (Peletier & Kolstee, Reference Peletier and Kolstee1986; Van den Bosch & Gaemers, Reference Van den Bosch and Gaemers2015). In 1989 a plug of dark argillaceous material was found amidst the Anisian limestone. The plug was c.2 m thick and c.30 m in diameter, and turned out to be the infill of a subrosion pipe or sinkhole (Oosterink et al., Reference Oosterink, Simon and Winkelhorst2005, Reference Oosterink, Simon, Hagdorn and Winkelhorst2006; Klompmaker & Van den Berkmortel, Reference Klompmaker and Van den Berkmortel2007). The dark sediments had fallen from a level about 10 m higher than where they were sampled, and appeared to be Rhaetian and/or Hettangian in age. Part of the plug was sampled by Adiël Klompmaker in 2005. Klompmaker & Van den Berkmortel (Reference Klompmaker and Van den Berkmortel2007) described a fauna of Hettangian psiloceratoid ammonites from the infilling sediments. The subrosion pipe and its infillings have been removed during exploitation of the quarry and cannot be studied or sampled anymore.
After the discovery of the subrosion pipe, a second and much larger outcrop of Rhaetian black claystones was found in 2004 and opened for extensive sampling in 2018. It is situated c.50 m north of the locality of the subrosion pipe and is supposedly the source rock of the infillings of the pipe, or at least of a part thereof. Total thickness is estimated to be about 5 m. Palynomorphs from these sediments indicate a Rhaetian age (Herngreen, Reference Herngreen2004; Herngreen et al., Reference Herngreen, Van Konijnenburg-van and Oosterink2005). Initial fossil collection there has so far resulted in the description of ophiuroid echinoderms (Thuy et al., Reference Thuy, Klompmaker and Jagt2012), and collection of a rich sample of chondrichthyan and actinopterygian teeth, dermal denticles and scales. As this constitutes a different locality it will be the subject of further study. Here, we describe the fish remains (Chondrichthyes and Actinopterygii) from the Rhaetian infillings of the subrosion pipe.
Until now, no Rhaetian vertebrate remains have been described from a locality in the Netherlands. Fossils from the Winterswijk subrosion pipe published so far include palynomorphs (Herngreen et al., Reference Herngreen, Van Konijnenburg-van and Oosterink2005; Klompmaker et al., Reference Klompmaker, Herngreen and Oosterink2010), bivalves (Klompmaker et al., Reference Klompmaker, Herngreen and Oosterink2010) and Hettangian (but not Rhaetian) psiloceratid ammonites (Klompmaker & Van den Berkmortel, Reference Klompmaker and Van den Berkmortel2007). The vertebrate remains that are the subject of the present paper are the first of this age so far described from the Netherlands and the neighbouring region of Germany; they add to our knowledge of Rhaetian biogeography.
Material and methods
Approximately 3 kg of the infilling of the subrosion pipe was collected by Adiël Klompmaker in 2005 and stored pending further study. Unfortunately, no more material from this interesting locality has been collected. The sample was treated with a c.5% solution of acetic acid (a technique loosely based on Jeppsson et al., Reference Jeppsson, Anehus and Fredholm1999), sieved under running tap water, dried and treated again with acetic acid until no further clay particles appeared. The remaining grit was hand-picked for fossils using a binocular microscope. The fossils themselves needed no further preservation. They are kept in the collection of Utrecht University (Department of Earth Sciences, working group Stratigraphy & Palaeontology), collection code WW-SP (for Winterswijk – subrosion pipe). Terminology of hybodont dental features used is after Duffin (Reference Duffin1985). Photographs were made with a Keyence VHX-500 digital microscope.
Systematic palaeontology
Class Chondrichthyes Huxley, 1880
Order Hybodontiformes Patterson, 1966
Genus Lissodus Brough, 1935
Lissodus minimus (Agassiz, 1839)
(Fig. 1a–d)

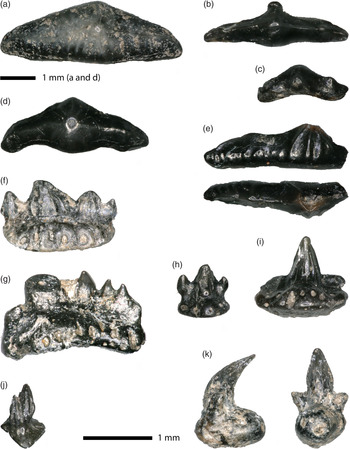
Figure 1. Chondrichthyan teeth. Lissodus minimus, (a) largest specimen WW-SP 001(c.4.5 mm), (b, d) intermediate-sized specimens (b) WW-SP 002 and (d) WW-SP 004), (c) smallest specimen WW-SP 003 (c.1.2 mm); Polyacrodus sp. indet., (e) partly damaged tooth (WW-SP 030) in labial view (above) and occlusal view (below); Rhomphaiodon minor, (f) asymmetrical tooth with one central cusp and three lateral cusplets (WW-SP 032), (g) asymmetrical tooth with one (broken) central cusp and four lateral cusplets (WW-SP 033), (h) tooth with two lateral cusplets (WW-SP 034), (i) tooth with central cusp only (WW-SP 035), (j) dermal denticle (WW-SP 045); Pseudodalatias barnstonensis, (k) tooth (WW-SP 031) with two lateral cusplets in mesial/distal view (left) and lingual view (right).
Lissodus minimus is by far the most abundant shark in the sample as far as the number of teeth is concerned. There are a total of 29 teeth, some of which are broken. The largest tooth measures c.4.5 mm in mesio-distal length, the smallest one c.1.2 mm. Teeth of L. minimus are curved in occlusal view (‘banana-shaped’), tapering towards both lateral (mesial and distal) ends. There is a strong but low central cusp and there may be one very low lateral cusplet on each (mesial and distal) side, but these may also be absent. An occlusal crest runs low over the entire mesiodistal length of the teeth. A more or less conspicuous peg may be positioned low on the labial side of the tooth, below the central cusp. These pegs may have functioned to lock the individual teeth in place with the teeth in the adjacent file (Allard et al., Reference Allard, Carpenter, Duffin and Benton2015). The larger teeth show a vertical striation, with the most central striae running towards the central cusp; the more distally and medially situated striae run towards the central crest. Smaller teeth have no striation. None of our teeth shows a root.
One elongate Lissodus-like tooth differs from the L. minimus teeth described above in being more mesio-distally elongate and in having a coarser striation. Unfortunately, it is broken in half and thus damaged, but on the anterior (lingual) side, we note four striations running towards the low central cusp. The labial side of the undamaged half possesses minute rudimentary striae showing only as inconspicuous little cuspules on the lower edge. We tentatively attribute this tooth to the genus Polyacrodus, but refrain from naming a species.
Order Synechodontiformes Duffin & Ward, 1993
Genus Rhomphaiodon Duffin, Reference Duffin1993
Rhomphaiodon minor (Agassiz, 1837)
(Fig. 1f–j)
In our sample, we possess 14 teeth that we here ascribe to R. minor. They show considerable variation in size, from small tricuspid teeth slightly less than 1 mm in size to one detached central cusp of 3 mm height. This agrees well with the description of R. minor given by Allard et al. (Reference Allard, Carpenter, Duffin and Benton2015), who noted that the teeth are small but vary in size, with a central cusp up to 3 mm in height. The central cusp of the teeth has vertical striations that do not continue to the apex. There are up to three pairs of lateral cusplets, which decrease in size towards the distal and mesial periphery. The central cusps and lateral cusplets are curved lingually, but to a varying degree.
In addition, we have one very small dermal denticle, c.0.9 mm in height, that sits on a thin basal plate of triangular shape; one of the sides of this triangle, the posterior side, is convex. The crown of the denticle is strongly curved in the posterior direction; it shows striations and one small lateral ‘cusplet’. We tentatively ascribe this hybodont denticle (Fig. 1j) also to R. minor as this is the most abundant hybodontiform shark with striated elements in our sample.
One of the hybodont teeth differs in morphology from the Rhomphaiodon minor teeth and is here identified as belonging to Pseudodalatias barnstonensis. According to Duffin (Reference Duffin, Swift and Martill1999) and Allard et al. (Reference Allard, Carpenter, Duffin and Benton2015), upper teeth of P. barnstonensis are characterised by the lingual curvature of the nearly circular central cusp, and by this central cusp being pronounced and flanked by two short lateral cusplets. The tooth sits on a narrow and ovoid root, with little vascularization. The relatively large central cusp is flanked by two short cusplets that do not sit on the root but emerge from the lower lateral (mesiodistal) sides of the central cusp. As lower teeth of P. barnstonensis have a different morphology with serrated central cusps, the tooth derives from the upper jaw.
Class Osteichthyes Huxley, 1880
Subclass Actinopterygii Cope, 1887
Family Saurichthyidae Owen, 1860 (sensu Stensiö, 1925)
Genus Saurichthys Agassiz, 1834
Saurichthys longidens Agassiz, Reference Agassiz1835
(synonym: Severnichthys acuminatus (Agassiz, Reference Agassiz1835) partim)
(Fig. 2a–d)

Figure 2. Teeth and cranial fragment of actinopterygians. Saurichthys longidens, (a) tooth (WW-SP 046), (b) tooth (WW-SP 047), (c) tooth (WW-SP 048), (d) paired cranial roof element with outline indicated (WW-SP 082); Birgeria acuminata, (e) large tooth (WW-SP 083) showing striated lingual side of the enamel cap (left) and smooth labial side (right), (f) small tooth (WW-SP 084); Gyrolepis albertii, (g, h) teeth showing unstriated and typically curved shafts (g) WW-SP 099, (h) WW-SP 100); cf. ‘Lepidotus’, (i) tooth (WW-SP 151) of the blunt type in side view (above) and occlusal view (below), (j) tooth (WW-SP 152) of the blunt type, (k) tooth (WW-SP 150) of the lentil-shaped type in buccal view (left) and lingual view (right), (l) tooth of the pointed type (WW-SP 142); Gnathostomata indet., (m, n) gill rakers (both WW-SP 155).
The dentition of Saurichthys longidens consists of elongated conical teeth with an often translucent cap. Generally speaking, the teeth show some morphological variation that has led to confusion in the literature on Rhaetian fishes (see Discussion). The enamel cap may show vertical striations, but there are also teeth that show a smooth, unstriated cap. Also, the relative length of the enamel cap may vary between one-third and about one-tenth of the total tooth length. However, as the teeth are often broken, it can be difficult to assess the relative length of the caps. The shafts of the teeth show distinct vertical ridges. Sometimes, the cap and the shaft are separated by a more or less distinct ridge. In our sample, we have attributed 36 teeth to this species, which were all loose teeth; jaws were not present.
The genus Saurichthys is also represented in our material by a small bony plate that we interpret on morphological grounds as being a pair of cranial roof elements (Fig. 2d). As it is only a fragment, yet shows a midline, it is impossible to decide whether we have frontals, parietals or dermopterotics (see e.g. Romano et al., Reference Romano, Kogan, Jenks, Jerjen and Brinkmann2012, fig. 3, for the appropriate configuration). The ornamentation of the surface consisting of closely arranged minute bumps leaves no doubt of the attribution to Saurichthys. Although the specific attribution of this cranial element cannot be ascertained on the basis of attached dental elements, we tentatively consider it to also be from S. longidens, as on the basis of the teeth this is the only recognised taxon in the sample. Size of the fragment: antero-posterior length 1.65 mm, total transverse width 1.75 mm.
Order Birgeriiformes Heyler, 1969
Family Birgeriidae Aldinger, 1937
Genus Birgeria Stensiö, 1919
Birgeria acuminata (Agassiz, Reference Agassiz1835)
(synonym: Severnichthys acuminatus (Agassiz, Reference Agassiz1835) partim)
(Fig. 2e–f)

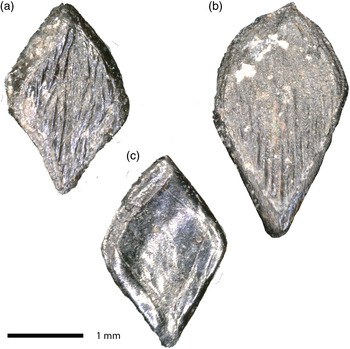
Figure 3. Gyrolepis albertii, scales. (a) WW-SP 156, (b) WW-SP 157, (c) WW-SP 158.
Sixteen teeth in the sample are attributed to Birgeria acuminata, on the basis of the following characters. The teeth are slightly curved, the lingual side being slightly concave and the labial side somewhat convex in side-view. If only caps are preserved, this may, however, be difficult or impossible to observe. The enameloid cap is mostly smooth on the labial side and striated on the lingual side, both sides being separated by a more or less distinct ridge. Due to these ridges, the cross-section of the teeth is somewhat biconvex or compressed, and not circular.
The teeth vary considerably in size. Such large variation was also reported in morphologically more complete material where partial dentitions could be observed instead of only loose teeth; e.g. by Romano et al. (Reference Romano, Jenks, Jattiot, Scheyer, Bylund and Bucher2017: fig. 4), Bürgin & Furrer (Reference Bürgin and Furrer1992: figs 2 and 3) and Schwarz (Reference Schwarz1970: fig. 20, a maxillary, and fig. 31, a fang tooth with surrounding ‘kleine und sehr kleine’ (small and very small) teeth).
Genus Gyrolepis Agassiz, Reference Agassiz1835
Gyrolepis albertii Agassiz, Reference Agassiz1835
A few dozen scales and scale fragments are preserved. Some show wear, probably as a result of an abrasive sedimentation process. There is a considerable size difference, but most are easily identifiable as Gyrolepis scales of the lozenge-shaped morphotype 2 sensu Landon et al. (Reference Landon, Duffin, Hildebrandt, Davies, Simms and Benton2017: fig. 6K, p. 367; see also Mears et al. (Reference Mears, Rossi, MacDonald, Coleman, Davies, Arias-Riesgo, Hildebrandt, Thiel, Duffin, Whiteside and Benton2016: fig. 10a–b, p. 491)), and some as the pear-shaped morphotype 4 sensu Landon et al. (Reference Landon, Duffin, Hildebrandt, Davies, Simms and Benton2017: fig. 6M, p. 367). Some of the worn scales show a concentric structure as of morphotype 3 sensu Landon et al. (Reference Landon, Duffin, Hildebrandt, Davies, Simms and Benton2017: fig. 6L, p. 367), but this configuration may be due to wear. No scales have a peg such as can be seen in morphotype 1 sensu Landon et al. (Reference Landon, Duffin, Hildebrandt, Davies, Simms and Benton2017: fig. 6J, p. 367) or morphotype S2 sensu Mears et al. (Reference Mears, Rossi, MacDonald, Coleman, Davies, Arias-Riesgo, Hildebrandt, Thiel, Duffin, Whiteside and Benton2016: fig. 10c–d and e–f, p. 491). The scales possess a ganoid enamel layer showing ridges running diagonally in the direction of the greatest length of the lozenge or pear. The striations may bifurcate near the centre of the scale, resulting in sometimes anastomosing ridges. Based on morphological and size similarity with scales described from the Rhaetian of the Penarth Group (e.g. Mears et al., Reference Mears, Rossi, MacDonald, Coleman, Davies, Arias-Riesgo, Hildebrandt, Thiel, Duffin, Whiteside and Benton2016; Landon et al., Reference Landon, Duffin, Hildebrandt, Davies, Simms and Benton2017), we identify the scales as belonging to Gyrolepis albertii (see Fig. 3).
Teeth of Gyrolepis somewhat resemble those of Saurichthys longidens, but differ from the latter in the relatively smaller acrodin cap not being larger than one-quarter (Nordén et al., Reference Nordén, Duffin and Benton2015) to one-third (Cross et al., Reference Cross, Ivanovski, Duffin, Hildebrandt, Parker and Benton2018) of the tooth, and by the absence of a prominent vertical striation of the shaft of the tooth, There may be a very subtle striation on the shaft, but it is far less obvious than in S. longidens. Furthermore, the teeth have a circular cross-section and are cone-shaped and curved (C-shaped) with an acute tip; sometimes they are slightly S-shaped. The small acrodin caps are often translucent and are always unornamented. In our sample, we have attributed 43 teeth to this species, making it the most abundant ray-finned fish in our sample of loose teeth. Although we realise that Gyropelis albertii was described on the basis of scales, we see no reason not to attribute our teeth to the same species, following, for example, Allard et al. (Reference Allard, Carpenter, Duffin and Benton2015) and Cross et al. (Reference Cross, Ivanovski, Duffin, Hildebrandt, Parker and Benton2018).
Furthermore, we have three morphotypes of unornamented actinopterygian teeth that are conical or bulbous and that could belong to either Semionotiformes (such as Lepidotus, Paralepidotus, Semiolepis or Serrolepis) or even Perleidiformes. One of our teeth has a lentil-shaped cross-section; the other twelve are conical. Four of these have a blunt tip and show a small wear facet most probably due to occlusion with an element from the opposing jaw. The remaining eight specimens have a more slender, pointed apex that is often somewhat translucent. It is difficult to attribute them with any certainty to a species, and often the waste-basket genus Lepidotus has been used to avoid giving no name at all. Thus, we tentatively name them cf. ‘Lepidotus’, realizing that they could well belong to other genera, such as the semionotiforms Paralepidotus Stolley, 1920 or Serrolepis Quenstedt, 1852.
Gnathostomata indet.
(Fig. 2m–n)
Finally, we have 19 gill raker teeth, long and slender elements that according to Duffin (Reference Duffin1998) and, for example, Cross et al. (Reference Cross, Ivanovski, Duffin, Hildebrandt, Parker and Benton2018) belong to the chondrichthyan Pseudocetorhinus pickfordi Duffin, Reference Duffin1998, but according to Landon et al. (Reference Landon, Duffin, Hildebrandt, Davies, Simms and Benton2017) to an unknown osteichthyan. Apparently, there is no consensus on the attribution of these small, needle-like teeth used for filter-feeding. Here, we refrain from assigning them to any taxon.
Discussion
Isolated teeth, especially those of actinopterygians, often pose a problem in identification. Large size differences in the teeth (e.g. the distinction between fangs and smaller teeth; Bürgin & Furrer, Reference Bürgin and Furrer1993), different positions in the jaws and ontogenetic size differentiation may result in the teeth originating from one individual fish or one single species showing a large variation. When found as loose elements, it may even be difficult to ascribe them with certainty to distinct taxa. What in fact we observe and describe are dental morphotypes that are then translated taxonomically into morphospecies.
Fossil fish taxa are ideally based on a suite of characters including cranial and postcranial morphology, number, size and position of the fins, the squamation and relative sizes of the animal. The morphology of (loose) dental elements, scales or dermal denticles is not always clearly described, and therefore they are not always unambiguously attributable to a certain taxon. The material available to us for the present study consists without exception of loose dental elements and some scales; bony cranial or postcranial material is lacking (with the exception of the Saurichthys skull fragment), as are more or less intact fishes. Hence, no correlation was possible between teeth on the one hand and other anatomical features on the other. We therefore based our identifications on comparison with material described in the above-mentioned literature. Comparison of our fauna with that from other European Rhaetian localities, especially those of the British Penarth Group (Korneisel et al., Reference Korneisel, Gallious, Duffin and Benton2015; Lakin et al. Reference Lakin, Duffin, Hildebrandt and Benton2016; Slater et al., Reference Slater, Duffin, Hildebrandt, Davies and Benton2016; Cavicchini et al., Reference Cavicchini, Heyworth, Duffin, Hildebrandt and Benton2018; Cross et al., Reference Cross, Ivanovski, Duffin, Hildebrandt, Parker and Benton2018) shows an overall resemblance in faunal composition, with the shark genera Lissodus and Rhomphaiodon, and the actinopterygians Gyrolepis, Saurichthys and Birgeria (the latter two often jointly referred to as Severnichthys) as the most abundant elements. See Table 1 for an overview.
Table 1. Faunal composition in number of dental elements of our sample from Winterswijk and selected British localities of the Rhaetian Penarth Group. Ref. 1: Cavicchini et al. (Reference Cavicchini, Heyworth, Duffin, Hildebrandt and Benton2018); ref. 2 : Cross et al. (Reference Cross, Ivanovski, Duffin, Hildebrandt, Parker and Benton2018); ref. 3: Slater et al. (2016); ref. 4: Lakin et al. (Reference Lakin, Duffin, Hildebrandt and Benton2016); ref. 5 : Korneisel et al. (Reference Korneisel, Gallious, Duffin and Benton2015). Decimals (ref. 3) represent partial examples of large teeth.

a ‘Severnichthys Birgeria-type’.
b ‘Severnichthys Saurichthys-type’.
The genus Rhomphaiodon was erected by Duffin (Reference Duffin1993) on account of its specific enameloid microscopic ultrastructure. Morphologically, however, there appeared to be no difference from teeth of the genus Hybodus, and subsequently other Hybodus species were incorporated into Rhomphaiodon, e.g. Hybodus minor Agassiz, 1837. We therefore choose to use the name Rhomphaiodon for the hybodont shark teeth.
The Severnichthys enigma
A vexing problem is the validity of the genus Severnichthys Storrs, Reference Storrs1994. This genus was described in order to encompass at least two different morphotypes that were originally identified as belonging to the two separate genera Birgeria and Saurichthys, but that were then considered to belong to a single species, for which Storrs (Reference Storrs1994) erected the taxon Severnichthys. In subsequent literature, these two morphotypes were labelled as ‘Severnichthys acuminatus (Birgeria acuminata-type)’ and ‘Severnichthys acuminatus (Saurichthys longidens-type)’, respectively (e.g. Duffin, Reference Duffin, Swift and Martill1999; Allard et al., Reference Allard, Carpenter, Duffin and Benton2015; Korneisel et al., Reference Korneisel, Gallious, Duffin and Benton2015; Nordén et al., Reference Nordén, Duffin and Benton2015; Lakin et al., Reference Lakin, Duffin, Hildebrandt and Benton2016; Mears et al., Reference Mears, Rossi, MacDonald, Coleman, Davies, Arias-Riesgo, Hildebrandt, Thiel, Duffin, Whiteside and Benton2016; Cross et al., Reference Cross, Ivanovski, Duffin, Hildebrandt, Parker and Benton2018).
Saurichthys and Birgeria are very different fish. Saurichthys is a very slender, garfish-like creature with an extremely elongate rostrum (Rieppel, Reference Rieppel1985; Romano et al., Reference Romano, Kogan, Jenks, Jerjen and Brinkmann2012; Werneburg et al, Reference Werneburg, Kogan and Sell2014; Maxwell et al., Reference Maxwell, Diependaal, Winkelhorst, Goris and Klein2016), while Birgeria is larger and possesses a strong and blunt rostrum (Schwarz, Reference Schwarz1970; Bürgin & Furrer, Reference Bürgin and Furrer1992). In the original description, Severnichthys was diagnosed with a ‘massive fused rostropremaxillary, subconical with a bluntly pointed tip’, which conforms to the morphology of Birgeria; and a heavily fragmented dentary ‘with vertically expanded posterior end, more so than in Birgeria or Saurichthys’ (Storrs, Reference Storrs1994). The mentioned type species was Severnichthys acuminatus (Agassiz, Reference Agassiz1835), formerly known as Birgeria acuminata.
Severnichthys, with its large and blunt snout, is morphologically a different fish from the strongly elongate and garfish-like Saurichthys. Interestingly, however, Storrs (Reference Storrs1994) listed Saurichthys longidens among the synonyms. In the suite of subsequent papers on the fishes from the Upper Triassic (Rhaetian) Penarth Group in the area of the Severn estuary (e.g. Duffin, Reference Duffin, Swift and Martill1999; Allard et al., Reference Allard, Carpenter, Duffin and Benton2015; Korneisel et al., Reference Korneisel, Gallious, Duffin and Benton2015; Nordén et al., Reference Nordén, Duffin and Benton2015; Lakin et al., Reference Lakin, Duffin, Hildebrandt and Benton2016; Mears et al., Reference Mears, Rossi, MacDonald, Coleman, Davies, Arias-Riesgo, Hildebrandt, Thiel, Duffin, Whiteside and Benton2016; Cross et al., Reference Cross, Ivanovski, Duffin, Hildebrandt, Parker and Benton2018), small actinopterygian teeth with acrodin caps were persistently identified as belonging to Severnichthys. No teeth from either Birgeria or Saurichthys were mentioned from these British localities; these were mere morphological ‘types’ within Severnichthys. The apparent absence of the genus Saurichthys itself is difficult to understand, as Saurichthys was an abundant taxon in Triassic fish assemblages (e.g. Duffin & Gazdzicki, Reference Duffin and Gaždzicki1977; Rieppel, Reference Rieppel1985; Romano et al., Reference Romano, Kogan, Jenks, Jerjen and Brinkmann2012; Werneburg et al., Reference Werneburg, Kogan and Sell2014; Maxwell et al., Reference Maxwell, Diependaal, Winkelhorst, Goris and Klein2016).
Both ‘types’ were originally described by Agassiz (Reference Agassiz1835) as Saurichthys longidens and Saurichthys acuminatus, respectively, based on material from the British Rhaetian at Aust, near Bristol. Savage & Large (Reference Savage and Large1966) transferred the latter species to the genus Birgeria: B. acuminata; they depicted a large and stout lower jaw that does not comply with the slender and elongate morphology of Saurichthys as now understood (see Rieppel, Reference Rieppel1985). This justifies the attribution to Birgeria. See also Bürgin & Furrer (Reference Bürgin and Furrer1993) for a discussion on the Birgeria vs Saurichthys problem.
Subsequently, Storrs (Reference Storrs1994) merged both taxa into Severnichthys acuminatus, which genus was diagnosed (as far as the teeth are concerned) by the following: ‘Very large recurved acrodont lingual tusks caudad, striated; coarsely ribbed at base to present labyrinthine folding in transverse section; folds lost in midsection of tooth; translucent enameloid cap sometimes removed by wear. Typical lingual dentition smaller and often sharper; less coarsely striated and recurved; more extensive enameloid cap; more pronounced carinae and cingulum.’
The two morphological types of Severnichthys teeth are difficult to separate as there tends to be an overlap in characters, especially when no complete jaws are available. Sometimes, the cap and the shaft are separated by a more or less distinct ridge. According to Cross et al. (Reference Cross, Ivanovski, Duffin, Hildebrandt, Parker and Benton2018), this is the case with the Birgeria-type teeth, while Nordén et al. (Reference Nordén, Duffin and Benton2015) mention this ridge as being present in the Saurichthys-type teeth. Korneisel et al. (Reference Korneisel, Gallious, Duffin and Benton2015) added to the confusion by naming Severnichthys acuminatus in the text, and Severnichthys longidens in the figure legend, although this might be due to an overlooked typing error.
To summarise, we consider the attribution of teeth from two rather different genera to Severnichthys to be a mistake. The apparent morphological similarities of the teeth can be explained as a convergency. There seems to be no other justification for the creation of Severnichthys, a conclusion also reached by Tintori & Lombardo (Reference Tintori, Lombardo and Tanner2017). We therefore propose to suppress Severnichthys as a separate genus; it is a nomen dubium. Loose teeth attributed to Severnichthys seem to belong to either Birgeria or Saurichthys; the cranial material used for the original description by Storrs (Reference Storrs1994) appears to conform to the morphology of Birgeria.
Conclusions
The fish fauna from the Rhaetian sediments found in the subrosion pipe in the Winterswijk quarry is very similar to the various faunas described from other localities known in Northwestern Europe, especially those from the British Triassic of the Penarth Group. Both chondrichthyan and osteichthyan teeth and scales are described. The most abundant taxa are the hybodontiform shark Lissodus minimus, the synechodontiform shark Rhomphaiodon minor and the actinopterygian Gyrolepis albertii. Other abundant bony fishes are Saurichthys longidens and Birgeria acuminata. The teeth of the latter two taxa were described under the name Severnichthys acuminatus by several British authors, but the genus Severnichthys is here considered a nomen dubium. In order to render taxonomy less complicated, its use should be avoided.
Acknowledgements
We are grateful to Adiël Klompmaker (now at the University of Alabama Museum of Natural History) who collected the material after the subrosion pipe was discovered; to our student Bart de Lange who did the washing, picking and initial identification of the material in the framework of his BSc thesis; and to Ilja Kocken (Utrecht) and Anne Schulp (Utrecht and Leiden) for technical assistance. Toni Bürgin (St-Gallen) and two anonymous reviewers made helpful remarks and suggestions that greatly improved the manuscript.