Introduction
A common conservation action for threatened species is to reintroduce them to areas of former habitat after the presumed threats have been mitigated (Seddon et al., Reference Seddon, Griffiths, Soorae and Armstrong2014). However, with faunal restoration a short- to medium-term view is often taken, focusing on manageable and achievable targets such as the removal of predators, the restoration of appropriate fire regimes or the maintenance or restoration of genetic vigour (Seddon et al., Reference Seddon, Strauss, Innes, Ewen, Armstrong, Parker and Seddon2012). Management actions are rarely targeted at longer-term threats that may render habitat unsuitable, such as climate change (Thomas, Reference Thomas2011; Stein et al., Reference Stein, Glick, Edelson and Staudt2014). Climate change, either directly, indirectly or in synergy with land-use change, is recognized as a major threat to biodiversity (Steffen et al., Reference Steffen, Richardson, Rockström, Cornell, Fetzer and Bennett2015) and its impacts are particularly pertinent in Mediterranean-type climates (Araújo et al., Reference Araújo, Alagador, Cabeza, Nogués-Bravo and Thuiller2011). Faunal restoration projects in these and other areas subject to rapid climate change would therefore be remiss if they did not attempt to quantify the future suitability of habitat (Thomas, Reference Thomas2011; Molloy et al., Reference Molloy, Davis and Van Etten2014).
Species distribution model algorithms have the capacity to determine a species’ potential distribution and predict how this will change in response to probable changes in predictive variables (Elith & Leathwick, Reference Elith and Leathwick2009; Pliscoff & Fuentes-Castillo, Reference Pliscoff and Fuentes-Castillo2011; Molloy et al., Reference Molloy, Davis, Dunlop and van Etten2017) but they are rarely used to inform reintroduction into parts of the former distribution of a target species or ecological community (Osborne & Seddon, Reference Osborne, Seddon, Ewen, Armstrong, Parker and Seddon2012). For example, if we reintroduce a species to an area where it is locally extinct, and mitigate those factors that we often assume to be the cause of this extinction, do we adequately consider the following questions? (1) Has a changing climate played a direct or indirect part in this extinction? (2) Given a changing climate, will this species be able to persist in this location, with or without the mitigation of other recognized threats? (3) In our attempts to mitigate climate change impacts are we potentially facilitating unforeseen threats (Brambilla et al., Reference Brambilla, Pedrini, Rolando and Chamberlain2016)?
Species distribution models use environmental data from known locations of a species to predict places where that species could potentially occur within landscapes or regions (Booth et al., Reference Booth, Nix, Busby and Hutchinson2014). They have been used to identify critical habitats for species with greatly reduced distributions (Jetz & Freckleton, Reference Jetz and Freckleton2015), facilitate identification of potential reintroduction sites for species based on known habitat requirements (Adhikari et al., Reference Adhikari, Barik and Upadhaya2012), identify potential areas for assisted colonization (Mitchell et al., Reference Mitchell, Hipsey, Arnall, McGrath, Tareque and Kuchling2012) and predict the movement of invasive species across landscapes under various scenarios (Kearney et al., Reference Kearney, Phillips, Tracy, Christian, Betts and Porter2008; Elith et al., Reference Elith, Kearney and Phillips2010). Spatially explicit probability of presence, or prediction of occurrence maps, generated using species distribution model algorithms, have been used to inform conservation planning and habitat management at both coarse and fine scales. Although often criticized for being phenomenological and for their inability to account explicitly for many ecological processes, species distribution models remain a powerful tool to learn about past, current and future species distributions, when limitations and assumptions are acknowledged (Engler et al., Reference Engler, Stiels, Schidelko, Strubbe, Quillfeldt and Brambilla2017). Consequently, we maintain that species distribution models can be used to guide or prioritize future survey efforts and aid in assessing the conservation status of target species.
Although applications of species distribution models to inform translocations under projected future climate scenarios are relatively rare, a study by Fortini et al. (Reference Fortini, Kaiser, Vorsino, Paxton and Jacobi2017) used this approach to identify potential translocation areas with suitable bioclimatic variables for critically imperilled Hawaiian birds. A number of studies have focused on refining the use of species distribution models in this context. For example, Payne and Bro-Jørgensen (Reference Payne and Bro-Jørgensen2016) emphasized the need to consider spatio-temporal dynamics within predicted climatically suitable ranges, and provided a framework for more refined identification of suitable translocation strategies. Similarly, Hällfors et al. (Reference Hällfors, Liao, Dzurisin, Grundel, Hyvärinen and Towle2016) addressed the need to consider local adaptation and population connectedness in species distribution models informing translocations. However, such information is not always available, particularly for extremely rare species that may be restricted to a single population. Furthermore, it is recognized that a common shortfall of species distribution models is a failure to recognize and incorporate the adaptive capacity of target species into the modelling process (Engler et al., Reference Engler, Stiels, Schidelko, Strubbe, Quillfeldt and Brambilla2017). This can lead to an overestimation of climate change impacts on target species (Bay et al., Reference Bay, Harrigan, Underwood, Gibbs, Smith and Ruegg2018). Here we have opted to take a cautious approach and disregard adaptive capacity in our translocation planning, on the assumption that such a small population would be lacking in physiological and genetic diversity (Hoffmann & Sgrò, Reference Hoffmann and Sgrò2011).
We present a case study of the application of species distribution models to investigate how knowledge of future habitat under predicted climate change scenarios could inform conservation translocations/reintroductions. We focus on a Critically Endangered bird in south-western Australia, the western ground parrot Pezoporus flaviventris (although this species has not yet been assessed for the IUCN Red List it has been categorized as Critically Endangered by the Australian Government; Australian Government, 2018). We constructed species distribution models to determine historical (realized) and future population distributions of this bird, a terrestrial parrot endemic to the South-West Australian Floristic Region. This region is a global biodiversity hotspot, with high plant species richness and endemism, and highly modified landscapes (Myers et al., Reference Myers, Mittermeier, Mittermeier, da Fonseca and Kent2000; Hopper & Gioia, Reference Hopper and Gioia2004).
While acknowledging that retaining and enhancing the existing population remains the highest priority, the current restriction of ground parrots to a single population in a small area within a high fire-risk environment, where ongoing fire and predator control remains problematic, leaves this population in a tenuous position. Furthermore, until this study was commissioned, the potential impacts of climate change were unknown but assumed to be potentially disastrous. Consequently, the recovery team charged with the conservation of this species decided that the establishment of additional populations in the wild is an essential step to reduce extinction risk. To that end, a translocation proposal has been approved and small numbers of birds have been moved to Perth Zoo. No wild-to-wild translocation has yet been attempted (Burbidge et al., Reference Burbidge, Comer, Lees, Page and Stanley2016).
Successful translocation of this species requires the identification of areas of suitable habitat, with consideration given to reintroduction into suitable previously occupied habitat following threat reduction activities such as predator control and the implementation of suitable fire regimes. However, to date, there has been a limited focus on how climate change has affected populations and will continue to affect them in the future. Given the rapid change in climate in this region (CSIRO & BoM, 2017) there is an urgent need to understand current and future climate impacts before suitable reintroduction sites can be nominated.
Our objective was to construct a species distribution model for the western ground parrot and then model predicted climate conditions within the current and former distribution of the species. This information will be used to inform the selection of translocation sites and conservation management actions for this threatened species.
Study area
The area modelled is the South-west Australia Floristic Region (Fig. 1; Hopper & Gioia, Reference Hopper and Gioia2004), which encompasses all known occurrences of western ground parrots. Although much of the region is, or was, woodland, there are extensive, mostly near-coastal areas of heathland on predominantly sandy soils that are potentially suitable habitat for the parrots. The area has a Mediterranean-type climate characterized by cool, wet winters and hot, dry summers, with fire being a recurring disturbance and driver of ecosystem dynamics (Yates et al., Reference Yates, Elith, Latimer, Le Maitre, Midgley, Schurr and West2010). Annual rainfall in these heathlands varies (c. 400–1,400 mm) but most areas are becoming hotter and drier (CSIRO & BoM, 2017).

Fig. 1 Presence records of the western ground parrot Pezoporus flaviventris (Department of Parks and Wildlife, 2014; S. Comer et al., unpubl. data), in the South-west Australian Floristic Region (SWAFR).
Methods
Species description and threats
The western ground parrot is a medium-sized bird weighing 84–108 g, with a wing length of 123–144 mm. Because of its cryptic mottled green colouring and the fact that it spends much of its time on the ground in dense heathlands, it is seen only rarely. Despite the substantial and increasing efforts invested in the management of this species it has continued to decrease in range and abundance, with a dramatic decline since the 1980s (Burbidge et al., Reference Burbidge, Comer, Lees, Page and Stanley2016).
Although estimating the abundance of such an elusive species is difficult, various attempts have been made to estimate its total population size. Determining exact numbers is challenging, but there is a consensus amongst researchers and conservation managers that decline is ongoing, and the total population is currently estimated to comprise < 150 individuals (Burbidge et al., Reference Burbidge, Comer, Lees, Page and Stanley2016). According to Burbidge et al. (Reference Burbidge, Comer, Lees, Page and Stanley2016), the drivers of this decline are introduced predators, especially feral cats Felis catus (Doherty et al., Reference Doherty, Davis, van Etten, Algar, Collier and Dickman2015), inappropriate fire regimes (Department of Parks and Wildlife, 2014), habitat loss and fragmentation (Hobbs, Reference Hobbs2001), and phytophthora dieback (Davis et al., Reference Davis, Valentine, Craig, Wilson, Bancroft and Mallie2014; Phytophthora cinnamomi is a fungal pathogen that kills many of the plant species that provide habitat for the western ground parrot).
The degree to which a rapidly changing climate has affected this species has been studied previously (Gibson et al., Reference Gibson, Barrett and Burbidge2007), but the development of new modelling tools, geographical information system (GIS) datasets and climate change models facilitates construction of more accurate and effective species distribution models. These tools also allow for this modelling process to be tailored towards addressing the conservation needs of the western ground parrot.
Presence data
All presence data for modelling were derived from a database of historical and current records of the western ground parrot maintained by the Western Australian Department of Biodiversity, Conservation and Attractions and the South Coast Threatened Birds Recovery Team (S. Comer et al., unpubl. data). This dataset includes c. 21,000 records, both targeted and opportunistic, recorded during 2001–2015. These presence records were sourced from the Western Australian Museum, published and unpublished literature, and fieldwork carried out under the auspices of the recovery team. Although some of these data are available through NatureMap (Department of Parks and Wildlife, 2007) and the Atlas of Living Australia (Flemons et al., Reference Flemons, Raymond, Brenton and Belbin2010), detailed current records are considered to be sensitive because of the species’ threatened status, and are not publicly available. Applications to access these data should be directed through the Western Australian Department of Biodiversity, Conservation and Attractions.
Variable selection
All GIS datasets used in the analyses are listed in Supplementary Material 1. For optimum efficiency, and to minimize multicollinearity and prevent overfitting, the suite of variables used should be kept compact (preferably ≤ 10) and comprise those variables that can best define the potential distribution of the target species or community (Beaumont et al., Reference Beaumont, Hughes and Poulsen2005; Hijmans, Reference Hijmans2012). To accomplish this we reviewed the literature on the western ground parrot and sought expert opinion from observers familiar with the species. All variable datasets were downloaded at, or converted to, a pixel resolution of 30 s (c. 1 km2) in the WGS84 datum, and clipped to the South-west Australia Floristic Region. The WGS84 datum was applied to all datasets, which were imported into an ASCII format grid (facilitating the creation of stacks) using ArcGIS 10.4 (ESRI, Redlands, USA).
We adopted a two-stage process to identify an appropriate suite of predictive variables. The first stage used a series of statistical tests (described below) to halve the number of potential variables and thus limit multicollinearity between model variables and remove those variables whose contribution to the model was low or counterproductive. We then used a stepwise elimination process to identify a final suite of predictive variables suitable for use in subsequent modelling.
To reduce multicollinearity between continuous variables we calculated both the Pearson and Spearman rank correlation coefficients between each pair of variables at western ground parrot presence points using the psych package in R v.3.5.1 (Revelle, Reference Revelle2014). For each pair of highly correlated variables (i.e. the Pearson correlation coefficient is > 0.70; Tsoar et al., Reference Tsoar, Allouche, Steinitz, Rotem and Kadmon2007) we selected only the single variable deemed to be the most relevant for identifying western ground parrot presence based on ecological relevance and expert opinion (Phillips & Dudík, Reference Phillips and Dudík2008). Categorical variables were tested for association with each other using Pearson's χ 2 test. Similarly, we tested relationships between categorical and continuous variables using linear models, with a 0.05 level of significance (Agresti & Kateri, Reference Agresti and Kateri2011).
The final selection was undertaken through a stepwise elimination process using the biomod2 package in R (Thuiller et al., Reference Thuiller, Georges and Engler2013), as described below. In this process we examined the contribution of each remaining variable and the consequences of its omission over a series of model runs. Our target for this process was a consistent area under the receiver operating characteristic curve (AUC; Swets, Reference Swets1988) value > 0.9. In this way we developed minimum sets of variables capable of producing realistic models that retained high test statistics (Elith et al., Reference Elith, Phillips, Hastie, Dudík, Chee and Yates2011).
Ensemble modelling
For our species distribution model we adopted an ensemble modelling process using the biomod2 package. Using this package we were able to apply a number of algorithms to our data, evaluate the output of each algorithm, remove under-performing algorithms, and combine the outputs of individual algorithms into a weighted mean species distribution model based on the performance of individual algorithms (Grenouillet et al., Reference Grenouillet, Buisson, Casajus and Lek2011; Thuiller et al., Reference Thuiller, Georges and Engler2013). The initial suite of algorithms tested was composed of generalized linear model, generalized boosted model, generalized additive model, classification tree analyses, artificial neural network, surface range envelope, flexible discriminant analysis, multiple adaptive regression splines, random forest, MAXENT.Phillips and MAXENT.Tsuruoka (Thuiller et al., Reference Thuiller, Georges and Engler2016).
We undertook this process twice. Firstly, we used bioclimatic data alone to model how predicted climate change would affect the population distribution of this species (Hijmans & Graham, Reference Hijmans and Graham2006; Booth et al., Reference Booth, Nix, Busby and Hutchinson2014). We then repeated the process using landscape variables alone. We could thus examine the contribution of those variables that help define habitat but would not, unlike bioclimatic variables, change dramatically over the modelled period. This provided a reference distribution, which, through comparison with the bioclimatic species distribution models, would provide us with a better understanding of how predicted climate change scenarios would affect the distribution of the western ground parrot.
Models were run for 100 iterations. In running all ensemble models, a random 30% of presences were used to calibrate the model and 70% of presences were withheld for testing. In the modelling process presences become binary; i.e. if any presences have occurred within a c. 1 km2 pixel, that pixel is considered to be a single presence regardless of the number of presences that may have occurred within that pixel. This reduced our original c. 20,000 records to 404 presences for the modelling process.
All outputs of all algorithms were evaluated with the true skill statistic (Allouche et al., Reference Allouche, Tsoar and Kadmon2006) and AUC. A weighting was given to each algorithm, based on AUC performance, and all model outputs were combined to produce a weighted mean species distribution model, which we used as our biomod2 output for both the bioclimatic and non-bioclimatic models. We note that AUC values can be problematic and that there is no golden rule to follow in their application (Lobo et al., Reference Lobo, Jiménez-Valverde and Real2008). However, during the course of this exercise we found this to be a consistent and effective metric.
To compensate for a lack of true absences, and possible restricted sample bias (Phillips, Reference Phillips2008), pseudo-absences were generated and used in all runs of all models. These took the form of 10,000 points selected randomly across the study area (Barbet-Massin et al., Reference Barbet-Massin, Jiguet, Albert and Thuiller2012). These were created with the biomod2 pseudo-absence function (Thuiller et al., Reference Thuiller, Georges and Engler2013). This approach has been shown to be an effective method of minimizing the restricted sample bias (Fourcade et al., Reference Fourcade, Engler, Rödder and Secondi2014), particularly when, as in this case, AUC values are used as the evaluation metric.
The bioclimatic datasets used in our modelling include historical baseline data (WorldClim 1.4, 1960–1990 means) and future scenarios (CMIP5 global climate models, as given by the Access 1.0, CNRM-CM5, HadGEM2-ES and MPI-ESM-LR models; Taylor et al., Reference Taylor, Stouffer and Meehl2012). These models were chosen for their high performance in Australian regional tests (Moise et al., Reference Moise, Wilson, Grose, Whetton, Watterson and Bhend2015). We performed future projections for 2050 and 2070 using the 4.5 and 8.5 representative concentration pathways, respectively. These pathways were chosen because studies indicate that the 2.6 (low) scenario is highly unlikely (Kim et al., Reference Kim, Webster and Curry2012; Rogelj et al., Reference Rogelj, den Elzen, Höhne, Fransen, Fekete and Winkler2016), leaving the 4.5 and 8.5 scenarios as the most probable minimum and maximum scenarios of the pathways chosen. Projections were made for 2050 and 2070 because it was assumed that more robust climate modelling would be available after 2070, and scenarios earlier than 2050 may not provide an adequate indication of climate change impact on the western ground parrot.
As our models were required to predict for places and times not sampled in the training data, we recognize the need to measure the similarity between the new environments and those in the training sample. We did this by using multivariate environmental similarity surface plots, following the methodology of Elith et al. (Reference Elith, Kearney and Phillips2010).
Application
The results of this exercise will inform the conservation management of the western ground parrot. The recovery team (a group of conservationists with expertise on the habitat requirements of this species) will consider predicted bioclimatic suitability along with other relevant factors, including vesting, size, connectivity, historical use by this species, floristics and other management constraints, to determine the most suitable site for translocation.
Results
Selected variables
From the broad suite of variables tested (Supplementary Material 1) we derived a final suite of variables with acceptable levels of covariance for use in both the bioclimatic and non-bioclimatic species distribution models (Table 1). Correlations between variables for both models are provided in the pairs-panel plots in Supplementary Material 2.
Table 1 Variables used in bioclimatic and non-bioclimatic species distribution models, with their contributions and importance as determined by the MaxEnt algorithm.

* Variable importance for each algorithm in each run is given in Supplementary Material 2.
Ensemble models
We selected the best performing modelling algorithms for our ensemble model. Six algorithms were chosen for their ability to produce high receiver operating characteristic and true skill statistic values consistently. The algorithms used in both the bioclimatic and non-bioclimatic species distribution models were classification tree analysis, generalized additive model, generalized boosted model, generalized linear model, multiple adaptive regression splines and MAXENT.Phillips (Thuiller et al., Reference Thuiller, Georges and Engler2016). Test scores for all algorithms are in Supplementary Material 2.
Our baseline bioclimatic model was projected with four scenarios (i.e. medium and high emission scenarios for both 2050 and 2070 timeframes) for each of four global climate models, yielding 16 future outcomes. For display purposes we combined our future global climate model outputs (in a GIS) to produce mean species distribution models for each of these scenarios. The site evaluation process involved a rigorous interrogation of individual model outputs.
The non-bioclimatic species distribution model (Fig. 2a) and the baseline bioclimatic species distribution model (Fig. 2b) conform strongly with each other and with the presence records (Fig. 1). However, there are some major differences between these two models; by restricting the non-bioclimatic potential distribution to remnant vegetation we obtained a more constrained, and therefore realistic, estimate of available habitat for the western ground parrot. Thus a major difference was apparent between a bioclimatically defined potential distribution and a distribution constrained by landscape features and modifications. There was a strong similarity between the species distribution models created from climate change models. This indicates that many of the areas identified as potential distribution by the non-bioclimatic model should remain as potential distribution, albeit with varying bioclimatically defined habitat value (Fig. 2c,d; Table 2). This conclusion was supported by the consistency between model outputs and multivariate environmental similarity surface plots (Fig. 3).
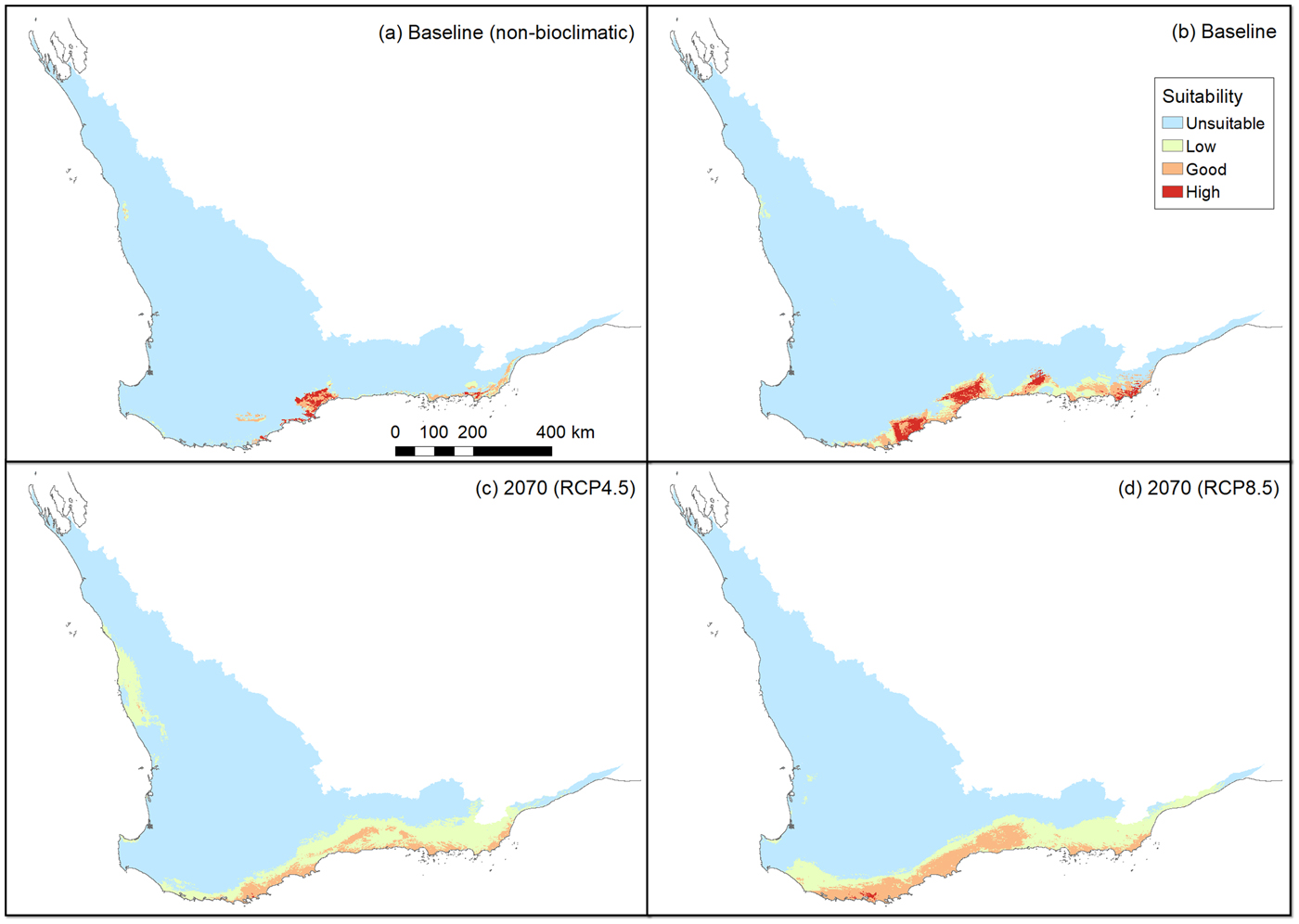
Fig. 2 Habitat suitability for the following scenarios: (a) non-bioclimatic species distribution model output identified using an equal sensitivity specificity cut-off (Jiménez-Valverde, Reference Jiménez-Valverde2012), (b) the baseline bioclimatic model (i.e. current potential distribution), (c) the bioclimatic model for RCP (relative concentration pathway) 4.5 (medium greenhouse gas emissions) in 2070, (d) the bioclimatic model for RCP 8.5 (high greenhouse gas emissions) in 2070.
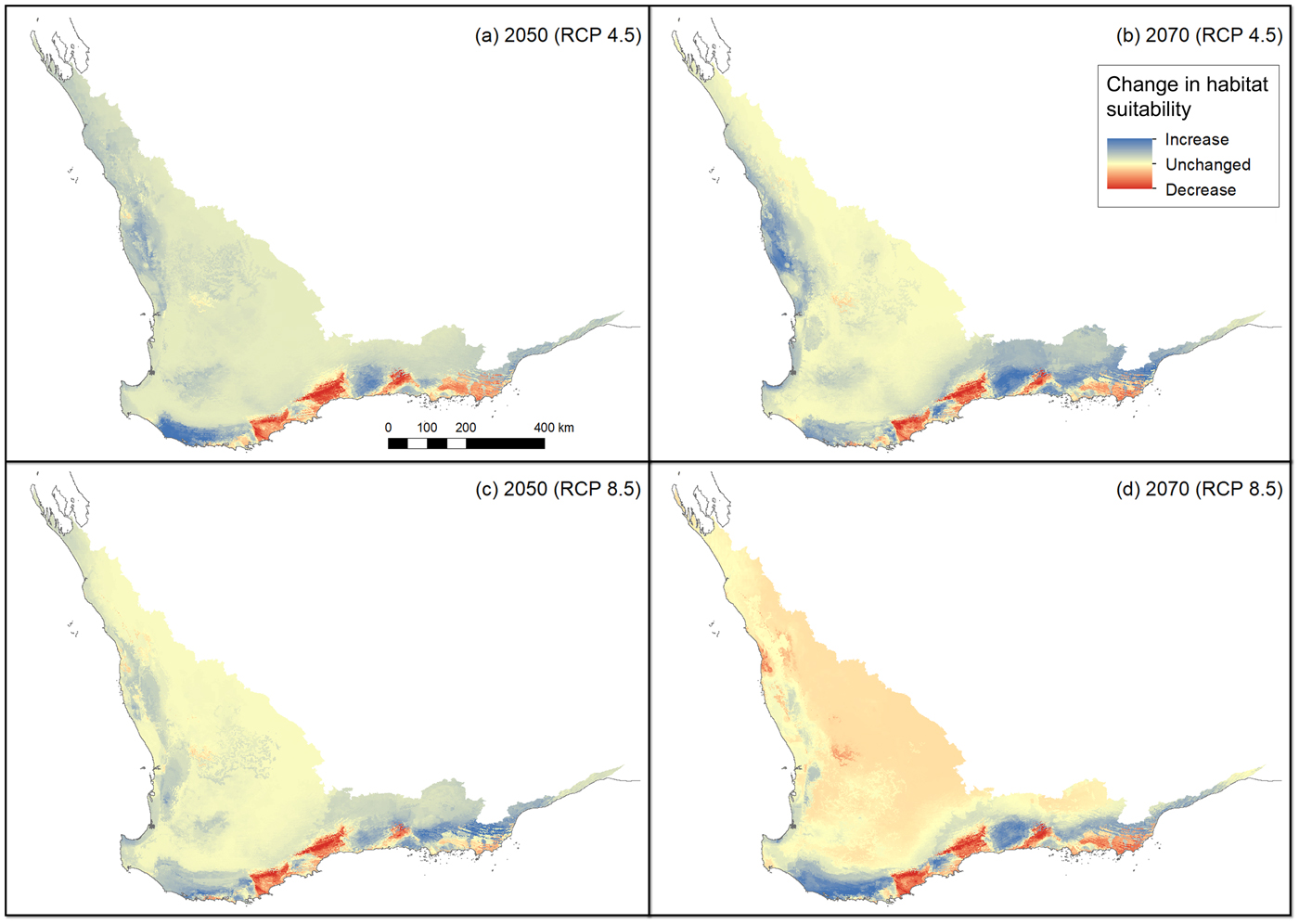
Fig. 3 Changes in habitat suitability in comparison to the baseline model (Fig. 2b) using all variables in a multivariate environmental similarity surface (Elith et al., Reference Elith, Kearney and Phillips2010) for the following scenarios: (a) median projection change for RCP 4.5 in 2050, (b) median projection change for RCP 4.5 in 2070, (c) median projection change for RCP 8.5 in 2050, (d) median projection change for RCP 8.5 in 2070.
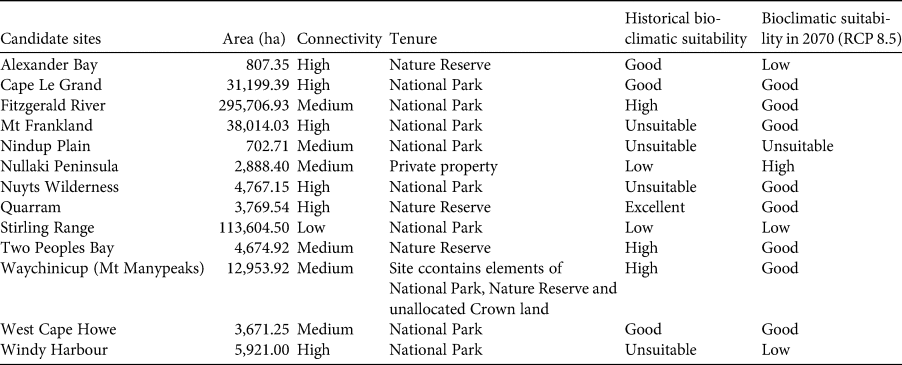
Table 2 Characteristics of candidate sites for translocation of the western ground parrot Pezoporus flaviventris, and a comparison of the historical bioclimatic suitability with the mean predicted suitability for these sites under the RCP 8.5 scenario in 2070. Suitability is based on the mean quartile value for the potential release site (Fig. 4).
Many of the potential release sites for the western ground parrot were predicted to remain in the species’ bioclimatic potential distribution (Fig. 4). Thus a suite of bioclimatically secure potential release sites was identified, which can be reviewed and prioritized for management action. We note a preference for sites within conservation estates, as these ensure secure tenure and allow the freedom to undertake fire and pest control measures as required for synergistic management outcomes.
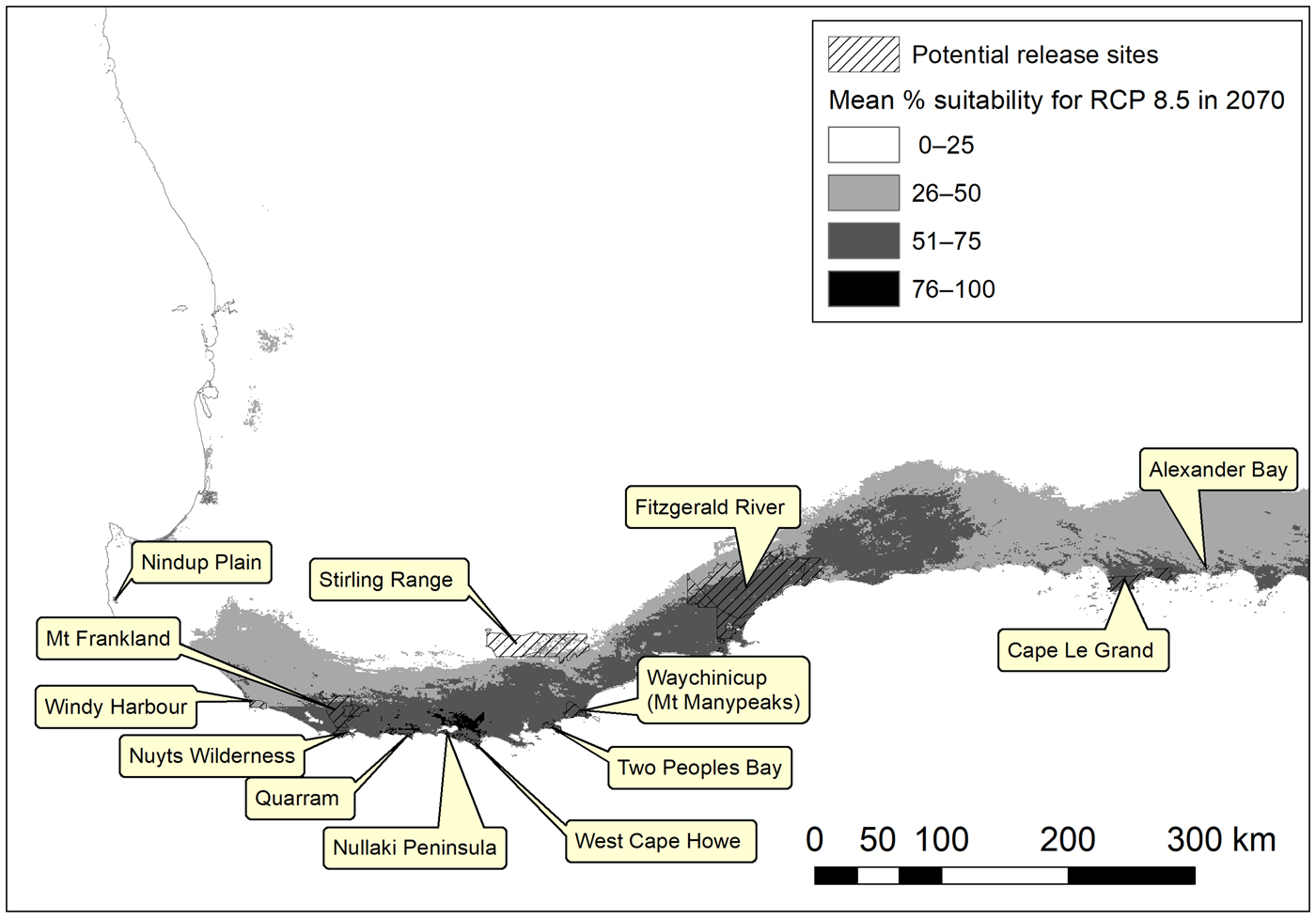
Fig. 4 Potential release sites for the western ground parrot in the South-west Australian Floristic Region overlain on the bioclimatic habitat suitability model for RCP 8.5 in 2070 (Fig. 2d).
Utilizing species distribution models, the recovery team was able to identify the most bioclimatically appropriate sites for reintroduction under future climate scenarios. Of 13 sites considered by the recovery team to be potentially suitable for translocation, four were ruled out immediately because their suitability was graded as ‘low’ or ‘unsuitable’. The suitability of eight sites was rated as ‘good’ and one as ‘high’, providing additional options for future translocation (Table 2).
Discussion
Selecting a suitable reintroduction site for western ground parrots requires assessment of all factors believed to be essential, and on the basis of this assessment sites will be prioritized and additional management actions commenced. The recovery team will consider not only resilience to climate change as a key parameter (e.g. Nullaki Peninsula), but also areas of suitable habitat within patches, security of tenure, and capacity to manage and respond to threats. With such low numbers of the parrots remaining in the wild, we believe that climate change impacts are but one, albeit important, factor in the decision-making process (Molloy et al., Reference Molloy, Davis and Van Etten2016). Factoring probable climate change impacts into planning for reintroductions of ground parrots is essential for maximizing the probability of long-term success.
Addressing key knowledge gaps regarding the condition or characteristics of habitat in translocation sites is a key element of the planning process. Although the suitability of many habitat characteristics (e.g. food resources and predator impacts) can be assessed in short time frames, the quantification of bioclimatic impacts over an extended time period is essential in assessing the potential suitability of sites over extended temporal scales. This is particularly important in justifying the significant cost of translocation, both in a monetary sense and in rationalizing the sourcing of individuals from already small populations.
Although meta-studies often find that climate change is a contributing factor in localized extinctions, few studies attribute such extinctions to climate change alone (Brook et al., Reference Brook, Sodhi and Bradshaw2008; Cahill et al., Reference Cahill, Aiello-Lammens, Fisher-Reid, Hua, Karanewsky and Ryu2013). Our research supports this finding in that, although our models show a contraction in potential distribution towards the south-west of the study region, they also show that relatively few (4 out of 13) previously inhabited areas have become, or are likely to become, bioclimatically unsuitable for the western ground parrot.
In light of our findings we can hypothesize that climate change may be a significant causal factor in the decline of the western ground parrot but is not the primary cause of decline. It is likely that climate change, when compounded with known impacts such as inappropriate fire regimes, habitat loss and predation by cats and foxes (Burbidge et al., Reference Burbidge, Rolfe, McNee, Newbey and Williams2007), may have contributed to the loss of populations and may continue to do so unless these impacts are mitigated. To undertake a future test of this hypothesis and to minimize the risk to western ground parrots, we recommend the use of surrogate species to test the effectiveness of our management actions before reintroducing this species to selected sites. For example, monitoring populations of species that are susceptible to introduced predators, such as the southern brown bandicoot Isoodon obesulus, can provide an indication of the effectiveness of predator control or exclusion actions. Similarly, monitoring a species with similar vegetation structural requirements, such as the tawny-crowned honeyeater Glyciphila melanops, may provide an indication of a suitable fire regime. Furthermore, the same impacts that are believed to have led to the loss of western ground parrot populations have also been implicated in declines in other species within the same landscapes. Through managing such impacts in appropriately selected sites for reintroduction of the western ground parrot, we therefore have the potential to simultaneously increase habitat value for other species of high conservation priority.
Through developing a series of species distribution models we have been able to address the eight considerations to guide the successful translocation of wildlife, as outlined by Osborne and Seddon (Reference Osborne, Seddon, Ewen, Armstrong, Parker and Seddon2012). In doing so we have attempted to demonstrate the best-practice application of bioclimatic modelling to conservation management, and we offer four recommendations for conservation practitioners: (1) There can be strong variation between climate change models and the outputs of various algorithms; use multiple, tested climate change models and algorithms to seek the most probable outcomes. (2) Bioclimatic factors cannot inform a successful translocation unless logistical, practical and ecological considerations are also addressed. (3) Incorporate expert ecological opinion into the design, evaluation and application of species distribution models. (4) Remember that the purpose of the species distribution model is to inform expert opinion, not to replace it.
Through the modelling we were able to identify sites of, in a bioclimatic sense, historical and current habitat for the western ground parrot, and that are predicted to remain so into the foreseeable future. We were able, with an acceptable level of certainty, to identify sites where factors other than climate change have been, either directly or indirectly, the most probable cause for the loss of western ground parrot populations. We therefore hypothesize that with the identification and management of these factors (e.g. habitat loss, predation by introduced predators, and fire), these areas can be made western ground parrot habitat once again, and managed in this capacity into the future. In this way conservation managers can develop a more efficient and cost-effective approach to threatened species management.
Acknowledgements
Data collation was supported by the Western Australian Department of Biodiversity, Conservation and Attractions (DBCA). Funding partners that have supported this work include the Western Australian State Natural Resource Management Program, South Coast Natural Resource Management Inc., the Commonwealth Biodiversity Fund, and the Friends of the Western Ground Parrot. We thank the South Coast Threatened Birds Recovery Team and project staff who provided expert opinions to guide this work, and all who have contributed to survey efforts for the western ground parrot. Data analysis and modelling were supported by DBCA and Edith Cowan University.
Author contributions
Lead author and species distribution modeller: SWM; project lead, study conception, fieldwork and provision of unpublished data: SC and AHB; assistance with data analysis and project administration: RAD. All authors contributed to writing and editing and approved the final text.
Conflicts of interest
None.
Ethical standards
All research undertaken complies with the Oryx Code of Conduct for authors. Fieldwork for this project was undertaken under Edith Cowan University animal ethics approval #15988 and Department of Parks and Wildlife animal ethics approval 2015/41.










