Introduction
Parasitic nematodes detrimentally impact the health of humans, wildlife, livestock and domestic animals (Cole and Viney, Reference Cole and Viney2018; Garcia-Bustos et al., Reference Garcia-Bustos, Sleebs and Gasser2019), and cause significant damage to agricultural crops (Jones et al., Reference Jones, Haegeman, Danchin, Gaur, Helder, Jones, Kikuchi, Manzanilla-López, Palomares-Rius, Wesemael and Perry2013). Infections by these parasites are non-trivial; an estimated ~1.5 billion (Garcia-Bustos et al., Reference Garcia-Bustos, Sleebs and Gasser2019) or as many as 3.5 billion (Iqbal and Jones, Reference Iqbal, Jones, Thomas, Murray and Murphy2017) people are infected with nematodes worldwide, and the impacts of infection in crops and livestock is enormous, resulting in the loss of billions of USD annually (Jasmer et al., Reference Jasmer, Goverse and Smant2003; Nicol et al., Reference Nicol, Turner, Coyne, den Nijs, Hockland, Maafi, Jones, Gheysen and Fenoll2011). Estimates of the number of species of helminths parasitizing vertebrates vary greatly, ranging from 75 000 (Poulin and Morand, Reference Poulin and Morand2004) to more than 300 000 (Dobson et al., Reference Dobson, Laffery, Kuris, Hechinger and Jetz2008). The checklist of human parasites (Ashford and Crew, Reference Ashford and Crew2003) included over 300 helminth species, of which 114 were nematodes. Human infection by parasitic nematodes is closely associated with economic status and disproportionately affects the poor, especially in tropical/subtropical and developing countries (Iqbal and Jones, Reference Iqbal, Jones, Thomas, Murray and Murphy2017).
Anthelmintic medications are available to treat some of the most notable parasitic infections, of both humans (e.g. Hu et al., Reference Hu, Ellis, Yiu, Miller, Urban, Shi and Aroian2013) and other animals (e.g. Gokbulut and McKellar, Reference Gokbulut and McKellar2018). However, anthelmintic treatments targeted at specific nematode infections are underdeveloped and the need for discovery of new compounds with more species-specific properties is urgent. The development of anthelmintics is historically a slow process (Nixon et al., Reference Nixon, Welz, Woods, Costa-Junior, Zamanian and Martin2020). Since 2001, only three new drugs have been introduced to the market (Epe and Kaminsky, Reference Epe and Kaminsky2013). Despite the World Health Organization's estimate of ~24% of the human population being infected with soil-transmitted helminths (WHO, 2020), development of new human anthelmintics has been viewed as unprofitable, curbing incentive for pharmaceutical companies to invest in doing so (Hotez et al., Reference Hotez, Brindley, Bethony, King, Pearce and Jacobson2008; Risi et al., Reference Risi, Aguilera, Ladós, Suárez, Carrera, Álvarez and Salinas2019). Because of high-financial cost associated with development of human therapeutics, the discovery of new anthelmintics is largely driven by animal health research, and successful compounds have been adopted subsequently for human treatment (Crump and Omura, Reference Crump and Omura2011; Krücken et al., Reference Krücken, Holden-Dye, Keiser, Prichard, Townson, Makepeace, Hübner, Hahnel, Scandale, Harder and Kulke2021).
Another serious concern is emerging drug resistance in human and livestock parasites (Geerts and Gryseels, Reference Geerts and Gryseels2000; Schwab et al., Reference Schwab, Boakye, Kyelem and Prichard2005; Matthews, Reference Matthews2014; Krücken et al., Reference Krücken, Fraundorfer, Mugisha, Ramünke, Sifft, Geus, Habarugira, Ndoli, Sendegeya, Mukampunga, Bayingana, Aebischer, Demeler, Gahutu, Mockenhaupt and von Samson-Himmelstjerna2017). The broad use of antiparasitics across host species and against multiple parasites may contribute to the problem, as drug resistance is probably enhanced when few treatment options are available; use of a single drug over a prolonged period of time leads to resistance (Reinemeyer et al., Reference Reinemeyer, Rohrbach, Grant and Radde1992). Therefore, development and use of multiple new anthelmintic therapeutics are imperative and could slow development of resistance (Camicia et al., Reference Camicia, Celentano, Johns, Chan, Maldonado, Vaca, Di Siervi, Kamentezky, Gamo, Ortega-Gutierrez, Martin-Fontecha, Davio, Marchant and Rosenzvit2018).
Development of antiparasitic drugs often employs Caenorhabditis elegans as a model organism, despite it being a free-living rather than parasitic nematode, because of the ease of rearing and caring for it in the laboratory (Giunti et al., Reference Giunti, Andersen, Rayes and De Rosa2021). However, the effectiveness of potential compounds does not always transfer from C. elegans to the parasitic helminth of interest (Geary and Johnson, Reference Geary and Johnson2001). Therefore, we modified an existing infrared-based motility assay that uses the wMicroTracker (Phylumtech, Argentina), an automated high-throughput scanning platform developed for C. elegans, to assess its use with a parasitic nematode, Angiostrongylus cantonensis. In areas where A. cantonensis is present in snail hosts, the infectious third-stage larvae (L3) of A. cantonensis can be maintained and easily accessed for testing without the need to maintain the definitive, mammalian host component of the life cycle in the laboratory, which is often a major difficulty of working directly with the parasite of interest. This approach provides the benefit of drug testing on a potential model parasitic (as opposed to non-parasitic) nematode that is more relevant to human and animal disease, and removes the cost of maintaining the definitive host.
Angiostrongylus cantonensis, commonly known as the rat lungworm, is widely present across the humid tropics and subtropics (Barratt et al., Reference Barratt, Chan, Sandaradura, Malik, Spielman, Lee, Marriott, Harkness, Ellis and Stark2016) as well as in a few more temperate regions, e.g. in Japan (Otsuru, Reference Otsuru and Cross1979), North America (York et al., Reference York, Creecy, Lord and Caire2015) and Australia (Barratt et al., Reference Barratt, Chan, Sandaradura, Malik, Spielman, Lee, Marriott, Harkness, Ellis and Stark2016), and is the main aetiological agent of eosinophilic meningitis globally (Barratt et al., Reference Barratt, Chan, Sandaradura, Malik, Spielman, Lee, Marriott, Harkness, Ellis and Stark2016). The disease, known as neuroangiostrongyliasis, is a serious, sometimes fatal but neglected disease, not only in humans but also in domestic animals and wildlife (Lunn et al., Reference Lunn, Lee, Smaller, MacKay, King, Hunt, Martin, Krockenberger, Spielman and Malik2012; Spratt, Reference Spratt2015; Barratt et al., Reference Barratt, Chan, Sandaradura, Malik, Spielman, Lee, Marriott, Harkness, Ellis and Stark2016). The current treatment for neuroangiostrongyliasis in humans and other animals often combines administration of both anthelmintics and anti-inflammatory corticosteroids; however, evidence of their efficacy in both humans and dogs (Lunn et al., Reference Lunn, Lee, Smaller, MacKay, King, Hunt, Martin, Krockenberger, Spielman and Malik2012; Prociv and Turner, Reference Prociv and Turner2018; Ansdell et al., Reference Ansdell, Kramer, McMillan, Gosnell, Murphy, Meyer, Blalock, Yates, Lteif, Smith and Melish2021; Odani et al., Reference Odani, Sox, Coleman, Jha and Malik2021) is somewhat equivocal, if not controversial, highlighting the need for treatment specifically targeting A. cantonensis.
This paper introduces the first use of the wMicroTracker system to evaluate the success of a large suite of natural products in arresting motility of A. cantonensis L3, the infectious stage of the parasite (Fig. 1). The existing protocol for C. elegans (for which the apparatus was developed) was modified to assess inhibition of A. cantonensis L3 motility (and hence reduce infection potential) by a suite of bioactive natural products derived from the secondary metabolites of Hawaiian fungi. The primary objective of the study was to validate this approach with A. cantonensis as an economical method that combines whole organism testing with high-throughput screening, thereby dramatically increasing the possibility of identifying novel anthelmintics, while alleviating the common downsides of whole organism testing, i.e. cost and time. This study adds to the short list of helminths for which screening has been validated using the wMicroTracker (Simonetta, Reference Simonetta2021), confirming the potentially broad utility of the approach beyond our specific study parasite and beyond our specific source of natural products, and thus the potential for development of drugs to treat diverse parasitic infections. The supplementary objective of the study was to use this approach as a preliminary investigation to identify anthelmintic activity of natural products derived from Hawaiian fungi, which are a bountiful source of bioactive compounds (16 recent studies referenced by Wang et al., Reference Wang, Wu, Bai, Zaman, Hou, Saito, Wongwiwatthananukit, Kim and Cao2020a), against infectious larvae of A. cantonensis, and to purify, characterize and identify selected compounds chemically, with a view to future development.
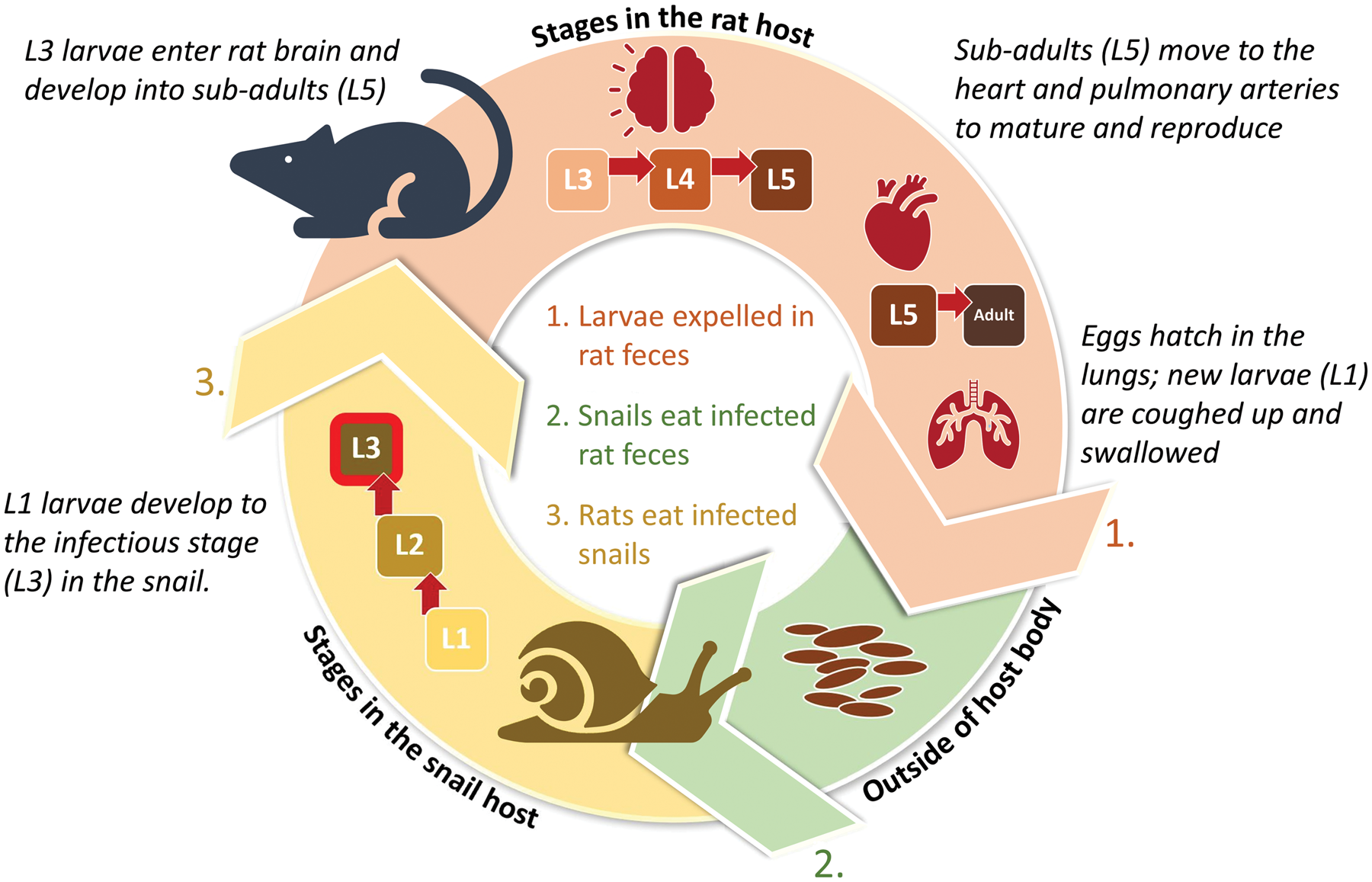
Fig. 1. Angiostrongylus cantonensis completes its life cycle in rats (definitive hosts) and snails (intermediate hosts) (Cowie, Reference Cowie2013). Snails become infected by ingesting rat faeces containing freshly hatched larvae. The larvae develop into infectious third-stage larvae (L3) in the snail and remain as such for the lifespan of the snail or until the snail is eaten by a definitive, paratenic or accidental host.
Materials and methods
Sources, isolation and preparation of A. cantonensis L3
Laboratory-reared and maintained snail (Parmarion martensi) colonies allowed easy access to the parasite for testing. The snails were infected with first-stage A. cantonensis larvae (L1) isolated from wild rat (Rattus rattus and Rattus norvegicus) faecal droppings and the L1 were given time to develop into infectious L3.
To obtain L3, two to three snails were finely minced and added to 50 mL tubes containing 25 mL 0.7% HCl and 0.125 g pepsin, then incubated at 37°C for 1 h and shaken vigorously by hand every 15 min. The tubes were centrifuged at 1100 rpm for 70 s and the supernatant decanted. The remaining pellets of snail tissue containing larvae were placed on two-ply gauze atop a metal mesh inserted in a modified Baermann apparatus (Baermann, Reference Baermann1917) filled with warm tap water. The tubes were rinsed with water to dislodge any remaining snail tissue, which was also poured onto the mesh. After 2 h, the clamp at the end of the Baermann tubing was opened and a small amount of liquid was released into a 1.7 mL or 5 mL tube. Once the larvae settled at the bottom of the tube, the water level was adjusted to 500 μL. The concentration of the larvae was determined by gently shaking the tube to disperse the larvae throughout the water, then quickly drawing six to eight 5 μL aliquots from the middle of the tube and pipetting them onto a microscope slide for counting. The tube was shaken after every three aliquots. The number of L3 in each aliquot was counted, and the number of larvae across the six to eight aliquots was averaged and taken as the concentration of L3 in 5 μL. Water was added or removed to achieve a concentration of ~2100 A. cantonensis larvae per mL in the tube.
Development of fungal natural product library
Sample collection and isolation of fungal species
Endophytic or associated fungi were isolated in Hawaii from soil or mud on seashores, from plant leaves and fruits and from soft corals. Locations with minimal human disturbance were selected. For isolation of fungi from plants or corals, samples were initially washed with running water to remove surface debris and dust, then the sterilized sample segments were cultured in solid nutrition media (potato dextrose agar and malt dextrose agar). Soil and mud samples were serially diluted 5–6 times with sterilized distilled water, then vortexed and 100 μL of the prepared suspension was evenly spread out on the solid nutrition media plates. These inoculated media plates were incubated at room temperature. After 7–10 days, the emerging fungal colonies were re-cultured to isolate pure fungi. The isolated pure fungal strains were maintained at −80°C in 50% glycerol and deposited at the Daniel K. Inouye College of Pharmacy, University of Hawaii at Hilo.
Fermentation and extraction
Pure fungal cultures grown in solid media were aseptically cut into 1 cm2 pieces together with media, transferred to 300 mL of sterilized liquid potato dextrose broth (potato extract 4 g and dextrose 20 g for 1 L distilled water) or C media (consists of mannitol 20 g, glucose 10 g, monosodium glutamate 5 g, KH2PO4 0.5 g, MgSO4⋅7H2O 0.3 g, yeast extract 3 g and sea salt 15 g for 1 L distilled water; pH 6.5 prior to sterilization) contained in 1 L conical flasks (three flasks for small scale and 50 flasks for large scale; see below). The contents were fermented at room temperature. After 28 days, solid mycelium and the liquid broth were separated. Solid mycelium was extracted with methanol (MeOH) and the liquid broth medium was treated with Diaion HP-20 polymeric resin. Treated Diaion HP-20 resins were loaded into an open glass column and eluted with MeOH–H2O (50, 90 and 100% MeOH) solvent systems. The resulting three crude fractions were collected separately and dried in vacuo. Our earlier studies have shown that 90 and 100% fractions contain similar, diverse metabolite patterns and biological activities compared to the 50% fraction. Hence, further studies focused on the combined 90 and 100% fractions (Wang et al., Reference Wang, Wu, Bai, Zaman, Hou, Saito, Wongwiwatthananukit, Kim and Cao2020a, Reference Wang, Sarotti, Jiang, Huguet-Tapia, Zheng, Wu, Li, Ding and Cao2020b; Yu et al., Reference Yu, Li, Kwon, Oh, Lee, Kim, Ahn, Ko, Kim, Cao and Kim2020; Qader et al., Reference Qader, Zaman, Hu, Wang, Wu and Cao2021; Zaman et al., Reference Zaman, Wu, Hu, Yoshida, Hou, Saito, Avad, Hevener, Alumasa and Cao2021).
Cell viability assay of small-scale extracts to prioritize strains for large-scale fermentation
The small-scale extracts prepared from isolated fungal strains were tested for the lack of toxicity against one or other of three human cell lines: HT1080 (fibrosarcoma cell line), T46D (breast cancer cell line) and A2780S (ovarian cancer cell line). Nontoxic extracts produced by the respective fungal strains were prioritized for large-scale fermentation (Wang et al., Reference Wang, Wu, Bai, Zaman, Hou, Saito, Wongwiwatthananukit, Kim and Cao2020a; Qader et al., Reference Qader, Zaman, Hu, Wang, Wu and Cao2021; Zaman et al., Reference Zaman, Wu, Hu, Yoshida, Hou, Saito, Avad, Hevener, Alumasa and Cao2021).
Pure compound isolation and structure characterization
Combined fractions were further fractionated using a reverse-phased high-performance liquid chromatography (RP-HPLC) system with a flow rate of 8 mL min−1 (system and column specifications are given in the Supplementary material file) eluted with 40–100% MeOH–H2O for 20 min to yield 15–20 sub-fractions. These sub-fractions were further fractionated using a semi-preparative HPLC system with a C18-ODC column with different solvent systems of MeOH–H2O (with 0.1% formic acid) at a flow rate of 3 mL min−1. The isolated pure compounds were further checked by analytical HPLC for their purity. Finally, the structures were characterized using nuclear magnetic resonance (NMR) and high-resolution mass spectroscopy (HRMS), and were identified by comparison with the literature.
Preparation of crudes, fractions and pure compounds for biological studies
Shugeng Cao's laboratory has a collection of pure compounds isolated during earlier research. For validation of the A. cantonensis motility method and for preliminary identification of samples with anthelmintic properties, some of these samples, as well as crude samples and fractions/sub-fractions, were used.
Dried extracts or fractions or pure compounds were prepared for the experiments by dissolving a small amount of product in the appropriate amount of neat dimethyl sulphoxide (DMSO) to obtain a final concentration of 1 mg mL−1 for crudes and fractions/sub-fractions and 0.1 mg mL−1 for pure compounds (two pure compounds, M200 and M201, also had a final concentration of 1 mg mL−1). The prepared samples were stored in a −20°C freezer until further use.
Motility assay development
The wMicroTracker is an automated locomotor activity tracker apparatus that detects movement of worms by shooting two 800 nm infrared beams through each well of a 96-well plate. The light-emitting diode (LED) beams are 150 μm in diameter, which is larger than the diameter of infectious A. cantonensis larvae (~30 μm diameter). The LED beams pulse every 1/384 s. Interruptions in the beams are recorded for each well. The number of interruptions per well are counted over a set period of time (i.e. a bin), which is adjustable by the user, and the totals are recorded as motility values. We initially tested bin sizes of 5 min to 6 h. One-hour bins were determined to be appropriate for the length of our plate runs (72 h) and for the purposes of this study.
The wMicroTracker system was originally designed for use with C. elegans, following prototype development by Simonetta and Golombek (Reference Simonetta and Golombek2007), and has subsequently been developed commercially and validated for use with a number of species, mostly as larval forms (Simonetta, Reference Simonetta2021). Therefore, a series of preliminary experiments was undertaken to optimize a protocol for A. cantonensis L3 prior to addressing the primary objective of developing a high-throughput testing approach. Details of these steps are provided here in order that others may benefit from this experience with A. cantonensis as they adapt the wMicroTracker protocols to their own experimental needs.
Plate design
Use of flat-bottom wells is the standard wMicroTracker protocol for C. elegans. However, flat-bottom wells gave inconsistent results because of the small size of A. cantonensis L3, which resulted in a high ratio of well space to total larval occupancy, and inconsistent laser detection. Instead, plates with round-bottom wells were used; these concentrate the larvae in the centre of the well, within the path of the laser beams, and permit consistent and meaningful motility results.
Well volume
The standard wMicroTracker protocol for C. elegans uses 100 μL liquid volume per well. To reduce the amount of sample product necessary for each test, as the amounts available were limited, the total well volume was reduced to 40 μL and this was found to be sufficient.
Number of larvae
Wells with 5, 10, 15, 20, 25, 40, 60, 80, 90, 120, 160 and 175 L3 were tested. Wells with 25 or fewer gave inconsistent motility results. No benefit was gained with 60 or more. Approximately 40 L3 per well was therefore determined to be the number required to obtain consistent motility results.
DMSO concentration
The library samples of natural products had to be dissolved in DMSO. Concentrations of 5, 2.5 and 0.5% DMSO were tested to determine the highest concentration in which the L3 could be suspended without affecting their motility. At 5%, motility was decreased but there was no effect at the lower concentrations. The samples were therefore dissolved in neat DMSO at a concentration necessary to allow for subsequent dilution to 2.5% DMSO in the well.
Thus, following these preliminary assessments, the optimum content of each well was determined to be as follows: ~40 larvae (suspended in 19 μL water), 20 μL 1× phosphate-buffered saline and 1 μL dissolved library sample (concentration 1 mg mL−1 for fractions, 0.1 mg mL−1 for pure compounds, with the exception of M200 and M201; see above), for a total well volume of 40 μL.
Motility assays
Once the wMicroTracker protocols had been optimized, it was possible to undertake the exploratory assays of potential motility inhibition of A. cantonensis L3 by our unique library of natural products isolated from Hawaiian fungi.
Sample testing and high-throughput protocol development
To develop an effective and efficient validated high-throughput screening method for testing each sample in a single well, samples of our natural product library were tested against A. cantonensis L3 larvae in a succession of well configurations. First, 24 samples were run in two replicates of three wells each (total six wells per sample). The mean motility values of the three wells in each replicate were used to verify the consistency of values between the two replicate triplets. After demonstrating consistency between triplet replicates, seven samples were run as single triplets (without a replicate). Next, four samples were run as both triplets and in an accompanying single well (singlet) to compare the averaged motility values of the triplets to the motility values of the singlets. Having shown that the singlet values appropriately tracked the mean values from the triplets, 76 samples were run as singlets to demonstrate the goal of a high-throughput screening method. The samples and whether they were screened as two replicate triplets, a single triplet, a triplet compared with a single well or a singlet alone (i.e. the high-throughput objective) are listed in Supplementary Table S1.
Each wMicroTracker run also included two to six wells with L3 suspended in buffer only, to obtain a baseline motility value, and 3–16 wells with only buffer to identify any spurious motility readings. Samples were run in the wMicroTracker for 72 h.
Data analysis
For samples run as triplets, the mean of the motility values of the three wells per 60 min time increment (bin) was used. These mean values, and the motility values obtained from the samples run as singlets, were scaled from 0 to 100, and these scaled motility values were used for assessment. For each sample, larval motility over the 72 h run period was plotted and compared visually to the larval motility in buffer (baseline motility), and subsequently categorized by their effect on motility: when motility was reduced to 0–25% of the maximum exhibited, the sample was categorized as high interest; when motility was reduced to 25–50%, as moderate interest; when motility was reduced to 50–75%, as low interest, and when motility was reduced to 75–100%, as no interest.
Results
Verifying the validity of single-well testing (high-throughput assay)
Consistency of the mean motility values from three wells when comparing the plotted means from each of the two triplet replicates was substantiated for 24 samples. Although some variation in the mean motility value was observed between the two triplets, the trends were the same (Fig. 2). Once this consistency had been demonstrated, it was considered that running each sample as a single triplet would give a reliable result. However, the objective of the study was to develop a high-throughput, single-well approach. Next, therefore, the mean motility values of four samples tested as triplets were compared to the values from the same samples tested in single wells; the overall reduction in motility caused by a sample was similar in the triplets and single wells (Fig. 3), supporting adoption of a single-well, high-throughput plate array for further testing of the natural product library (Fig. 4).

Fig. 2. Mean L3 motility values over 72 h of natural product samples run as two replicate triplets, left and right panels; lines of the same colour (blue, green, etc.) are from the same sample. Samples (n = 24) were run on three plates; for clarity, these graphs illustrate results from just one plate (n = 7). Sample numbers are internal S. Cao laboratory codes (Supplementary Table S1).

Fig. 3. Comparison of mean L3 motility values of triplicates (samples with T suffix) with motility values of single wells (S suffix) for four natural product samples. Each sample is represented by one colour (orange, green, purple and blue), with lighter shades representing the S and darker shades representing the T values. Sample numbers are internal S. Cao laboratory codes (Supplementary Table S1).

Fig. 4. Top: L3 motility values of the 76 natural product samples tested in a high-throughput plate layout plotted over time. Bottom left: High-throughput plate layout: blue wells, buffer only; green wells, buffer and L3 only; red wells, 76 natural product samples in single wells with L3 in buffer.
Screening of natural product samples
Overall, using the optimized protocol for A. cantonensis, 108 samples (55 pure compounds along with their nontoxic parent crude extracts, fractions and sub-fractions) were screened, either as triplets or as singlets, and 13 samples (two pure compounds and 11 fractions or sub-fractions) were identified as being in the high-interest category (reduction to 0–25% motility) (Supplementary Table S2). Of these 13 samples, five (M251, M252, M272, M273 and M275) caused essentially immediate and dramatic (close to 99%) motility reduction, which was sustained throughout the 72 h period with little variability (Fig. 5A). These five samples (all were fractions) were considered of priority interest.

Fig. 5. Among the high-interest natural product samples, five showed a fast and persistent effect on L3 motility (A), while six showed a slightly less immediate and more variably persistent but still dramatic reduction in L3 motility (B). Sample M1018, and the rebounding sample, M1025 (see text), are also shown (in panels A and B, respectively). Sample numbers are internal S. Cao laboratory codes (Supplementary Table S1).
Another six (M259, M260, M274, M282, M283 and M285) of the 13 high-interest samples were especially effective at reducing motility: larval movement was essentially halted, although, unlike the five samples above, the effect took longer, >3000 min, and/or was not fully sustained throughout the 72 h period (Fig. 5B).
The remaining two of the 13 high-interest samples were pure compounds (M1018 – emethacin B, M1025 – epicoccin E). Sample M1018 showed almost complete inhibition of motility from the very start of the run (Fig. 5A). Sample M1025 reduced motility to nearly 0% within ~3700 min, although larval motility rebounded and was recorded in the 50–75% range before the end of the run (Fig. 5B).
Eleven samples, including five pure compounds (Supplementary Table S1), were also identified as being of moderate interest (reduction to 25–50% motility) (Supplementary Table S2). Although of lower priority than the high-interest samples above, these samples still caused great reduction in larval movement and may be considered for further testing. Twenty-four samples were categorized as low interest because their motility values were reduced only to the 50–75% range. The other 60 samples showed little to no effect on motility (Supplementary Table S2).
Case study: bioassay-guided separation
An example of the process of bioassay-guided separation is described here based on the analysis of the crude extract from Hawaiian fungus FS112. Initial prioritization of the extract via cell viability assays against the HeLa cell line showed that the extract was nontoxic. Large-scale culturing, fractionation (three fractions: M250, M251 and M252), and further fractionation via preparative RP-HPLC (flow rate 8 mL min−1 eluted with 40–100% MeOH/H2O for 20 min) resulted in 16 sub-fractions, which were assessed with the high-throughput assay. Figure 6 summarizes the chemical breakdown of the extract from FS112 using different chromatographic methods and the anthelmintic activity assay used to prioritize the fractions for isolation of anthelmintic compounds.

Fig. 6. Process of bioassay-guided isolation of pure compounds causing motility inhibition of A. cantonensis L3, exemplified by isolation and identification of the pure compound M200 as lamellicolic anhydride.
According to the results of the motility assay, of the three extracts (50, 90 and 100%) obtained from the Diaion HP-20 open column, only the 90 and 100% extracts resulted in reduction of motility to 0–25%, such that they were categorized as high-interest extracts. Since the chemical profile of both 90 and 100% extracts showed similar patterns in analytical HPLC, we combined them for further separation, which resulted in 16 fractions (M253–M268) that were re-assayed. Among these 16 fractions, only two sub-fractions (M259 and M260) showed potent activity against the L3 at the level of that provided by the parent 90 and 100% extracts. Since both active sub-fractions (M259 and M260) were chemically similar and adjoining, we combined them and purified them using semi-preparative HPLC (60% isocratic MeOH–H2O in 0.1% formic acid, flow rate 3.0 mL min−1 for 20 min). This led to the isolation of one major compound, lamellicolic anhydride (M200, 20 mg), which showed moderate anthelmintic activity, reducing larval motility in less than 3000 min post-treatment (Figs 7 and 8). The structure characterization was carried out using molar mass (Supplementary Fig. S1) based dereplication with the SciFinder database (https://scifinder.cas.org) and further confirmed by 1H (Supplementary Fig. S2) and 13C NMR (Supplementary Fig. S3) and comparison with the literature (Gao et al., Reference Gao, Zhang, Garcia-Borràs, Hung, Billingsley, Houk, Hu and Tang2018). The toxicity of M200 to human cell lines was tested using a 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl tetrazolium bromide assay against the HT1080 (fibrosarcoma), T46D (breast cancer) and A2780S (ovarian cancer) cell lines and the compound did not show any toxicity at 50 μ m concentration.

Fig. 7. L3 motility reduction by fractions and sub-fractions of the crude extract from Hawaiian fungus FS112 (M209), as screened during the process (Fig. 6) of isolating and identifying the pure compound M200 (red line) as lamellicolic anhydride.

Fig. 8. Structures of M200 (lamellicolic anhydride), which reduced L3 motility to 50–75%, and of the two pure compounds M1018 (emethacin B) and M1025 (epicoccin E), which reduced motility to 0–25%, and were therefore considered of high interest.
Discussion
The study demonstrated a validated, whole organism, automated, high-throughput and low-cost methodology for screening anthelmintic properties of a large library of fungal secondary metabolites against A. cantonensis L3. Two pure compounds (emethacin B and epicoccin E; Fig. 8) were identified with potential for development into effective anthelmintics, neither of which has been reported in any anthelmintic or antiviral activity assays.
The high-throughput approach permitted screening of far more products than did the more conventional approaches of screening products at least in duplicate (Liu et al., Reference Liu, Kipanga, Mai, Dhondt, Braeckman, De Borggraeve and Luyten2018, Reference Liu, Landuyt, Klaassen, Geldhof and Luyten2019) and often in triplicate or quadruplicate (e.g. Hahnel et al., Reference Hahnel, Roberts, Heisler, Kulke and Weeks2021). However, it is conceivable that by not replicating screening of a single sample, an abnormally high motility value (i.e. minimal inhibition of motility) could lead to overlooking a potentially effective product, and vice versa that an abnormally low value could lead to incorrectly identifying a product as being of interest but that in fact had low to no effectiveness. Nonetheless, the likelihood of an abnormally high value is low, because if a product has significant anthelmintic activity, the test worms in the well would not be able to escape it. On the other hand, the likelihood of an abnormally low value might be greater, because various factors (e.g. contamination, low worm numbers due to pipetting error, and uneven activity among the worms) could reduce recorded motility despite the test sample possessing minimal anthelmintic activity. However, in the case of an abnormally low value, confirmatory investigation of what seemed like a product with potential would rapidly screen it out from consideration. In addition, the consistency demonstrated in our study, both between paired triplet replicates and between triplicates and singlets (Figs 3 and 4) of the same product, indicated that the possibility of such errors is low. The advantages of screening products in single wells only using an efficient, high-throughput method such as demonstrated here, as opposed to a low-throughput replicated method, seem to outweigh the disadvantages associated with the increased possibility of false positives and false negatives, and indicate that the high-throughput approach could significantly speed up the identification of novel compounds with pharmacological potential and reduce the economic costs of the process.
Although reduced or halted motility does not necessarily mean death of the worm, many anthelmintics act on the nervous system such that reduced motility is often assayed as an important criterion for identifying compounds of interest (e.g. Liu et al., Reference Liu, Landuyt, Klaassen, Geldhof and Luyten2019; Risi et al., Reference Risi, Aguilera, Ladós, Suárez, Carrera, Álvarez and Salinas2019; Hahnel et al., Reference Hahnel, Roberts, Heisler, Kulke and Weeks2021). Furthermore, A. cantonensis larvae do not necessarily need to be killed for successful mitigation of severe neuroangiostrongyliasis. If L3 can be prevented from reaching the brain (in the first few days it takes them to reach the brain naturally post infection), illness would be essentially prevented. Even if they reached the brain (because anthelmintic treatment was not sufficiently early), much of the damage caused by their movement and feeding would be precluded. Furthermore, the lack of movement and feeding would preclude growth and development, such that when they do die, the enhanced immune reaction normally expected when larger worms die would be reduced, as it may be related to the amount of dying worm tissue. Motility reduction or cessation is thus an appropriate objective. Even so, anthelmintic treatment would be most effective the sooner it is begun (Prociv and Turner, Reference Prociv and Turner2018; Ansdell et al., Reference Ansdell, Kramer, McMillan, Gosnell, Murphy, Meyer, Blalock, Yates, Lteif, Smith and Melish2021).
The wMicroTracker was developed for use with C. elegans of all larval to adult stages, length ranging from 250 to 1700 μm (InVivoBiosystems, 2021). It has been used with C. elegans, as a model system, e.g. for assessing the anthelmintic activities of a diversity of compounds (Liu et al., Reference Liu, Kipanga, Mai, Dhondt, Braeckman, De Borggraeve and Luyten2018; Risi et al., Reference Risi, Aguilera, Ladós, Suárez, Carrera, Álvarez and Salinas2019) and to study neurodegeneration (Vérièpe et al., Reference Vérièpe, Fossouo and Parker2015), and its performance has been compared favourably with more traditional assays of anthelmintic activity (Hahnel et al., Reference Hahnel, Roberts, Heisler, Kulke and Weeks2021). It has also been validated for use with ten other helminth species (Simonetta, Reference Simonetta2021). These include nematodes of human and animal concern, the tested stages ranging from 200 to 2000 μm in length, i.e. Cooperia oncophora, Haemonchus contortus, Teladorsagia circumcincta and Ostertagia ostertagi (Liu et al., Reference Liu, Landuyt, Klaassen, Geldhof and Luyten2019), Dirofilaria immitis (Simonetta, Reference Simonetta2021), Brugia pahangi (Simonetta, Reference Simonetta2021), a plant pest nematode, Meloidogyne incognita (350–450 μm; Simonetta, Reference Simonetta2021), two cestode species causing human diseases, i.e. Echinococcus granulosus (200 μm; Camicia et al., Reference Camicia, Herz, Prada, Kamenetzky, Simonetta, Cucher, Bianchi, Fernández, Brehm and Rosenzvit2013, Reference Camicia, Celentano, Johns, Chan, Maldonado, Vaca, Di Siervi, Kamentezky, Gamo, Ortega-Gutierrez, Martin-Fontecha, Davio, Marchant and Rosenzvit2018) and Mesocestoides corti (750–1000 μm; Simonetta, Reference Simonetta2021) and the trematode Schistosoma mansoni (100 μm; Simonetta, Reference Simonetta2021). Angiostrongylus cantonensis L3 are ~450 μm long (Bhaibulaya, Reference Bhaibulaya1975), within the range of the stages of other species for which the use of the wMicroTracker has been validated.
This study therefore adds to the growing body of research that demonstrates the potential of high-throughput and uncomplicated assays to screen high numbers of compounds for possible anthelmintic properties, thereby reducing both the cost and time associated with the initial development of novel anthelmintic drugs. Such assays, by facilitating the discovery of new anthelmintics will aid in mitigating drug resistance and reducing disease burden, which is especially important for neglected tropical diseases and diseases of economically disadvantaged people and nations.
Supplementary material
The supplementary material for this article can be found at https://doi.org/10.1017/S0031182022000191.
Acknowledgements
The authors thank Todd Cullison and the Hawaii Nature Center for permitting the trapping of rats at their facility/property. The authors also thank Ana-Melissa Kea for assistance with protocol validation. This is publication number 11475 of the University of Hawaii School of Ocean and Earth Science and Technology, and a publication of the Manoa Angiostrongylus Research Group.
Author contributions
S. C. in collaboration with W. L. G. conceived the project; W. L. G. facilitated purchase of wMicroTracker; R. L. R. developed and conducted the motility assays in conjunction with W. L. G. and discussion with R. H. C.; fungal samples were collected and prepared by M. Q. who conducted the assay-guided separation; the active compounds were identified by M. Q., C. W. and S. C.; larvae were collected and prepared by R. L. R. and snail colonies were maintained by R. L. R. The manuscript was written primarily by R. L. R. and R. H. C., with sample source, preparation and characterization sections by S. C. and M. Q., and with input from W. L. G. All authors reviewed and agreed to the final draft.
Financial support
This project was supported by a grant from the National Institutes of Health (NIH), National Institute of General Medical Sciences (NIGMS), IDeA Networks of Biomedical Research Excellence (INBRE), Supplemental Award number P20GM10346-18S1. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Conflict of interest
The authors declare that they have no competing interests.
Ethical standards
The study was conducted according to ethical standards for research involving vertebrates under the University of Hawaii IACUC protocols 20-3257 and 20-3257-2.