Childhood and adolescence as ‘critical periods’
The prevalence of cardiometabolic disease risk markers, including insulin resistance and dyslipidaemia, and even the manifestations of certain cardiometabolic diseases are increasing globally among young people(Reference Andes, Cheng and Rolka1–Reference Lawrence, Divers and Isom3). Given that cardiometabolic disease develops over time, an understanding of disease precursors rather than manifestation is more relevant to young people. Even so, in the absence of global data, recent data of 3⋅47 million 10–19-year-olds in the US reported a 95⋅3 % relative increase in estimated type 2 diabetes prevalence over 16 years between 2001 and 2017(Reference Lawrence, Divers and Isom3). Furthermore, US National Health and Nutrition Examination Survey data have shown that 18⋅0 % of 12–18-year-olds have prediabetes(Reference Andes, Cheng and Rolka1) and 20⋅2 % of 8–17-year-olds have an adverse lipid concentration(Reference Kit, Kuklina and Carroll4). With rises in obesity among young people being a key driver, these data suggest cardiometabolic disease is set to manifest at an earlier age in future generations, prolonging the health and economic burden(Reference Andes, Cheng and Rolka1,Reference Weiss, Bremer and Lustig5) . Importantly, targeting young people to aid disease prevention rather than focusing efforts towards treatment in later life may also benefit the planet through reduced greenhouse gas emissions associated with disease diagnosis and treatment within healthcare systems; for example, due to reduced manufacturing of blood analysis consumables and drugs, motorised transportation of goods and services, and clinical waste disposal (see Fig. 1)(Reference Eckelman and Sherman6).
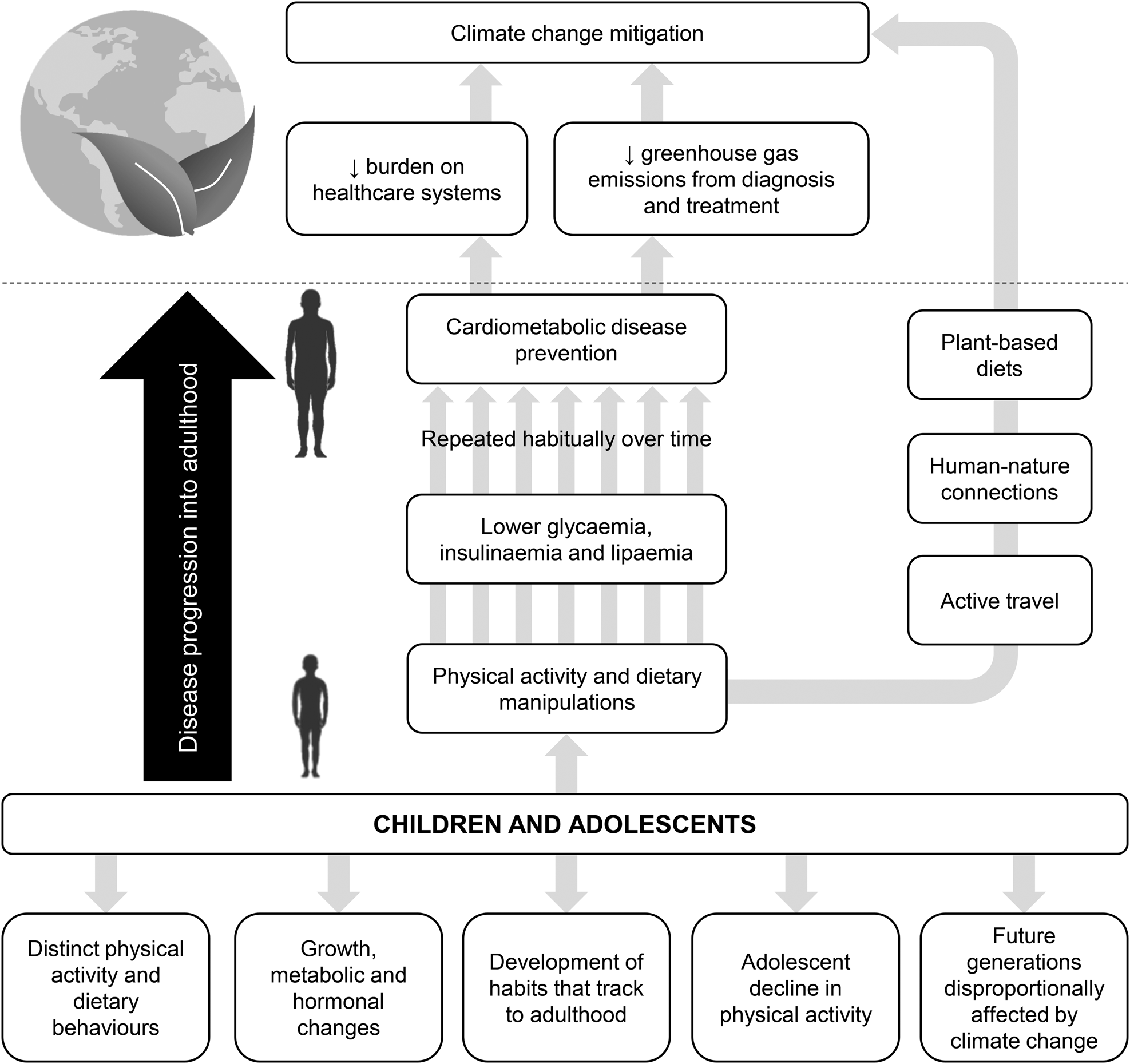
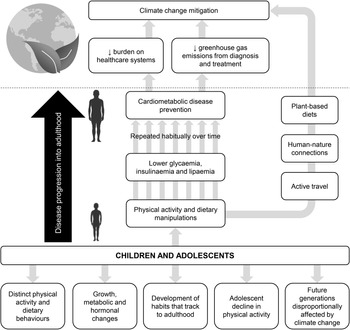
Fig. 1. Potential avenues that support a focus on children and adolescents (young people) for cardiometabolic disease prevention through physical activity and/or diet with co-benefits to mitigating climate change. Note that examples of pathways linking physical activity and dietary manipulations in young people with climate change mitigation include, but are not limited to, active travel, human-nature connections and plant-based diets.
Moderating postprandial glycaemia, insulinaemia and lipaemia is at the cornerstone of cardiometabolic disease prevention(Reference Blaak, Antoine and Benton7,Reference Heine, Balkau and Ceriello8) . Such risk markers share common physiological pathways, leading to their ‘clustering’ and placing young people at further risk(Reference Weiss, Bremer and Lustig5). As such, public health recommendations that address multiple cardiometabolic disease risk markers stand to create the largest impact. Most waking hours are spent inducing marked metabolic fluctuations, emphasising the importance of postprandial rather than fasting measures. Postprandial glycaemia can increase cardiometabolic disease risk through oxidative stress, inflammation and atherosclerosis(Reference Blaak, Antoine and Benton7,Reference Heine, Balkau and Ceriello8) with repeated rises and falls indicative of high glycaemic variability appearing particularly harmful(Reference Ceriello, Esposito and Piconi9,Reference Sun, Zhao and Teng10) . Even in ‘healthy’ populations, higher postprandial glucose concentrations increase type 2 diabetes, cardiovascular disease (CVD) and all-cause mortality risk(Reference Abdul-Ghani, Abdul-Ghani and Ali11–Reference Ning, Zhang and Dekker14). Likewise, regular exposure to elevated postprandial triacylglycerol (TAG) concentrations promotes a pro-atherogenic lipid phenotype, including low HDL concentrations and the accumulation of small, dense LDL(Reference Cohn15) that increases susceptibility to future clinical cardiovascular events. Taken together, these findings may explain the lack of sensitivity of fasting measures alone for the diagnosis of cardiometabolic disease(Reference Cosson, Hamo-Tchatchouang and Banu16).
Childhood and adolescence are vulnerable times of life and have been associated with entering the initial stages of cardiometabolic disease development. Among girls and boys, pubertal insulin resistance reduces the ability of insulin to increase glucose uptake and utilisation, resulting in a compensatory increase in insulin secretion and plasma insulin concentrations to maintain normal blood glucose concentrations(Reference Goran and Gower17,Reference Moran, Jacobs and Steinberger18) . Pubertal insulin resistance is transient, typically reported to begin during early puberty, peak during mid-puberty and recover by the end of puberty(Reference Goran and Gower17). Yet, more recent longitudinal data have demonstrated that the first stages are detectable from about 7 years of age(Reference Jeffery, Metcalf and Hosking19). Unfortunately, the diets of many young people are not conducive to the puberty-related reduction in insulin sensitivity; for example, free sugar intakes exceeded the recommended level in 87 % of 4–10-year-olds and 92 % of 11–18-year-olds in the UK in 2020(20). The factors contributing to pubertal insulin resistance remain elusive, but appear to include rises in insulin-like growth factor-1 and adiposity(Reference Jeffery, Metcalf and Hosking19,Reference Kelsey and Zeitler21,Reference Roemmich, Clark and Lusk22) , and affect girls more than boys(Reference Moran, Jacobs and Steinberger18,Reference Jeffery, Metcalf and Hosking19,Reference Cooper, Dring and Morris23) . Due to the additive effect of adiposity, unfortunately pubertal insulin resistance in young people with obesity does not fully resolve, resulting in an increased risk of β-cell exhaustion and cardiometabolic disease at a younger age(Reference Kelsey and Zeitler21). Likewise, the antecedents of atherosclerotic disease originate in the formative years(Reference McGill, McMahan and Herderick24), where aortic and coronary atherosclerotic lesions can develop in the first two decades of life and progress in prevalence and severity over time(Reference Holman, McGill and Strong25,Reference Strong and McGill26) . Further, CVD risk markers that manifest in youth are predictive of subclinical atherosclerosis in adulthood(Reference Davis, Dawson and Riley27,Reference Raitakari, Juonala and Kähönen28) and may increase the risk of premature death in adults(Reference Franks, Hanson and Knowler29). These data highlight the importance of protecting cardiometabolic health from an early age.
For effective cardiometabolic disease prevention, childhood and adolescence have been identified as a critical time to establish healthy, sustainable physical activity and dietary behaviours for life-long impact(Reference Eccles, Midgley and Wigfield30,Reference Todd, Street and Ziviani31) . Yet, young people remain under-represented in the field of exercise and nutrient metabolism. Drawing on data from adult samples, acute exercise is a potent stimulus for moderating postprandial glycaemia, insulinaemia and lipaemia for up to 2–3 d(Reference Henriksen32–Reference Maraki and Sidossis34). Critically, such findings may not apply to the distinct hormonal, metabolic and behavioural profiles of children and adolescents, including pubertal insulin resistance(Reference Goran and Gower17,Reference Moran, Jacobs and Steinberger18) , fuel utilisation(Reference Riddell35,Reference Timmons, Bar-Or and Riddell36) and physical activity patterns(Reference Armstrong and Welsman37). The lack of paediatric-specific data is concerning given the low physical activity levels of many young people; for example, about 71 % of European 2–18-year-olds do not meet the physical activity guidelines when measured objectively(Reference Steene-Johannessen, Hansen and Dalene38), and about 81 % of 11–17-year-olds were classified as insufficiently physically active globally based on population survey data of 1⋅6 million participants(Reference Guthold, Stevens and Riley39). Attention to adolescents is of particular relevance due the adolescent decline in physical activity, which is more pronounced among girls than boys and supported by a large body of longitudinal research employing objective physical activity measures(Reference Farooq, Martin and Janssen40). During this time, adolescents often begin to skip breakfast(Reference Monzani, Ricotti and Caputo41), which may interact with physical activity and glycaemic control(Reference Betts, Richardson and Chowdhury42–Reference Gonzalez48). However, a comprehensive summary of the available paediatric evidence on the acute glycaemic, insulinaemic and lipaemic responses to physical activity and the role of breakfast–physical activity interactions have not yet been undertaken.
This review paper will critically summarise the extant literature on the cardiometabolic responses up to 48 h after completing physical activity bouts among children and adolescents. The possible interactions of breakfast with physical activity on cardiometabolic disease risk are then discussed. Reflecting traditional cardiometabolic disease risk markers and the available paediatric literature, this review paper will pay specific attention to glycaemia, insulinaemia and lipaemia, notwithstanding that an array of other markers exists. Given the seriousness and timeliness of global climate change with responsibility to act across disciplines, this review paper offers new insights into the inherent interactions between child–adolescent behaviour and cardiometabolic health in the context of environmental sustainability to aid climate change mitigation efforts, including exploring future research avenues. Based on these themes, Fig. 1 provides a platform for the concepts integral to this review paper, offering potential avenues that support a focus on young people for cardiometabolic disease prevention with co-benefits to mitigating climate change through direct and indirect pathways. From the outset, it should be highlighted that the term ‘physical activity’ is used to describe bodily movements with an energy expenditure >1⋅5 metabolic equivalents (METs), whereas the term ‘exercise’ is used to describe the subset of physical activity that has pre-planned, structured characteristics. The collective term ‘young people’ is used where findings apply to mixed samples of children and adolescents and where it is not essential to distinguish between chronological age (referred to as ‘age’ for simplicity) and pubertal stage.
Cardiometabolic responses to acute physical activity
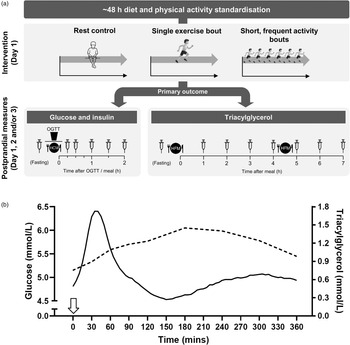
As illustrated in Fig. 2a, experimental investigations on acute physical activity have typically employed crossover designs using a 1 or 2 d study paradigm, whereby the physical activity stimulus is either applied before measuring fasting and/or postprandial cardiometabolic responses or is accumulated throughout the measurement period. These experimental designs over multiple days reflect the expected lag between exposure to the physical activity stimulus and cardiometabolic benefit. The test day protocols for the measurement of outcome variables are designed in line with the differing pattern, duration and magnitude of postprandial glycaemic, insulinaemic and lipaemic responses to a single or multiple meals consumed across the day, as shown in Fig. 2b.
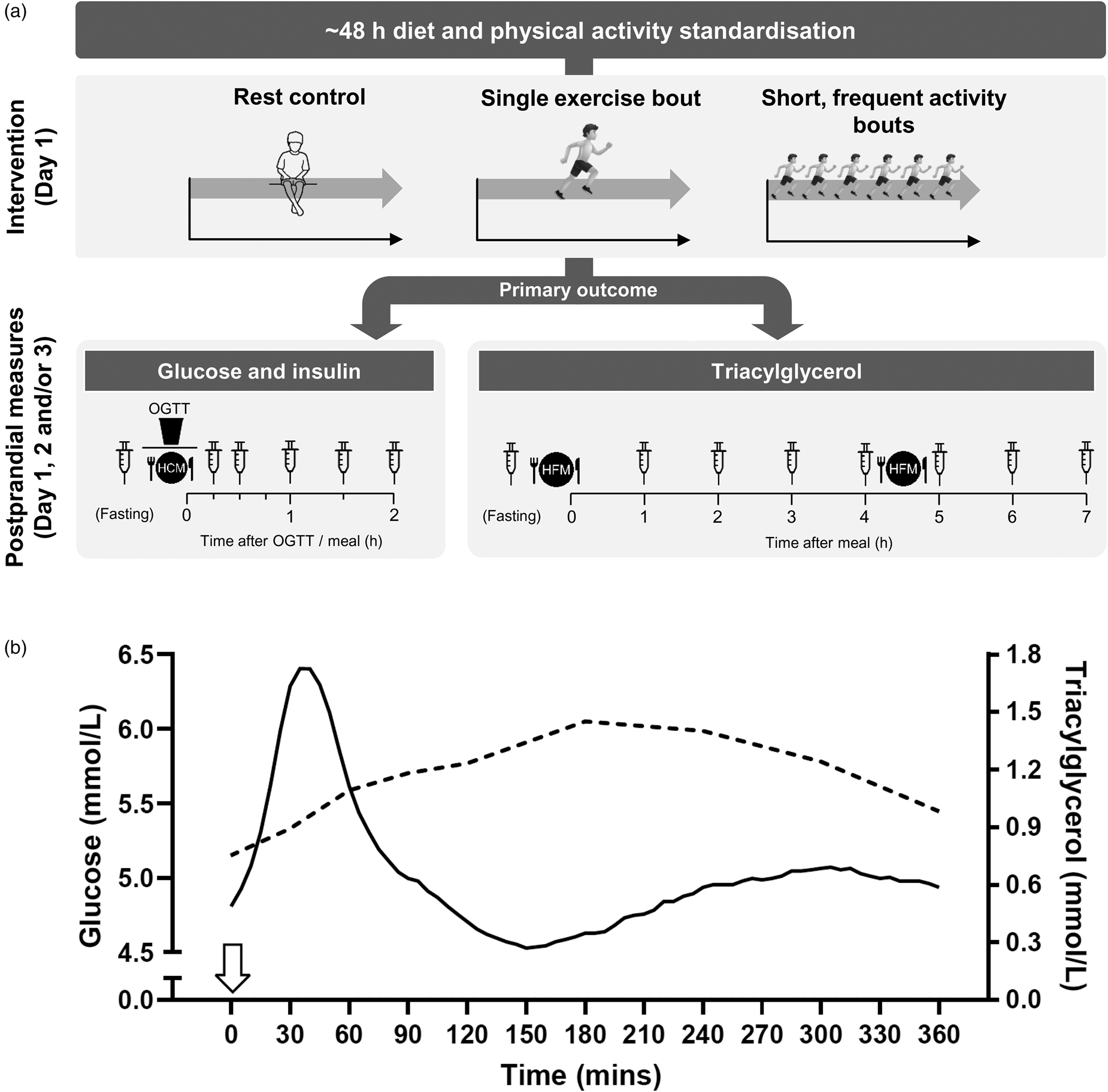
Fig. 2. (a) Schematic of typical protocols adopted to assess the postprandial cardiometabolic responses to acute exercise or physical activity bouts. Typically, a 1 or 2 d protocol is used, with exercise/physical activity manipulations and rest (control) conditions performed on day 1 and postprandial cardiometabolic risk marker responses assessed on day 1 and/or day 2 and in some cases day 3. Protocols differ according to whether the primary outcome is plasma glucose and insulin or TAG. Repeated capillary or venous blood samples are collected at pre-determined time-points in the fasted and postprandial state. OGTT, oral glucose tolerance test; HCM, high-carbohydrate meal; HFM, high-fat meal. (b) Illustration of the typical circulating plasma glucose and TAG response to consumption of a glucose load or meal at 0 h (indicated by the arrow). Postprandial glycaemic and insulinaemic responses are typically immediate, peaking at about 15–30 min and returning to baseline within about 2 h(20,Reference Zakrzewski and Tolfrey49,Reference Dring, Cooper and Williams56,Reference Zakrzewski, Stevenson and Tolfrey114) , whereas postprandial TAG rises slower to reach a peak at about 3–4 h before returning to baseline after 6–8 h in the absence of a second-meal challenge(Reference Tolfrey, Doggett and Boyd65–Reference Barrett, Morris and Stensel68). As such, glucose and insulin concentrations require more frequent sampling over a shorter duration often in response to a carbohydrate-based test meal/drink, whereas less frequent blood sampling over a longer duration is required to capture postprandial TAG profiles, often in response to a high-fat meal. The macronutrient content of the test meal is typically designed to induce a sufficient metabolic challenge specific to the primary cardiometabolic risk marker under investigation to ensure ample scope for intervention effects. Mixed macronutrient meals have also been employed for the measurement of both glycaemia and lipaemia or to enhance ecological validity.
Single exercise bouts
Glycaemia and insulinaemia
With children and adolescents being under-represented in the field of exercise metabolism, research on the glycaemic and insulinaemic responses to acute exercise in paediatric populations began to emerge about 10–15 years after(Reference Zakrzewski and Tolfrey49–Reference Cockcroft, Williams and Jackman52) the adult-based literature was reviewed(Reference Henriksen32). To date, studies in samples of healthy adolescent boys have shown that acute moderate-intensity exercise and high-intensity interval exercise (HIIE) can improve indices of glucose control and insulin sensitivity assessed during an oral glucose tolerance test (OGTT) immediately(Reference Cockcroft, Williams and Tomlinson50) and for up to 24 h(Reference Cockcroft, Williams and Weaver51) compared with no exercise. In contrast, 7–10-year-old boys had limited scope for improvement in OGTT-derived indices of glucose control and insulin sensitivity after single bouts of HIIE and moderate-intensity exercise, perhaps due to their younger age(Reference Cockcroft, Williams and Jackman52). The OGTT is primarily intended for diabetes diagnosis and does not necessarily reflect the glycaemic challenges of habitual dietary intakes. Using more ‘ecologically valid’ test meals among female and male healthy adolescents, 60 min of games-based activity improved insulin sensitivity in response to a standardised lunch consumed 60 min post-exercise(Reference Dring, Cooper and Morris53). However, a 60 min school-based football session was only sufficient to reduce glucose concentrations 60 min post-exercise and did not affect the total glucose response (i.e. the area under the curve) or the insulin response(Reference Williams, Cooper and Dring54). Thus, the glycaemic and insulinaemic benefits of acute exercise among young people reported in controlled laboratory environments may not necessarily translate to more ecologically valid settings.
The persistence of improvements in glycaemia and insulinaemia in the days after exercise v. rest has been examined in adolescents described as ‘healthy’ and ‘at risk’ of cardiometabolic disease. Beneficial effects in indices of insulin sensitivity and glucose control were reported 17 h after a 45 min aerobic exercise bout at 75 % peak heart rate in response to a standardised mixed meal among female and male adolescents with habitually low physical activity levels(Reference Short, Pratt and Teague55) and after moderate-intensity exercise and HIIE in response to an OGTT 24 h post-exercise among healthy adolescent boys(Reference Cockcroft, Williams and Weaver51). Such effects may interact with participant characteristics, including weight status; indeed, a bout of exercise at Fatmax (i.e. the intensity that elicits individual peak fat oxidation) with an estimated energy expenditure of 2092 kJ performed 16 h before a high-glycaemic-index breakfast reduced fasting and postprandial insulin concentrations in girls classified as non-overweight (i.e. ‘healthy’ weight), but not in girls classified as overweight/obese(Reference Zakrzewski and Tolfrey49). The lack of change in the girls with overweight/obesity may reflect a degree of metabolic inflexibility or a higher energy and carbohydrate intake to compensate for the exercise-induced energy deficit in this group(Reference Zakrzewski and Tolfrey49). In healthy mixed-sex or male-only samples of adolescents, no effects of 30 or 60 min of high-intensity intermittent running were seen 24 h post-exercise in response to an ecologically valid breakfast meal(Reference Dring, Cooper and Williams56) or at 24 or 48 h after moderate-intensity, HIIE or games-based activity using fasted measures(Reference Cockcroft, Williams and Weaver51,Reference Dring, Cooper and Morris53) . Thus, the time-course of potential post-exercise improvements in glycaemia and insulinaemia among young people requires further investigation, taking into account participant characteristics.
A higher exercise intensity and/or longer duration may enhance the potential for exercise-induced improvements in glucose control and insulin sensitivity due to increased glycogen depletion providing a stimulus for muscle glycogen repletion via GLUT-4, as suggested by adult data(Reference Henriksen32). In line with this, more pronounced glycaemic and insulinaemic benefits among adolescents were seen in response to a 60 v. 30 min bout of high-intensity intermittent running in girls and boys(Reference Dring, Cooper and Williams56) and in response to work-matched high-intensity interval cycling v. moderate-intensity exercise in boys(Reference Cockcroft, Williams and Weaver51). Given the importance of muscle glycogen depletion, it should be highlighted that the nutritional status of participants varies between studies, with exercise performed in the fed(Reference Zakrzewski and Tolfrey49,Reference Williams, Cooper and Dring54,Reference Dring, Cooper and Williams56) or fasted(Reference Cockcroft, Williams and Tomlinson50,Reference Cockcroft, Williams and Weaver51) state. Although dietary intakes on the day of the exercise intervention can often be controlled, this is challenging when participants are unsupervised by the researchers. Thus, control of dietary intake between experimental trials has often been implemented within, but not between participants, using replication of habitual intakes via food diaries(Reference Zakrzewski and Tolfrey49,Reference Cockcroft, Williams and Weaver51) . Such issues may contribute to conflicting findings across studies.
Related to the exercise-induced energy deficit and muscle glycogen depletion, enhanced fat oxidation may partly underpin improvements in insulin sensitivity after acute exercise through a reduced accumulation of fatty acid metabolites (e.g. diacylglycerol, ceramides) within the muscle that can interfere with insulin signalling(Reference Holloway, Bonen and Spriet57,Reference Schenk and Horowitz58) . Such mechanisms could have high relevance to young people with obesity who can already exhibit markers of fatty acid-induced insulin resistance(Reference Sinha, Dufour and Petersen59). Although studies that have measured fatty acid metabolite accumulation in response to exercise in young people are lacking, increased fat oxidation rates have been reported immediately after moderate-intensity exercise and HIIE in adolescent boys(Reference Cockcroft, Williams and Tomlinson50) and about 16 h after exercise among adolescent girls classified as overweight/obese or non-overweight(Reference Zakrzewski and Tolfrey49), whereas 7–10-year-old boys exhibited an increase in fat oxidation immediately after HIIE but not moderate-intensity exercise(Reference Cockcroft, Williams and Jackman52). It is possible that such effects may not apply equally across different age groups and pubertal stages because the child–adolescent–adult transition is accompanied by a reduced reliance on fat and exogenous carbohydrate with a concomitant increased reliance on total and endogenous carbohydrate as fuels during exercise, which is governed by advances in puberty rather than age(Reference Riddell35,Reference Timmons, Bar-Or and Riddell36) . Yet, studies have not typically controlled for pubertal stage, often due to ethical issues associated with the recruitment of participants based on this sensitive marker. Although girls may have higher glucose and insulin responses than boys during puberty, they appear to respond similarly to acute exercise(Reference Dring, Cooper and Morris53,Reference Dring, Cooper and Williams56) ; thus, the influence of sex on between-study comparisons may be minimal.
Although important paediatric literature has accumulated over recent years, an understanding of exercise-induced improvements in glycaemia and insulinaemia remains in its infancy. These data suggest that improvements in glucose control and insulin sensitivity can be achieved through acute exercise bouts. Yet, the extent of such improvements appears to depend on the exercise characteristics (e.g. intensity, duration and mode), sample characteristics (e.g. pubertal stage, sex, baseline insulin resistance, habitual physical activity levels, adiposity) and possible compensatory physical activity and dietary intake responses during the often uncontrolled period between the intervention and outcome measures. Such factors require further investigation to inform physical activity recommendations. With much of the existing paediatric literature based on healthy young people, further research in samples of girls and boys who are at risk of cardiometabolic disease would have valuable public health impact. An insight into the underpinning physiological mechanisms is also required. Indeed, different mechanisms may be at play when compared with adults due to the limited glycogen storage capacity and lower reliance on endogenous glycogen as a fuel in younger, less mature children and adolescents(Reference Riddell35,Reference Timmons, Bar-Or and Riddell36,Reference Eriksson, Gollnick and Saltin60) . That said, it may be possible to apply findings based on adults more readily to adolescents in the later stages of puberty once an ‘adult-like’ metabolic profile has been developed(Reference Riddell35) and after exercise training in boys, where an increased capacity to store and use muscle glycogen has been shown(Reference Eriksson, Gollnick and Saltin60). Yet, since the early muscle biopsy studies in the 1970s and 1980s(Reference Eriksson61), the invasive measures often required to provide insight at the cellular level have been bound to more stringent ethical and technical constraints than the adult literature. More promisingly, advances in technologies have enabled minimally invasive techniques to be employed with young people, including stable isotope tracer techniques and magnetic resonance spectroscopy(Reference Riddell35,Reference Timmons, Bar-Or and Riddell36,Reference Sinha, Dufour and Petersen59) .
Lipaemia
Scientific interest exploring postprandial lipaemic responses to exercise in young people has grown in the last two decades. The maximal TAG-lowering effect of acute exercise is typically delayed until 12–20 h after exercise, coinciding with the exercise-mediated increase in lipoprotein lipase activity that facilitates circulating TAG clearance(Reference Maraki and Sidossis34). Consequently, most paediatric studies have adopted a 2 d model whereby the exercise stimulus is applied the day before measuring metabolic responses to high-fat meals (Fig. 2a). This body of work has shown consistently that moderate- to high-intensity exercise bouts performed 12–18 h before a high-fat meal reduces postprandial TAG concentrations. Although the magnitude of reduction varies highly across studies, the TAG-lowering effect of acute exercise has been demonstrated in both boys and girls classified as lean, overweight and moderately obese. In contrast, using a 1 d protocol, moderate- and high-intensity exercise bouts have yielded negligible effects(Reference Bond, Williams and Isic62,Reference Bond, Gates and Jackman63) . Along with increased lipoprotein lipase activity, the secretion of fewer, TAG-richer, VLDL particles from the liver that have a higher affinity for lipoprotein lipase have been proposed in adults as a central mechanistic pathway responsible for the exercise-induced reduction in postprandial lipaemia(Reference Ghafouri, Cooney and Bedford64).
A key determinant of the reduction in postprandial lipaemia the day after exercise in adults is the exercise-induced energy expenditure(Reference Freese, Gist and Cureton33,Reference Maraki and Sidossis34) . In contrast, there is little evidence of clear dose-dependent relationships between exercise energy expenditure and reduced postprandial TAG concentrations in young people(Reference Tolfrey, Doggett and Boyd65–Reference Tolfrey, Engstrom and Murphy67). Specifically, similar exercise-induced reductions in postprandial lipaemia were observed in 12–14-year-old boys when comparing two exercise bouts varying in intensity (60 min at 53 v. 75 % of peak oxygen uptake)(Reference Tolfrey, Doggett and Boyd65) and duration (30 v. 60 min at 55 % of peak oxygen uptake)(Reference Tolfrey, Bentley and Goad66) despite a 2-fold difference in gross energy expenditure. Furthermore, a 60, but not 30 min, exercise bout (1536 v. 777 kJ, respectively) reduced postprandial TAG concentrations in 10–14-year-old girls, and does not support a dose-dependent relationship given the 30 min bout did not induce an ‘intermediate’ effect(Reference Tolfrey, Engstrom and Murphy67). Nevertheless, about 56 % of the female participants experienced a reduction in postprandial TAG concentrations after 30 min of exercise, suggesting some girls may accrue metabolic health benefits from lower volumes of exercise(Reference Tolfrey, Engstrom and Murphy67).
The TAG-lowering effects of single exercise bouts have been demonstrated consistently in response to laboratory-based exercise protocols involving moderate- to vigorous-intensity treadmill walking or running and cycling that meet the current physical activity guidelines for young people(Reference Tolfrey, Doggett and Boyd65–Reference Thackray, Barrett and Tolfrey74). Alternatively, other studies have also highlighted the efficacy of acute intermittent(Reference Barrett, Morris and Stensel68,Reference Smallcombe, Barrett and Morris75) or high-intensity(Reference Thackray, Barrett and Tolfrey76–Reference Sedgwick, Morris and Nevill79) exercise regimens for reducing postprandial lipaemia early in life. Such protocols may better match the sporadic stop–start activity habits of young people(Reference Armstrong and Welsman37) and offer an alternative form of activity that can evoke important metabolic health benefits. Desirable reductions in postprandial lipaemia have been reported in response to repeated 1 min running intervals performed at 100 % of peak oxygen uptake(Reference Thackray, Barrett and Tolfrey76,Reference Thackray, Barrett and Tolfrey77) in addition to repeated short (about 6 s) maximal running(Reference Smallcombe, Barrett and Sherar78) and cycling(Reference Sedgwick, Morris and Nevill79) sprints. In the earliest exercise postprandial study in young people, simulated games activity comprising repeated blocks of walking, sprinting, cruising, jogging and rest reduced postprandial lipaemia by 26 % in adolescent boys (mean age 15⋅4 years)(Reference Barrett, Morris and Stensel68). A similar reduction in postprandial TAG concentrations was reported more recently the day after 11–13-year-old boys completed a 48 min session of school-based free-living soccer(Reference Smallcombe, Barrett and Morris75). The magnitude of reduction after the soccer activity was comparable to that observed after time-matched moderate-intensity treadmill exercise (25 v. 18 %, respectively), but importantly provides a more representative form of activity for young people and advances insights gained from laboratory-based studies that dominate the paediatric literature(Reference Smallcombe, Barrett and Morris75).
Beneficial effects of single exercise bouts on postprandial lipaemia have been observed in boys and girls classified as overweight or moderately obese(Reference MacEneaney, Harrison and O'Gorman69,Reference Lee, Burns and White70) . A single bout of 60 min treadmill exercise at 65 % of peak oxygen uptake evoked an equivalent reduction (about 20 %) in postprandial lipaemia in late-adolescent boys classified as lean or overweight(Reference MacEneaney, Harrison and O'Gorman69). More recently, 60 min of moderate-intensity cycling reduced postprandial TAG concentrations in black and white adolescents (12–18-years-old) classified as overweight(Reference Lee, Burns and White70). The TAG-lowering effects of exercise were more pronounced in the white than black adolescents (19 v. 8 %, respectively), with increased visceral adipose tissue identified as a strong determinant of the exercise-induced reduction in postprandial lipaemia in the white adolescents(Reference Lee, Burns and White70). Despite the elevated adiposity status, the participants in these studies were otherwise healthy. Nevertheless, these findings provide a foundation for future work in young populations at elevated cardiometabolic disease risk (e.g. hypertriglyceridaemia, insulin resistant) where augmenting postprandial metabolic health may have most clinical relevance.
Another avenue of inquiry has investigated whether the acute benefit of exercise on postprandial lipaemia is due to the associated energy deficit or driven by skeletal muscle contraction itself. Immediate dietary replacement of the exercise energy expenditure counteracted the reduction in postprandial TAG concentrations observed the day after a bout of moderate-intensity exercise in 11–13-year-old boys(Reference Thackray, Barrett and Tolfrey74). Thus, maintenance of the energy deficit in the immediate post-exercise period may be required to maximise the metabolic health benefits. Other studies have explored the potential differential effects of imposing energy deficits from acute exercise and/or energy intake restriction. This work has demonstrated that a moderate-intensity exercise (about 1⋅5 MJ) bout was a more potent stimulus for reducing postprandial lipaemia than an equivalent diet-induced energy deficit in 11–13-year-old girls(Reference Thackray, Barrett and Tolfrey73). Subsequently, Thackray et al.(Reference Thackray, Barrett and Tolfrey77) compared the effect of 10 × 1 min high-intensity treadmill runs and 5 × 1 min high-intensity treadmill runs combined with a controlled restriction in habitual energy intake at the evening meal (about 0⋅82 MJ) in girls (mean age 12⋅1 years). The reduction in postprandial TAG concentrations reported after high-intensity running alone was similar in magnitude when the smaller exercise dose was superimposed with energy intake restriction (10 v. 9 % respectively)(Reference Thackray, Barrett and Tolfrey77). Therefore, combinations of exercise and mild dietary energy intake restriction may offer a practical alternative for young people to acquire metabolic health benefits, particularly those who struggle to accumulate a sufficient level of physical activity.
Short, frequent physical activity bouts
Short, frequent physical activity bouts that break up sedentary behaviour have received growing interest as evidence of the high prevalence of sedentary behaviour across the lifespan continues to accumulate(Reference Bauman, Petersen, Blond, Leitzmann, Jochem and Schmid80). Sedentary behaviours are any waking behaviours characterised by an energy expenditure ≤1⋅5 METs, while in a sitting, reclining or lying posture(Reference Tremblay, Aubert and Barnes81), which can be replaced by physical activity of a light (1⋅6–2⋅9 METs), moderate (3⋅0–5⋅9 METs) or vigorous (≥6⋅0 METs) intensity during waking hours. This avenue of research is timely as sedentary behaviour has emerged as a strong determinant of mortality and cardiometabolic disease risk in adults(Reference Ekelund, Tarp and Steene-Johannessen82,Reference Patterson, McNamara and Tainio83) and is associated with adverse cardiometabolic health outcomes in young people(Reference Carson, Hunter and Kuzik84). Efforts to mitigate the deleterious effects of prolonged sitting (a common proxy for sedentary behaviour) through short, frequent physical activity bouts are likely to have greater appeal for those who are not motivated to perform continuous bouts of structured exercise, where paediatric populations typically perceive prolonged exercise as more demanding than adults(Reference Timmons and Bar-Or85). This pattern of activity may also more closely reflect the sporadic physical activity patterns of children and adolescents(Reference Armstrong and Welsman37) and be more conducive to integrate into the school day.
A meta-analysis of studies across the lifespan showed that interrupting sitting with short, frequent light- to moderate-intensity physical activity bouts reduces postprandial glucose and insulin, but not TAG, concentrations(Reference Saunders, Atkinson and Burr86). Although the impact of disrupting sitting with regular activity bouts on postprandial cardiometabolic risk markers was not modified by age, a closer examination of the paediatric-specific studies presented here suggests relatively modest and inconsistent effects. In this regard, breaking up prolonged sitting with 3 min moderate-intensity walking bouts performed every 30 min during a 3 h OGTT reduced glucose and insulin concentrations but did not alter postprandial lipaemia in 7–11-year-old children with healthy weight(Reference Belcher, Berrigan and Papachristopoulou87) and reduced insulin concentrations in 7–11-year-old children who were overweight or obese(Reference Broadney, Belcher and Berrigan88). Using less frequent physical activity bouts in 13–14-year-old boys, accumulating four bouts of work-matched high-intensity physical activity (2 × 1 min intervals at 90 % peak power) reduced the postprandial glucose response on the intervention day when compared with continuous moderate-intensity (about 6 min at 90 % gas exchange threshold) exercise and a rest control trial, although no effect was observed for postprandial lipaemia(Reference Bond, Williams and Jackman89). In contrast, postprandial glucose, insulin and TAG concentrations were all unaffected in response to interrupting sitting with 2 min light-intensity walking bouts every 20 min for 8 h compared to 8 h of uninterrupted sitting in 10–14-year-olds; this lack of effect was maintained even when the 2 min light walking breaks were supplemented with two, 20 min moderate-intensity treadmill bouts(Reference Saunders, Chaput and Goldfield90). Similarly, breaking up sitting time with bouts of self-paced walking and standing accumulated during two consecutive simulated school days did not alter postprandial glucose, insulin or TAG concentrations assessed on the second simulated school day or the next day in 11–13-year-old physically inactive girls(Reference Smallcombe, Biddle and Slater91). Thus, the immediate and delayed effect of an ecologically valid standing and walking intervention appears to be negligible.
Discrepancies between studies are most likely explained by differences in participant characteristics and study design features, including variations in the physical activity or exercise stimulus, meal content or glycaemic load, timing of activity bouts and frequency of outcome assessments. Specifically, the relatively low frequency of blood sampling of ≥90 min intervals in some studies may have missed important glycaemic fluctuations in close proximity to the meals and activity/standing bouts(Reference Saunders, Chaput and Goldfield90,Reference Smallcombe, Biddle and Slater91) . Using continuous glucose monitoring devices that measure interstitial glucose concentrations at about 5 min intervals, interrupting prolonged sitting with 2 min resistance activity bouts every 18 min reduced the incremental, but not total, glucose area under the curve for 3 h after both breakfast and lunch meals in adolescents aged 14–17 years(Reference Fletcher, Salmon and McNaughton92). Yet, these changes were relatively subtle in magnitude with detection likely facilitated by the improved sensitivity of continuous glucose monitors to capture glucose dynamics.
The lack of consistent reductions in postprandial lipaemic responses may be a consequence of the 1 d study protocol often employed in these studies(Reference Belcher, Berrigan and Papachristopoulou87–Reference Saunders, Chaput and Goldfield90). In this regard, accumulating 6 × 10 min bouts of running at 70 % of peak oxygen uptake did not alter next day postprandial glucose and insulin concentrations, but did reduce total and incremental area under the TAG curve by 11 and 16 %, respectively, in adolescent boys aged 12–14 years(Reference Sedgwick, Morris and Nevill72). These differences did not reach statistical significance, but are similar in magnitude to other exercise postprandial studies in children and adolescents. In support of the delayed effect of exercise on postprandial lipaemia, an in-school protocol in 11–13-year-old boys comparing the effect of 40 × 30 m sprints in one uninterrupted after school bout and accumulated in four bouts of 10 × 30 m sprints strategically placed during natural breaks in the school day reported reductions in postprandial TAG concentrations the next day, despite no effect on the day of exercise(Reference Smallcombe, Barrett and Sherar78). This may provide a viable model for translating the metabolic health benefits reported with laboratory-based exercise protocols into free-living settings providing the exercise stimulus is of sufficient duration and/or intensity. Importantly, since favourable effects among adults are most consistently seen in clinical, overweight/obese and physically inactive populations(Reference Saunders, Atkinson and Burr86), further research in such paediatric populations is warranted and has high public health relevance.
Acute breakfast consumption–physical activity interactions for cardiometabolic health
Cross-sectional and prospective observational studies show that frequent breakfast consumption is associated with reduced cardiometabolic disease risk in young people(Reference Monzani, Ricotti and Caputo41,Reference Souza, Neves and Gorgulho93) and manifestation in adults(Reference Ballon, Neuenschwander and Schlesinger94,Reference Chen, Zhang and Ge95) . Such findings may in part be explained by higher free-living physical activity and enhanced glucose control in response to breakfast consumption v. breakfast omission, as reported in randomised controlled trials and crossover trials in adults(Reference Betts, Richardson and Chowdhury42–Reference Jovanovic, Leverton and Solanky47). An eating pattern that includes breakfast to aid the provision of a continuous carbohydrate supply may be of greater importance to young people v. adults due to their elevated resting and physical activity energy expenditures(Reference Harrell, McMurray and Baggett96,Reference Rogol, Clark and Roemmich97) and higher reliance on exogenous carbohydrate as a fuel(Reference Timmons, Bar-Or and Riddell36). Indeed, increased exogenous glucose availability (i.e. a physiological response) as well as perceived energy levels (i.e. a psychological response) are potential mediators of the breakfast–physical activity relationship. An understanding of such responses is particularly relevant to adolescent girls due to the adolescent decline in breakfast consumption frequency and physical activity levels as well as pubertal insulin resistance disproportionately affecting girls(Reference Moran, Jacobs and Steinberger18,Reference Jeffery, Metcalf and Hosking19,Reference Cooper, Dring and Morris23,Reference Farooq, Martin and Janssen40,Reference Monzani, Ricotti and Caputo41) . Depending on the country and definition of breakfast employed, the prevalence of skipping breakfast can range from 10 to 30 % among young people, and is higher among adolescents (v. children) and girls (v. boys)(Reference Monzani, Ricotti and Caputo41).
Inconsistent cross-sectional findings on the association between breakfast consumption and objectively measured physical activity among young people(Reference Corder, van Sluijs and Steele98–Reference Vissers, Jones and Corder100) highlight the need for experimental trials to provide more definitive causative interpretations of the findings with reduced interference from confounders. Using a 3 d crossover trial to compare standardised breakfast consumption v. breakfast omission in adolescent girls, physical activity was unaffected despite incomplete energy intake compensation of the breakfast meal(Reference Zakrzewski-Fruer, Plekhanova and Mandila101). If repeated habitually, this lack of energy compensation would be expected to result in weight gain in response to breakfast consumption v. omission, contradicting the higher adiposity reported among habitual breakfast skippers(Reference Monzani, Ricotti and Caputo41,Reference Souza, Neves and Gorgulho93) . Yet, it is possible that wrist-worn accelerometry used in this study was not sensitive enough to detect differences in physical activity in response to breakfast manipulation. Using combined heart rate accelerometry, a 7 d crossover trial reported that adolescent girls spent more time in light-intensity physical activity before 10:30 and after school, and less time sedentary after school during daily v. intermittent (i.e. alternate breakfast omission and consumption) standardised breakfast consumption(Reference Zakrzewski-Fruer, Wells and Crawford102). The 19⋅8 min/d higher light-intensity physical activity during daily v. intermittent breakfast consumption complemented previous findings among adults that breakfast-induced changes in physical activity may be characterised by the replacement of sedentary behaviours with spontaneous light-intensity physical activity, whereas moderate- to vigorous-intensity physical activity is typically planned and structured(Reference Betts, Richardson and Chowdhury42). Interestingly, no differences were reported during the 10:30–15:00 time segment, possibly because the school timetable restricts physical activity opportunities, limiting the ‘window of opportunity’ among school-aged young people. Thus, previous reports that morning physical activity typically before around 12:00 has been shown to be particularly sensitive to breakfast manipulation in adults(Reference Betts, Richardson and Chowdhury42–Reference Yoshimura, Hatamoto and Yonekura44) may not apply to adolescents during school days. Although it is plausible that contrasting the extremes of daily breakfast omission v. consumption across 7 d may confer more pronounced effects, no difference in physical activity quantified via combined heart rate accelerometry was found when comparing these extremes in adolescent girls who habitually skipped breakfast(Reference Zakrzewski-Fruer, Seall and Tolfrey103). Nevertheless, the girls were able to accurately compensate after 10:30 for the missed energy at breakfast through increased energy intake, primarily in the form of carbohydrate, during breakfast omission v. breakfast consumption. The lack of physical activity response may have been because the girls were already accustomed metabolically and behaviourally to skipping breakfast, suggesting that habituation to breakfast omission could alter responses to breakfast manipulation. However, ethical restrictions pose challenges in asking girls who habitually consume breakfast to engage in extended periods of breakfast omission.
Inconsistent extant findings may be due to environmental constraints inherent to the lives of adolescent girls, including the school timetable, extra-curricular activities, family routines and commitments. As such, further research that includes opportunities to be physically active would be useful in extending current knowledge. Adding a further degree of complexity, the nature of the causal relationship between breakfast and physical activity may also depend on physical activity intentions. Indeed, breakfast may promote physical activity or, alternatively, intentions to engage in physical activity may prompt breakfast consumption in preparation, and early morning exercise may result in ‘breakfast consumption’ (i.e. within 2–3 h of waking)(Reference O'Neil, Byrd-Bredbenner and Hayes104). Studies in this area have used carbohydrate-based breakfasts to examine the potential impact on free-living physical activity as this is the primary fuel for physical activity(Reference Zakrzewski-Fruer, Plekhanova and Mandila101–Reference Zakrzewski-Fruer, Seall and Tolfrey103); nevertheless, the effect of breakfast quantity and quality requires investigation. Additionally, data among boys, younger children and ‘at risk’ young people are required to inform population-specific recommendations.
Due to the lack of clarity on the potential association and/or causal link between breakfast consumption and physical activity among adolescents, alternative mediating factors may play a more prominent role in explaining the favourable cardiometabolic health profiles of habitual breakfast consumers. Among adults, breakfast consumption attenuates glycaemic and insulinaemic responses to subsequent meals when compared to breakfast omission, as termed the ‘second-meal effect’(Reference Chowdhury, Richardson and Tsintzas45–Reference Jovanovic, Leverton and Solanky47). If repeated habitually, a series of moderated glycaemic responses induced by breakfast consumption could reduce cardiometabolic disease risk through a reduced magnitude of glycaemic excursions and glycaemic variability(Reference Blaak, Antoine and Benton7,Reference Heine, Balkau and Ceriello8) . A key mechanism for the ‘second-meal effect’ in adults is increased glucose conversion into muscle glycogen(Reference Jovanovic, Leverton and Solanky47). Such responses may differ in adolescents due to their lower reliance on endogenous glucose and reduced capacity for muscle glycogen storage, particularly in younger, less mature individuals(Reference Riddell35,Reference Timmons, Bar-Or and Riddell36,Reference Eriksson and Saltin105) , and with an estimated 32 % reduction in insulin sensitivity occurring from pre- to mid-puberty(Reference Goran and Gower17). The slower rate of gastric emptying that has also been proposed to contribute to attenuations in glycaemia in response to breakfast consumption v. omission in adults(Reference Gonzalez48) may, again, not apply equally to young people. Indeed, puberty, age and sex may affect gastric emptying in healthy children and adolescents, with slower rates reported in girls v. boys and during mid-late puberty v. pre-puberty(Reference Kovacic, Zhang and Nugent Liegl106). Unfortunately, current knowledge on breakfast consumption and glycaemia among adolescents relies on a few novel studies that have pooled data from adolescents and adults aged 13–20-year-olds with little consideration of pubertal status, age and sex(Reference Alwattar, Thyfault and Leidy107,Reference Bauer, Reynolds and Douglas108) ; hence, the responses of children and adolescents specifically remain unknown.
The available research based on pooled adolescent–adult data suggests that the ‘second-meal’ effect depends on factors such as habitual breakfast patterns and breakfast meal composition. In a novel study of girls classified as overweight/obese (mean age 19 years), 3 d of skipping breakfast v. consuming a normal-protein (12 g protein) breakfast v. consuming a high-protein (32 g protein) breakfast did not affect post-lunch glycaemic and insulinaemic responses when assessed on day 4 among habitual breakfast skippers(Reference Alwattar, Thyfault and Leidy107). However, the habitual breakfast consumers, who did not complete the breakfast skipping trial, had lower afternoon and daily glucose responses following the higher-protein breakfast v. the normal-protein breakfast(Reference Alwattar, Thyfault and Leidy107). Extending a similar protocol over 12 weeks with a similar yet mixed-sex sample, high protein (35 g) breakfast consumption appeared to be more beneficial for free-living glycaemia than normal protein (13 g) breakfast consumption(Reference Bauer, Reynolds and Douglas108); yet, this was not an acute, controlled study and reductions in energy intake, unhealthy evening snacking of high-fat/high-sugar foods may have contributed to the findings(Reference Leidy, Hoertel and Douglas109). Other than age and/or puberty, a potential reason for the unclear findings to date may be that the repeated ingestion of glucose appears to be key to inducing the ‘second-meal effect’ among adults, with low glycaemic index breakfasts being particularly beneficial(Reference Bonuccelli, Muscelli and Gastaldelli110,Reference Liljeberg and Björck111) . Further, the energy content of the breakfast meals(Reference Alwattar, Thyfault and Leidy107,Reference Bauer, Reynolds and Douglas108) was modest when considering data in adults(Reference Chowdhury, Richardson and Tsintzas45–Reference Jovanovic, Leverton and Solanky47) and recommendations that breakfast should provide at least 20 % of daily energy needs(Reference Gibney, Barr and Bellisle112,Reference Monteagudo, Palacin-Arce and Mdel113) . Considering daily glucose profiles, low glycaemic index breakfasts also induce a lower immediate response compared with high glycaemic index breakfast among adolescents(Reference Cooper, Dring and Morris23,Reference Zakrzewski, Stevenson and Tolfrey114) . Using a standardised carbohydrate-rich low glycaemic index breakfast, our recent work with adolescent girls who consumed breakfast habitually demonstrated significant and large reductions in peak, post-lunch (2 h) and total trial (5 h) glycaemia and insulinaemia in response to breakfast consumption v. omission followed by a standardised high glycaemic lunch provided at 3 h, with minimal inter-individual variability(Reference Zakrzewski-Fruer, Morari and Champion115). Further, enjoyment during exercise performed in the afternoon was enhanced with breakfast consumption v. omission(Reference Zakrzewski-Fruer, Morari and Champion115). Although this work did not target adolescents ‘at risk’ of poor glycaemic control, it suggests that moderations in glycaemia with breakfast consumption may be important in driving a favourable cardiometabolic health profile. Further research on the possible interactive effect of breakfast consumption and physical activity requires investigation due to the potential link between these two variables(Reference Corder, van Sluijs and Steele98–Reference Vissers, Jones and Corder100,Reference Zakrzewski-Fruer, Wells and Crawford102) . Such experimental research would help move closer to developing an evidence-based standardised definition of breakfast consumption for health that can be employed by researchers and practitioners.
Future directions: opportunities to impact human and planetary health using active travel to school as an example
With preventable cardiometabolic diseases and climate change progressively escalating over recent decades due to a complex interplay of modern human practices, multi-disciplinary approaches that target certain human behaviours may contribute to addressing these global challenges simultaneously. Results on the co-benefits of physical activity and nutrition in young populations for human and planetary health are of high interest, particularly due to cardiometabolic disease risk reaching concerning levels among young people and global climate change placing uncertainty on their futures (see Fig. 1)(Reference Gasparri, Eissa and Imbago-Jácome116). Active travel to school offers a physical activity opportunity for school-aged young people that can benefit both (their) human and planetary health. Further, morning active travel is in close proximity to the breakfast meal, providing a pathway for potential breakfast–physical activity interactions, as discussed previously(Reference Corder, van Sluijs and Steele98–Reference Vissers, Jones and Corder100,Reference Zakrzewski-Fruer, Wells and Crawford102) . Concurrently, active travel reduces the greenhouse gas emissions associated with motorised vehicle transport, thus assisting climate change mitigation. In contrast to many physical activity opportunities, transport is most often a stipulation of everyday life with almost all school-aged young people travelling to school across the school year. Further, active travel may offer a convenient route to build physical activity into the day with a minimal time burden and interference with daily tasks; this is important given that lack of time or competing activities for time are common barriers to physical activity(Reference Martins, Costa and Sarmento117). Thus, encouraging school-based active travel may be a feasible approach to increase population-level physical activity levels.
Accumulating evidence among children and adolescents outlined in this review paper suggests that the likelihood of achieving cardiometabolic health benefits through active travel will depend on the duration, intensity (affected by the nature of the route, inclines and terrain), mode (e.g. walking, cycling, scooting) and frequency of the physical activity characterising the active travel(Reference Zakrzewski and Tolfrey49–Reference Dring, Cooper and Williams56,Reference Tolfrey, Doggett and Boyd65–Reference Sedgwick, Morris and Nevill79) . Thus, it is important that such factors are amenable to change. The weight of the evidence from intervention and prospective studies among young people and adults combined supports the positive effects of active travel over longer periods and distances on cardiometabolic health outcomes(Reference Saunders, Green and Petticrew118). Yet, further research utilising study designs that can infer causality, including acute studies, with measures of cardiometabolic disease risk other than obesity is required to draw stronger conclusions among children and adolescents specifically(Reference Saunders, Green and Petticrew118). Determining the active travel characteristics that can confer health benefits would inform a health-based definition of ‘active travel’ that could be used in research and practice, which is currently lacking(Reference Saunders, Green and Petticrew118).
Considering real-life application, it may be most feasible for active travel to contribute to light- or moderate-intensity physical activity; although, active commutes have potential to reach vigorous intensities in certain instances, such as up-hill cycling. It is also crucial that the duration of the commute is sufficient to contribute to daily physical activity, which may be a challenge in young children who often live in close proximity to school(Reference Metcalf, Voss and Jeffery119). However, high levels of air pollution on some commutes may negate some of the positive health benefits, especially for children who are more susceptible than adults due to greater physiological sensitivity to air pollutants and higher respiration rates(Reference Al-Kindi, Brook and Biswal120). Additionally, active travel may interact with daily diet and physical activity behaviours, and such potential compensatory responses might either negate or enhance any effects of active travel. With future research, an understanding of the acute and chronic cardiometabolic risk marker responses to active travel across different intensities, modes, durations and frequencies has potential to inform messages that could help promote active travel for both health and climate change mitigation. Additionally, research focused on adaptation to climate change, such as strategies to keep cool during physical activity in extreme heat, will be important to promote active travel in our changing climates.
Conclusions
Cardiometabolic disease prevention is more effective than a cure and offers co-benefits for humans and the planet. Focusing on young populations to understand their distinct responses to physical activity and dietary manipulations from an early age can help inform disease prevention efforts. Knowledge of the cardiometabolic responses to acute physical activity and breakfast manipulations using controlled experimental designs among young people is growing, yet remains in its infancy when compared with the adult literature. Existing evidence suggests that acute bouts of moderate- to high-intensity exercise can attenuate postprandial plasma glucose, insulin and TAG concentrations for up to 24–48 h in young people. Accumulating physical activity throughout the day with short, frequent bouts offers another potential route to attenuate postprandial cardiometabolic responses, although the benefits have not been reported consistently among young people. Likewise, although further evidence is required to draw firmer conclusions, breakfast consumption may reduce glycaemic responses to subsequent meals consumed throughout the day and enhance free-living physical activity for a possible additive health impact. If repeated habitually, such responses to physical activity and breakfast manipulations would be of benefit to long-term cardiometabolic health, yet appear to depend on the study design and the physical activity, test meal and individual characteristics. To help maximise the potential cardiometabolic effects for public health, further understanding of such moderating factors and more research in samples of ‘at risk’ young people (e.g. overweight/obese, physically inactive, prediabetic) would be beneficial. Further, technological advances may offer minimally invasive avenues to understand cellular-level responses for greater mechanistic insights. Determining whether findings based within a controlled laboratory environment translate to free-living settings with ecologically valid interventions also requires further study. Given that climate change is a serious threat that will disproportionally affect our younger generations and is relevant to cardiometabolic health, this future research should pay consideration to important planetary co-benefits.
Acknowledgements
A. E. T. acknowledges support from the National Institute for Health Research (NIHR) Leicester Biomedical Research Centre. The views expressed are those of the authors and not necessarily those of the NHS, the NIHR or the Department of Health.
Financial Support
This research received no specific grant from any funding agency, commercial or not-for-profit sectors.
Conflict of Interest
None.
Authorship
The authors had sole responsibility for all aspects of preparation of this paper.