Ipilimumab is a novel monoclonal antibody used in the treated of metastatic melanoma. Sarcopenia has recently emerged as an important predictor chemotherapeutic drug efficacy and toxicity ( Reference Prado, Lieffers and McCargar 1 , Reference Mourtzakis, Prado and Lieffer 2 ) and as well as poor performance status and shortened survival in cancer patients( Reference Prado, Lieffers and McCargar 1 ). Sarcopenia can develop rapidly after a diagnosis of cancer, particularly in the metastatic setting. To-date no studies have examined the prevalence of sarcopenia in metastatic melanoma or the impact of ipilimumab on muscle mass and clinical outcomes.
The aim of this study was to examine the prevalence of sarcopenia in metastatic melanoma and to examine the impact of ipilimumab on longitudinal changes in body composition. Patients with metastatic melanoma, treated with ipilimumab between 2009–2015 at two university teaching hospitals were included. Body composition was assessed by CT scan( Reference Mourtzakis, Prado and Lieffer 2 ) at the 3rd lumbar vertebra using OsiriX® software (Pixmeo, Geneva, Switzerland) at baseline and after 4 cycles of Ipilmumab (12 weeks) and included measurement of skeletal muscle, visceral and subcutaneous adipose tissue. Cut off points for sarcopenia were set at 55·4 cm2/m2 for men and 38·9cm2/m2 for women( Reference Mourtzakis, Prado and Lieffer 2 ). Estimates of whole body fat free mass (FFM) were calculated using published regression equations( Reference Mourtzakis, Prado and Lieffer 2 ).
Seventy-four patients were treated with Ipilimumab, however only 42 patients had both baseline and 3-month imaging available for analysis. There were 24 men (57 %) and 18 women. The mean age was 53 (±17) years and the mean BMI was 28·15 ± 6·5 kg/m2. 71 % of patients were considered to be overweight or obese according to WHO standards. Sarcopenia was present at baseline in 31 % of patients and it was prevalent across all BMI categories. Skeletal muscle area (SMA) was significantly reduced from baseline to follow up (150·1 ± 38·8 vs 145·4 ± 39·7cm2, p = 0·047). Mean SMA change was 4·74 ± 14·9cm2. 38 % of patients experienced a meaningful muscle loss of >6cm2 between the baseline and follow up scan (14·3 % experienced meaningful muscle gain). This cut point was chosen, as it is equivalent to 1 kg of skeletal muscle and is associated with physical function( Reference Prado, Sawyer and Gosh 3 ). Fat free mass significantly decreased from 51·09 kg (±11·65) to 49·67 kg (±11·18) (p = 0·04) and muscle density was also significantly reduced (mean loss of 4·15 mean HU) (P = 0·001). The prevalence of sarcopenia thereby increased significantly from 31 % (n = 13) at baseline to 52·4 % (n = 22) by completion of Ipilimumab (p = 0·004). Adipose tissue index (cm2/m2) and fat mass (kg) were also significantly reduced (p = 0·01,p = 0·026 respectively). 45 % of patients experienced a meaningful adipose tissue loss of >14·7cm2(3), with a mean loss of 28·6 ± 70·15cm2.
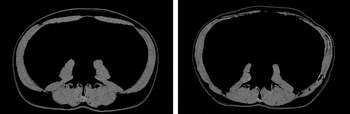
Fig. 1. Male, 47years old. SMA at L3 before (left) and after (right) treatment. Images taken 3 months apart. 26·8cm2 loss of muscle. SMI reduced from 62cm2/m2 (non-sarcopenic) to 54·7cm2/m2 (sarcopenic)
While this study was limited in terms of sample size, we observed significant and rapid loss of muscle within this patient group over a short time frame. These changes in muscle mass cannot be explained by the age related loss of muscle since measurements were taken within 5 months. The reduction in muscle mass may relate to fatigue experienced by patients during treatment resulting in reduced physical activity. 52·4 % of patients experienced fatigue (any grade), while 19 % reported experiencing grade 3 or 4 fatigue.
In conclusion metastatic melanoma patients treated with Ipilimumab experience significant and rapid loss of muscle mass during treatment with Ipilimumab. This muscle loss is masked by excessive adiposity. Patients should be encouraged toward isometric exercise and nutritional intervention to maximise protein intake in the hope of retaining muscle mass.





