Introduction
Body type theories, proposed by Kretschmer and Sheldon, are historical concepts that attempt to correlate somatotypes and personalities or psychiatric disorders (Kretschmer, Reference Kretschmer1925; Sheldon, Reference Sheldon1940). Accordingly, Kretschmer classified four types of people: (1) the asthenic type who has a slender body (‘leptosome’) and is more prone to schizophrenia (SCZ); (2) the pyknic type who has a round body and is likely to become manic-depressive illness [bipolar disorder (BD)]; (3) the athletic type with a muscular body who may suffer from epilepsy; and (4) the dysplastic type who cannot be classified as any of the other three types (Kretschmer, Reference Kretschmer1925). Sheldon had a similar insight, classifying people into ectomorphic (corresponding to Kretschmer's asthenic type), endomorphic (corresponding to the pyknic type), and mesomorphic (corresponding to the athletic type). Interestingly, Sheldon also claimed that there was a deeper connection, with genetic components being linked to both the somatotypes and personality (Sheldon, Reference Sheldon1940). Such classifications proposed by these celebrated psychiatrists or psychologists were based on their observational surveys in the beginning of the twentieth century; however, scientific evidence thereof is limited (Sorensen et al. Reference Sorensen, Mortensen, Reinisch and Mednick2006; Zammit et al. Reference Zammit, Rasmussen, Farahmand, Gunnell, Lewis and Tynelius2007).
Nevertheless, recent genetic studies have re-evaluated their body type theories by conducting genetic correlation analysis, which employs genetic association results from genome-wide association studies (GWASs) to examine the genetic relationship between two independent phenotypes, that is, theoretically, excluding environmental factors. The first study reported a significant correlation between SCZ and low body mass index (BMI) in a European (EUR) ancestry (Bulik-Sullivan et al. Reference Bulik-Sullivan, Finucane, Anttila, Gusev, Day and Loh2015); however, no correlation was observed between BD and BMI (Bulik-Sullivan et al. Reference Bulik-Sullivan, Finucane, Anttila, Gusev, Day and Loh2015). Following this, we conducted a study in which we replicated a significant negative correlation between SCZ and BMI, but again found no correlation between BMI and BD in the Japanese samples (Akiyama et al. Reference Akiyama, Okada, Kanai, Takahashi, Momozawa and Ikeda2017). More recently, the PGC2 major depressive disorder (MDD) group has reported a positive correlation between BMI and MDD (Major Depressive Disorder Working Group of the PGC, Reference Wray and Sullivan2017), using EUR samples.
Method
These results motivated us to conduct a meta-analysis (using the ‘metafor’ package in R project: https://www.r-project.org/) to estimate the combined polygenic relationships between two datasets from different populations:
(1) for SCZ and BD in EUR datasets, we updated the genetic correlation using LD score regression analysis (Bulik-Sullivan et al. Reference Bulik-Sullivan, Finucane, Anttila, Gusev, Day and Loh2015) (removing MHC SNPs from 26Mb to 34Mb and using SNPs with imputation INFO >0.7) based on the Psychiatric GWAS Consortium data only for the EUR [PGC2(49) for SCZ (Schizophrenia Working Group of the Psychiatric Genomics Consortium, 2014) and PGC-BD (Psychiatric GWAS Consortium Bipolar Disorder Working Group, 2011: https://www.med.unc.edu/pgc/results-and-downloads/] and GIANT GWAS 2015 (Locke et al. Reference Locke, Kahali, Berndt, Justice, Pers and Day2015: https://portals.broadinstitute.org/collaboration/giant/images/1/15/SNP_gwas_mc_merge_nogc.tbl.uniq.gz).
(2) For the Japanese SCZ samples, we used our published results (Akiyama et al. Reference Akiyama, Okada, Kanai, Takahashi, Momozawa and Ikeda2017). For the Japanese BD subjects, we updated the genetic correlation between the BMI (Akiyama et al. Reference Akiyama, Okada, Kanai, Takahashi, Momozawa and Ikeda2017) and the BD results (INFO >0.7), which we also published recently (Ikeda et al. Reference Ikeda, Takahashi, Kamatani, Okahisa, Kunugi and Mori2018).
(3) In the MDD analyses, we used the results of the PGC2-MDD (Major Depressive Disorder Working Group of the PGC, Reference Wray and Sullivan2017) in a EUR dataset [BMI dataset was GIANT GWAS 2015 (Locke et al. Reference Locke, Kahali, Berndt, Justice, Pers and Day2015)], and
(4) calculated genetic correlations using the CONVERGE Consortium [Chinese females only: INFO >0.816 because summary statistics over this threshold were publicly available: (CONVERGE consortium, 2015)] and our BMI results [Japanese females only: INFO >0.816: (Akiyama et al. Reference Akiyama, Okada, Kanai, Takahashi, Momozawa and Ikeda2017)] for the dataset for East Asian ancestry (EAS).
As an exploratory investigation, and in an attempt to replicate the findings from the current meta-analysis, we also conducted trans-ethnic genetic correlation analysis (BMI in EUR and psychiatric disorders in EAS, and vice versa) using the Popcorn software (Brown et al. Reference Brown, Ye, Price and Zaitlen2016), following a default protocol (https://github.com/brielin/Popcorn).
All procedures contributing to this work complied with the ethical standards of the relevant national and institutional committees on human experimentation and with the Helsinki Declaration of 1975, as revised in 2008.
Results
In Fig. 1, the forest plots for the genetic correlations between BMI and SCZ/BD/MDD are shown. The combined estimate of r g for SCZ was −0.094 [fixed effect model: standard error (s.e.) = 0.017, p = 4.5 × 10−8], and for BD, there was a marginally significant correlation at −0.069 (fixed effect model: s.e. = 0.032, p = 0.028) with no statistical heterogeneities (p > 0.05). Interestingly, the meta-analysis for MDD showed significant heterogeneity (p = 2.0 × 10−6), in the opposite direction of the correlation between the EUR (i.e. positive correlation) and EAS (i.e. negative correlation) samples.
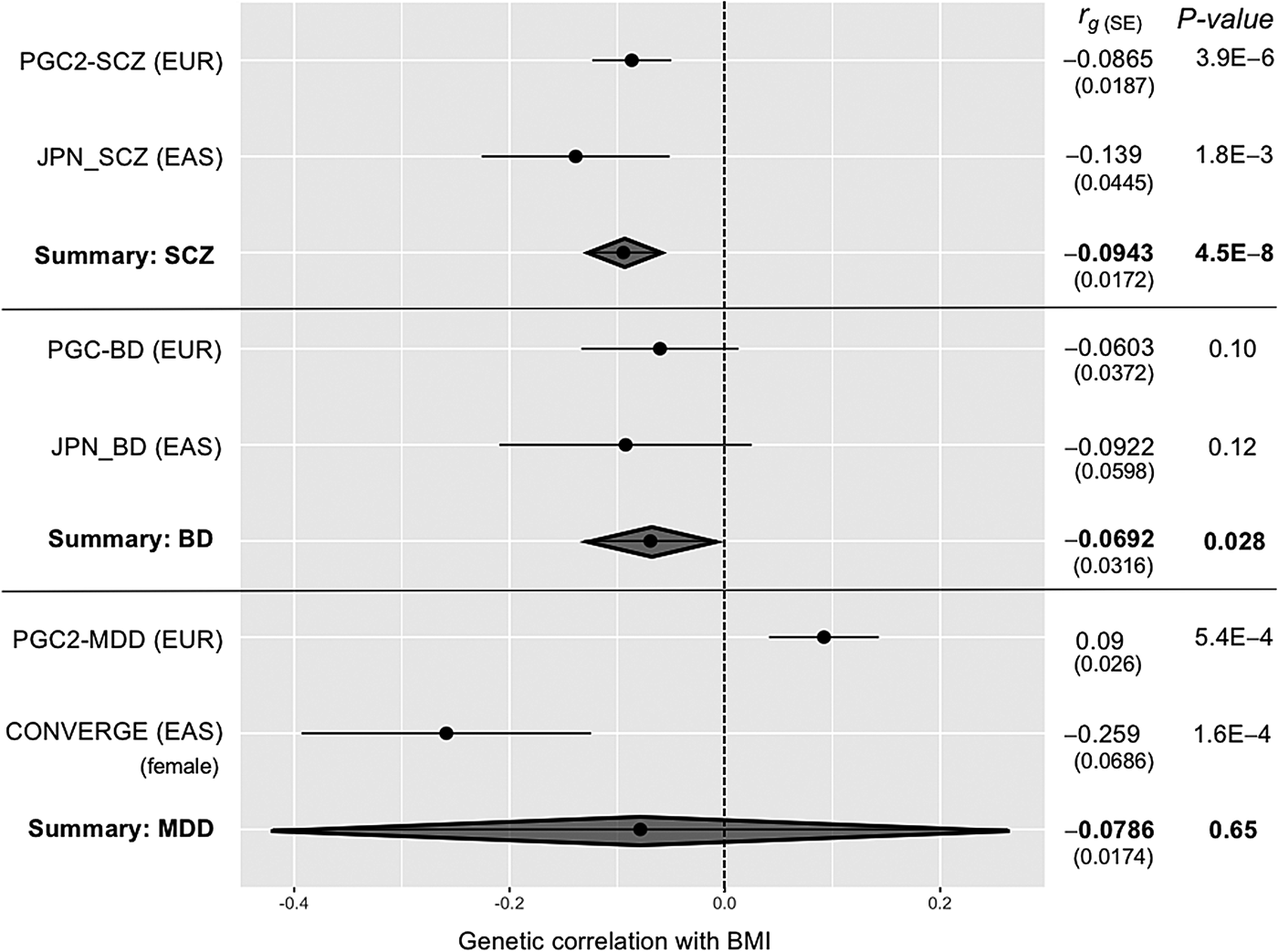
Fig. 1. Meta-analysis of the genetic correlations (r g) between body mass index (BMI) and schizophrenia/bipolar disorder/major depressive disorder (SCZ/BD/MDD). s.e.: standard error. No heterogeneity was observed either in SCZ/BD analyses (p = 0.28 and p = 0.65 for SCZ and BD, respectively) but significant heterogeneity was found in MDD analyses (p = 2.0 × 10−6). Therefore, random-effect model was applied in the meta-analysis for MDD. PGC2-SCZ (EUR): SCZ genome-wide association study (GWAS) in the European (EUR) ancestry (33 640 SCZ v. 43 456 controls: Schizophrenia Working Group of the Psychiatric Genomics Consortium, 2014). BMI was calculated on the basis of maximum 322 154 subjects in the European ancestry (Locke et al. Reference Locke, Kahali, Berndt, Justice, Pers and Day2015). JPN-SCZ: SCZ GWAS in the Japanese ancestry (1987 SCZ v. 9788 controls: Akiyama et al. Reference Akiyama, Okada, Kanai, Takahashi, Momozawa and Ikeda2017). BMI was calculated on the basis of 158 284 subjects in the Japanese ancestry (Akiyama et al. Reference Akiyama, Okada, Kanai, Takahashi, Momozawa and Ikeda2017). PGC-BD: BD GWAS in the European ancestry (7481 BD v. 9250 controls: Psychiatric GWAS Consortium Bipolar Disorder Working Group, 2011). BMI was calculated on the basis of maximum 322 154 subjects in the European ancestry (Locke et al. Reference Locke, Kahali, Berndt, Justice, Pers and Day2015). JPN-BD: BD GWAS in the Japanese ancestry (2964 BD v. 61 887 controls: Ikeda et al. Reference Ikeda, Takahashi, Kamatani, Okahisa, Kunugi and Mori2018). BMI was calculated on the basis of 158 284 subjects in the Japanese ancestry (Akiyama et al. Reference Akiyama, Okada, Kanai, Takahashi, Momozawa and Ikeda2017). PGC2-MDD: MDD GWAS in the European ancestry (130 664 MDD v. 330 470 controls: Major Depressive Disorder Working Group of the PGC, Reference Wray and Sullivan2017). BMI was calculated on the basis of maximum 322 154 subjects in the European ancestry (Locke et al. Reference Locke, Kahali, Berndt, Justice, Pers and Day2015). CONVERGE: MDD GWAS in the East Asian (EAS) ancestry (females only: 5303 MDD v. 5337 controls: CONVERGE consortium 2015). BMI was calculated on the basis of 72 390 female subjects with Japanese ancestry (Akiyama et al. Reference Akiyama, Okada, Kanai, Takahashi, Momozawa and Ikeda2017).
The exploratory trans-ethnic genetic correlation analysis partially supported the findings from the meta-analysis between SCZ/BD and BMI: negative correlation between BMI defined by EUR and SCZ defined by EAS (and vice versa; see online Supplementary Table S1). For BD, we replicated the finding of the meta-analysis in our examination of [BMI (from EUR)/BD (from EAS)], but in the reverse analysis [BMI (from EAS)/BD (from EUR)], we observed non-significant trends between BMI and BD (e.g. ρ~−0.04, p value for ‘non-correlation test’ ~0.10; see online Supplementary Table S1). However, it is difficult to interpret these results, since (1) the sample sizes for BMI GWAS differed substantially (the EUR sample was twice the size of EAS) and (2) this analysis aimed at examining not only trans-ethnic but trans-phenotype factors as well.
Discussion
The results of the polygenic correlation between low BMI and SCZ, which was measured or diagnosed by modern practice, provide some support for the body type theories by Kretschmer and Sheldon, although as noted previously, they proposed the theory on the basis of their observation of people or diagnosis in their era. Therefore, this result for SCZ sheds light on Kretschmer and Sheldon's insight, which proposed diagnostic categories for psychiatric disorders based on the somatology; it is surprising that their classical and well-known hypothesis could be explained by genetic factors, although Kaplan and Sadock's textbook, for example, regarded Kretschmer's achievement as a ‘history’ (Sadock et al. Reference Sadock, Sadock and Ruiz2014). Nevertheless, our genetic correlation analysis did not correspond to the ‘body type’ analysis for BD, in which Kretschmer and Sheldon assumed the tendency of the round body in BD patients; rather, the correlation was in the opposite direction (i.e. low BMI was associated with BD), despite the relatively low statistical significance of both the meta-analysis and the trans-ethnic/phenotype analysis.
In addition, from a clinical perspective, the current genetic results may indicate that patients with SCZ (and possibly BD) tend to have lower BMI presumably before the ‘onset’ (i.e. influenced by fewer confounding ‘environmental factors’). In a real clinical setting, the major and defining problem of the patients with SCZ and BD is obesity (Manu et al. Reference Manu, Dima, Shulman, Vancampfort, De Hert and Correll2015; Strassnig et al. Reference Strassnig, Kotov, Cornaccio, Fochtmann, Harvey and Bromet2017). Therefore, we speculate, on the basis of our current results, that obesity in SCZ or BD may not be attributable entirely to genetic factors but might be greatly influenced by several environmental factors, such as medications (e.g. antipsychotics), lifestyle, and living conditions of modern society, especially during the prodromal period and after the ‘onset’ (Correll et al. Reference Correll, Robinson, Schooler, Brunette, Mueser and Rosenheck2014) or gene–environment correlation/interaction.
The results based on MDD are also of interest. The high level of heterogeneity between BMI and MDD in each population might reflect the different clinical backgrounds of the MDD samples (PGC2 for EUR and CONVERGE for EAS). For MDD samples from EUR (correlated with high BMI), the proportion of MDD individuals with atypical features, characterized by increased appetite and body weight during the depressive phase, was modest. In this context, a recent subgroup study targeting the same PGC2 sample found that MDD with atypical features was correlated with high BMI (r g = 0.53, p = 6.3 × 10−4). In contrast, more typical features of MDD (decreased appetite and body weight, probably including melancholia) exhibited a nonsignificant trend of negative correlation with BMI (r g = −0.28, p = 0.06; Milaneschi et al. Reference Milaneschi, Lamers, Peyrot, Baune, Breen and Dehghan2017). Therefore, it could be inferred that the evidence of a shared genetic component between MDD with atypical features and high BMI is attributable to the significant positive correlation between overall MDD and BMI in EUR datasets (Major Depressive Disorder Working Group of the PGC, Reference Wray and Sullivan2017). On the contrary, for MDD samples from EAS (CONVERGE consortium, 2015), the primary feature related to phenotyping is the predominance of melancholia (about 85%), which is generally associated with low appetite and body weight loss during the depressive course; the result in our study, a negative correlation between BMI and MDD in EAS, is presumably compatible with the sample proportion of CONVERGE. Nevertheless, there were a few limitations in the interpretation of the results; first, our results for genetic correlation in EAS are based solely on female subjects because the CONVERGE consortium targeted female MDD to select a purer phenotype (CONVERGE consortium, 2015). Second, it should also be stressed that the comparison of MDD in the EAS was not perfectly matched in terms of ethnicity; there were MDD subjects with Chinese and BMI subjects having Japanese ancestry.
In conclusion, a trans-ancestry meta-analysis of the genetic correlation between psychiatric disorders and BMI indicated that the negative correlation with SCZ supported classical body type theories proposed in the last century, but found a negative correlation between BD and BMI, opposite to what would have been predicted. In addition, our results indicate that clinical subtypes of MDD play a role in the genetic correlations with BMI; however, further study is required to achieve conclusive results. Genetic correlation analysis is clearly a powerful tool for (re)discovering unexpected connections between psychiatric disorders and other phenotypes (Bulik-Sullivan et al. Reference Bulik-Sullivan, Finucane, Anttila, Gusev, Day and Loh2015). Therefore, the current trend toward conducting such analyses intensively could detect new links or reinforce previously identified ones, thereby helping us to better understand psychiatric disorders.
Supplementary material
The supplementary material for this article can be found at https://doi.org/10.1017/S0033291718000685
Acknowledgements
The authors thank Dr Tetsufumi Kanazawa, M.D., Ph.D. (The University of Melborune, Osaka Medical Colleage), members of Nagoya Psychopathology Research, and Dr Michael O'Donovan, Ph.D., FRCPsych (Cardiff University) for their helpful and critical comments. Dr Kanazawa and Dr O'Donovan have no conflicts of interest to declare.
This work is the result of the Strategic Research Program for Brain Sciences (SRPBS) from the Japan Agency for Medical Research and Development (AMED); part of the BioBank Japan Project from the Ministry of Education, Culture, Sports, and Technology (MEXT) of Japan; GRIFIN of P3GM from AMED; JSPS Kakenhi Grant Numbers JP25293253, JP16H05378, JP26293266, JP17H04251, and JP16K19785; the Private University Research Branding Project from MEXT.
Declaration of interest
None.





