The efficacy of providing ready-to-use therapeutic food has been demonstrated in the management of severe acute malnutrition(Reference Diop, Dossou and Ndour1–Reference Patel, Sandige and Ndekha4) in institutional settings, as well as in home-based therapy for moderate acute malnutrition(Reference Briend5, Reference Ciliberto, Sandige and Ndekha6). Because of this success, there has been growing interest in the efficacy of lipid-based nutrient supplements (LNS), modelled on ready-to-use therapeutic food, on growth and other health benefits in moderately undernourished children. Among mild to moderately underweight and/or stunted children, LNS has been shown to have modest effects on weight and height gains by several studies in different settings(Reference Patel, Sandige and Ndekha4, Reference Adu-Afarwuah, Lartey and Brown7–Reference Phuka, Thakwalakwa and Maleta9). Most of the information on the efficacy of LNS has been shown in controlled research settings where compliance is intensely encouraged. It is well documented that the effectiveness of supplementation approaches differs in controlled v. operational settings(Reference Beaton and Ghassemi10). The present study attempted to determine whether the documented efficacy of LNS on growth and other health benefits was achievable in operational settings when supplementation was through the national health system.
Fortified blended foods, such as fortified cereal and legume mixtures that resemble the indigenous diet and are prepared in a manner similar to staple food, have been recommended for supplementary feeding of moderately undernourished children(Reference Perez and Klein11). In Malawi, treatment of moderate undernutrition (underweight, weight-for-age Z-score (WAZ) <−2) and wasting (weight-for-length Z-score (WLZ) <−2)) is done through the national health system whereby guardians are given fortified corn–soya blend (CSB) to make a soft porridge and feed the children in their homes. However, the effectiveness of using CSB remains under debate(Reference Navarro-Colorado, Shoham and Mason12). Apart from the appropriateness of the intervention, there is paucity of good data to document its effectiveness when supplementation is through the national health system.
To assess the effectiveness of LNS and CSB when administered through the national health system, we conducted a three-arm clinical trial, where moderately underweight infants and children aged 6–15 months received monthly either CSB or LNS or no supplementation (control) during the lean season of the year.
Methods
Study design
The study was a randomised, controlled, assessor-blinded clinical trial conducted at four health centres (Kukalanga, Koche, Katema and Jalasi) in Mangochi District, rural Malawi, south-east Africa. The main aim of the trial was to test the growth-promoting effect and other health benefits to infants and children of daily provision of CSB or LNS. The primary outcome measure was weight change during the 12-week follow-up period. Secondary outcomes included mean changes in length (mm), blood Hb concentration (g/l), anthropometric indices (WAZ, WLZ and length-for-age Z-score (LAZ)), mid-upper arm circumference (MUAC), head circumference and incidence of adverse events (AE) and serious adverse events (SAE).
Study participants
Participants were identified and screened at home. During the home visit, infants were weighed and their WAZ calculated. During the implementation of the trial, WAZ was being used nationally to assess the nutritional status of children <5 years old; as such, it was also used in screening participants for the present trial. Those whose guardians showed initial interest, were aged between 5·0 and 14·5 months and had WAZ <−1·7 were invited to the health centre for more thorough screening for enrolment criteria. The inclusion criteria were: a signed informed consent from at least one guardian, aged between 6·0 and 15·0 months, WAZ <−2·0, availability during the period of the study and permanent residence in the catchment area. Exclusion criteria included WLZ <−3·0 or presence of oedema, history of peanut allergy, history of any serious allergic reaction to any substance requiring emergency medical care, history of anaphylaxis, severe illness warranting hospital referral and concurrent participation in another clinical trial with intervention to the child. Those who met all inclusion criteria and whose guardians signed a consent form were randomised into one of three study groups. An independent statistician not involved in the study developed the random list, packed and sealed the randomisation codes into envelopes, then handed the sealed envelopes over to the research team. There was block randomisation. Participants picked an envelope from the remaining reshuffled envelopes which contained the randomisation group. The randomisation code was broken when all data collection was finished.
Enrolment in the trial was during the lean season between November 2007 and January 2008. No enrolment took place between 21 December 2007 and 6 January 2008 due to Christmas holiday. The 12-week follow-up of the last participant ended in April 2008 at the beginning of harvest season. The period between December and March is the rainy season during which the staple food (maize) and other crops are grown, and food levels and weight gains are at their lowest.
Sample size
The target sample size was ninety-seven infants per group (291 in total), calculated from the expected difference in the primary outcome, i.e. weight gain, among infants provided with either CSB or LNS and those provided with nothing. This expected difference was based on an assumed mean weight gain of 550 (sd 440) g in the control infants and 750 (sd 440) g among infants receiving either CSB or LNS supplementation(Reference Kuusipalo, Maleta and Briend13). This gave the trial 85 % power and a type 1 error rate of no more than 5 % to detect a difference of 200 g or more in mean weight gain between the control and intervention groups and allowed a 10 % loss to follow-up since the trial was done in less controlled conditions than the earlier studies.
Interventions and follow-up
Participants in the control group did not receive any food supplement during the trial period. Those in the first intervention group received 2 kg of CSB while those in the second intervention group received 1·2 kg of LNS. Both supplements were received from the health centre once every 4 weeks for 12 weeks. All participants were scheduled for a follow-up visit at the health centre every 4 weeks. CSB contains corn, soya and sugar. This was locally produced in Malawi by Rab Processors (Blantyre, Malawi). LNS was produced at a Malawian non-governmental organization, Project Peanut Butter (Blantyre, Malawi), from locally purchased peanut butter (26 %), dried skimmed milk (25 %), vegetable oil (20 %), icing sugar (27·5 %) and a pre-made mineral and vitamin mix (1·5 %; Nutriset Inc., Malaunay, France), where all percentages are by weight. All the supplements were fortified with micronutrients, but the level of fortification varied between the products.
During the follow-up visits every 4 weeks, the participants’ medical condition was checked and their anthropometric measurements taken. Rations for the CSB and LNS participants were handed out during these visits. The guardians were provided with spoons and advised to give their infants, twice daily, either three spoonfuls of LNS or porridge containing five spoonfuls of CSB. The guardians were encouraged to give the supplement in addition to breast milk. Interventions were handed out by a research assistant not involved in outcome assessment. Table 1 provides the energy and nutrient contents of a daily ration of each supplementation.
Table 1 Nutrient composition of the participants’ daily dose of corn–soya blend (CSB) or lipid-based nutrient supplement (LNS)
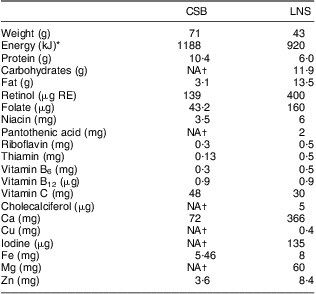
RE, retinol equivalants.
*1 kcal = 4·184 kJ.
†No information provided by the manufacturer.
Measurement of outcome variables
Infant nude weights were measured to the nearest 10 g using an electronic infant weighing scale (SECA 735; Chasmors Ltd, London, UK). Head circumference and MUAC were measured to the nearest 1 mm by a non-stretchable measuring tape (Lasoo-o-tape; Harlow Printing Ltd, South Shields, UK). Length was measured to the nearest 0·1 cm using a length board (infantometer; Child Growth Foundation, London, UK). Anthropometric indices (WAZ, WLZ, LAZ) were calculated using Epi Info 3·3·2 software (Centers for Disease Control and Prevention, Atlanta, GA, USA), based on the Centers for Disease Control and Prevention 2000 growth reference(Reference Kuczmarski, Ogden and Guo14). All anthropometric measurements were done in triplicate by one investigator (M.J.), assisted by one trained research assistant. The first measurements were taken on enrolment day and then every 4 weeks until the end of the 12-week follow-up period. The last follow-up measurements were taken within 2 weeks of the scheduled date.
In the trial, the following medical occurrences were recorded as AE: abdominal discomfort, vomiting or diarrhoea for more than two consecutive days; skin rash for two or more consecutive days; noisy, wheezy, rapid or difficult breathing; and any other medical conditions that were judged abnormal or not typical childhood illnesses by the study physician. The SAE included: death; life-threatening condition; in-patient hospitalisation or prolongation of existing hospitalisation; persistent or significant disability or incapacity; or any other serious medical condition. Participants were advised to report to the clinical officer at the health centre if they had any of these symptoms or any problems during the study period. The clinical officer determined if the problem was an AE or SAE. An independent data safety and monitoring board (DSMB) also determined if the condition was an AE or SAE.
Ethics
The trial was performed according to International Conference of Harmonization–Good Clinical Practice guidelines (ICH-GCP) and the ethical standards of the Helsinki Declaration. The protocol was reviewed and approved by the College of Medicine Research and Ethics Committee, University of Malawi and the Ethical Committee of Pirkanmaa Hospital District, Finland. At least one guardian signed or thumb-printed an informed consent form before enrolment of each participant. The trial was registered with a registration ID of NCT00420368 at http://www.clinicaltrials.gov/. The DSMB monitored the incidence of suspected SAE.
Statistical analysis
All statistical analyses were conducted using the STATA statistical software package version 9·0 (StataCorp, College Station, TX, USA) based on an intention-to-treat principle. We compared group means between control and each of the supplemented groups at a single time point using the t test and results are expressed as absolute differences and their 95 % confidence intervals. We estimated the risk ratio and risk difference for comparison of binary endpoints between the control and each of the supplemented groups and tested the difference in proportions using Fisher's exact test. To prevent inflated type 1 errors due to multiple comparisons, we began hypothesis testing by testing the global null hypothesis of all three groups being identical, using Fisher's exact test (categorical variables) and ANOVA (continuous variables). Only if the global null hypothesis was rejected would the pairwise comparisons be interpreted in a confirmatory manner. No post hoc test was used for pairwise comparison after the aforementioned procedures. Linear regression modelling was used to determine whether, if controlled for baseline age, the type of food supplement given would be associated with weight gain. Values in the text are given as mean (sd), mean difference (95 % CI) or n ( %). All confirmatory analyses were considered significant if P < 0·05.
Results
Of the 1711 infants and children screened at home, 1066 (62 %) did not meet criteria for enrolment screening at the health centre (they were older and/or had WAZ >−2·0); sixty-two (10 %) of the 645 invited did not show up. Of the 583 who attended enrolment screening, fourteen (2 %) refused to participate and 270 (46 %) did not meet enrolment criteria. We randomised the remaining 299 infants and children into three groups: control, CSB and LNS (Fig. 1). Two hundred and eighty-two participants (94 %) completed the follow-up, i.e. underwent medical and anthropometric assessment after the 12-week intervention. All participants were breast-fed from enrolment to the end of the follow-up period. Baseline WAZ values of participants who completed the follow-up were −3·14, −2·95 and −3·03 (P = 0·352) for the control, CSB and LNS groups, respectively; corresponding values for those who died or were lost to follow-up were −2·88, −3·33 and −3·67 (P = 0·793). These did not suggest that they came from different populations. The losses to follow-up were not significantly different between the intervention groups and the control group (P = 0·341 for deaths and P = 0·929 for total loss to follow-up; Fisher's exact test; Fig. 1).
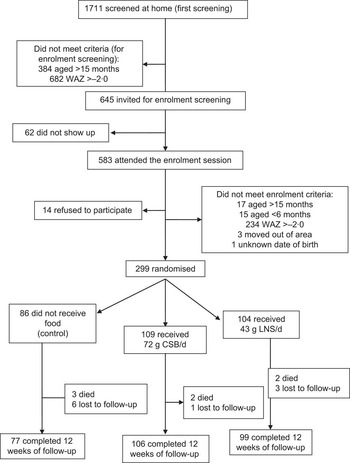
Fig. 1 Participant flow (WAZ, weight-for age Z-score; CSB, corn–soya blend; LNS, lipid-based nutrient supplement)
At baseline, children in the LNS group were thinner and shorter compared with children in the CSB and control groups. There were more children in the younger age group, 6–9 months, in the LNS group than in the control and CSB groups: 38 % v. 15 % and 23 %, respectively. The proportions of children between the ages of 9 and 12 months and 12 and 15 months were, however, comparable across the groups (Table 2). At baseline, forty (47 %), forty-two (39 %) and seven (7 %) of the study participants were severely underweight in the control, CSB and LNS groups, respectively. None of the participants were severely wasted but about 66 %, 59 % and 56 % of the participants in the control, CSB and LNS groups, respectively, were moderately wasted (data not shown). Attendance to the scheduled health centre visits was good in all groups. Among participants who did not die or drop out of the follow-up, the proportion that attended up to the last scheduled health centre follow-up visit was 94 %, 99 % and 98 % among control, CSB, and LNS groups, respectively.
Table 2 Characteristics of participants at enrolment by study group: underweight infants and children aged 6–15 months (n 299), rural Malawi, 2007–2008
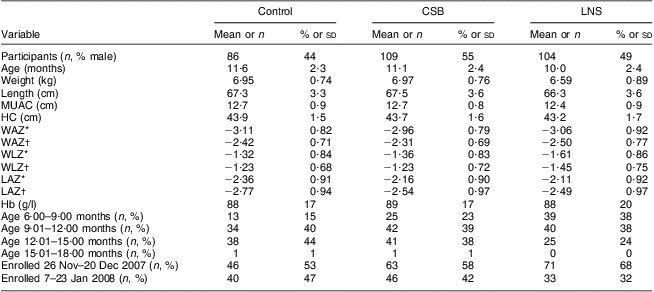
CSB, corn–soya blend; LNS, lipid-based nutrient supplement; MUAC, mid-upper arm circumference; HC, head circumference; WAZ, weight-for-age Z-score; WLZ, weight-for-length Z-score; LAZ, length-for-age Z-score.
Data are presented as mean and standard deviation unless indicated otherwise.
*Using Centers for Disease Control and Prevention 2000 growth reference(Reference Kuczmarski, Ogden and Guo14).
†Using WHO 2006 growth reference(26).
During the 12-week follow-up, mean weight gain was 630 g, 680 g and 750 g in the control, CSB and LNS groups, respectively, with no statistically significant differences among the groups (P = 0·211). Changes in the secondary outcomes (WAZ, LAZ, WLZ, length, head circumference, MUAC and Hb concentration) were not statistically significantly different in the three groups (Table 3). When controlled for baseline age, children receiving LNS gained on average 90 g more weight (P = 0·185) and their WLZ increased by 0·22 more (P = 0·049) compared with those receiving no supplementation. Children receiving CSB gained 40 g more weight (P = 0·571) and their WLZ increased by 0·14 more (P = 0·205) compared with controls. Changes in weight and other secondary outcomes (length, WAZ, LAZ, Hb) were comparable among the groups (Table 4).
Table 3 Comparison of mean quantitative anthropometric outcomes among participants receiving CSB or LNS for 12 weeks or no supplementation (control): underweight infants and children aged 6–15 months (n 299), rural Malawi, 2007–2008

CSB, corn–soya blend; LNS, lipid-based nutrient supplement; MUAC, mid-upper arm circumference; HC, head circumference; WAZ, weight-for-age Z-score; WLZ, weight-for-length Z-score; LAZ, length-for-age Z-score.
*ANOVA.
†Differences are mean value in intervention group – mean value in control group.
‡t Test.
Table 4 Comparison of mean quantitative outcomes among participants receiving CSB or LNS for 12 weeks or no supplementation (control), controlling for baseline age: underweight infants and children aged 6–15 months (n 299), rural Malawi, 2007–2008
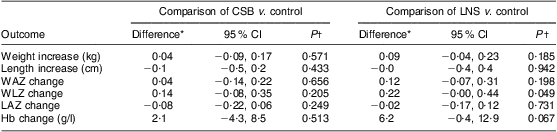
CSB, corn–soya blend; LNS, lipid-based nutrient supplement; WAZ, weight-for-age Z-score; WLZ, weight-for-length Z-score; LAZ, length-for-age Z-score.
*Differences are mean value in intervention group – mean value in control group.
†From multiple linear regression models of changes in weight, length, WAZ, WLZ, LAZ and Hb with intervention (CSB, LNS and no food) and age.
At the end of the follow-up period, slightly more children in the control group (56 %) were severely underweight compared with CSB (45 %) and LNS (46 %) groups. For severe stunting, the prevalence was 22 % in controls, 17 % in the CSB group and 13 % in the LNS group. Severe wasting was comparable among the groups: five (6 %), six (6 %) and seven (7 %) children in the control, CSB and LNS groups, respectively (data not shown).
During the intervention period, the incidence of AE did not differ significantly among the three groups (all P > 0·05). Fifty-one children experienced a clinician-documented AE, nine of which were SAE, with no differences in the incidence of any AE among the groups (Table 5). All SAE were assessed as unlikely related to the intervention since they did not follow a reasonable time sequence from administration of the study food.
Table 5 Proportion with confirmed AE and SAE among participants who received CSB or LNS for 12 weeks or no supplementation (control): underweight infants and children aged 6–15 months (n 299), rural Malawi, 2007–2008
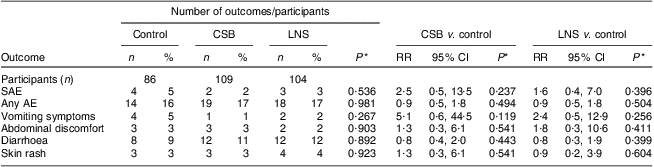
AE, adverse effect.; SAE, severe adverse effect; CSB, corn–soya blend; LNS, lipid-based nutrient supplement; RR, risk ratio.
*Fisher's exact test.
Discussion
In this sample, LNS supplementation to moderately underweight 6- to 18-month-old children through the national health system was associated with a modest increase in weight gain compared with supplementation with CSB or no supplementation. The amount of weight gain improvement (120 g/12 weeks) was in the same direction but lower than weight gains reported when LNS has been offered for a similar duration in more controlled settings (150 g)(Reference Thakwalakwa, Ashorn and Phuka15). Although the findings were statistically only marginally significant with or without adjustment for covariates, the effectiveness estimate is plausible in the context of the previous efficacy trials.
The utility of any dietary supplementation regimen is dependent on its biological efficacy and its operational feasibility and practical acceptability. Results from the current study suggest that the effectiveness of LNS is maintained in a less controlled setting, albeit at a rather modest level. In a recent efficacy trial under controlled conditions from the same study area, there was a modest weight gain with LNS supplementation as compared with control(Reference Thakwalakwa, Ashorn and Phuka15). The current observation from less controlled conditions is therefore not surprising. Weight gain in this setting is likely to be lower than that in a controlled setting because the LNS might be shared with family members and siblings(Reference Flax, Phuka and Cheung16). The effects observed could easily be applied to similar settings where interventions are given at health facilities and the patients are not followed up to check compliance. However, the weakness is that compliance/adherence to the study food was not checked, limiting our understanding of how the supplement was used by each child and within the household. The positive impact of LNS in these settings is likely to be associated with its good taste and acceptability among infants and young children(Reference Adu-Afarwuah, Lartey and Zeilani17–Reference Phuka, Ashorn and Ashorn19), its high energy and nutrient density, and its non-resemblance to normal family foods(Reference Briend5, Reference Maleta, Kuittinen and Duggan20). CSB, on the other hand, is normally consumed by infants as high-volume porridge, similar to many traditional foods. All of these characteristics make LNS more likely than CSB to actually complement existing diets for the intended beneficiaries, rather than just displacing traditional foods or ending up as shared commodities with other individuals than the undernourished child.
Apart from their intrinsic differences, the modest effects of LNS and CSB could also be explained by the higher proportion of infants and children recruited at the beginning of the lean season (68 % and 58 %, respectively), when some families still had some food left, compared with the non-supplemented children, who were mostly evenly distributed. We have previously documented reduced rates of weight gain in these months(Reference Maleta, Virtanen and Espo21) compared with the latter months of the year. This could result in underestimation of the effect size of either of the interventions.
The efficacy of any intervention programme is also dependent on the age of the participant(Reference Schroeder, Martorell and Rivera22) and the length of the supplementation(Reference Simondon, Gartner and Berger23). In the present study, the intervention length was relatively short and the groups were not homogeneous in age, such that we had to estimate if the intervention had a differential effect based on baseline age. Controlling for age, a higher but still modest effect was observed on changes in WLZ. The modest impact of the intervention in the current trial could therefore also have been explained by the shorter duration of supplementation.
Undernutrition impacts on morbidity and morbidity affects an individual's nutritional status. Undernutrition and morbidity have a synergistic relationship in such a way that, on the one hand, nutritional deficiencies increase the susceptibility of the child to infectious diseases such as diarrhoea, fevers and malaria, and on the other, illness can suppress a child's appetite leading to undernutrition(Reference Brabin and Coulter24, 25). In the current study, the incidence of any AE (diarrhoea, vomiting and skin rash) in the CSB and LNS groups was similar to that in the control group, a finding that others have documented from supplementary programmes(Reference Ciliberto, Sandige and Ndekha6). However, a recent trial documented more episodes of vomiting and skin rash with LNS supplementation(Reference Thakwalakwa, Ashorn and Phuka15). One reason for the difference might be because the current study was done in a less controlled setting such that some cases may not have been reported, unlike in the previous trial where the participants were visited weekly and encouraged to report to the hospital with any problem.
The current results did not show any protective effect of CSB nor LNS against morbidity.
The current findings, however, should be regarded as preliminary, given that the period of supplementation was relatively short and did not cover both seasons. Caution must be taken when extrapolating the results since the current study was not strictly operational in that the participants were recruited into the study rather than them coming through the health system by themselves. This might affect the generalizability of the results from the study. However, all the infants and children aged 6–15 months in the catchment area were screened and given a chance to participate in the study.
The participants’ diet in the study area is dominated by foods of vegetable origin with very limited intake of animal-source foods, making the quality of the diet poor. Supplementation of LNS could therefore add value and improve the nutritional quality of the diet, contributing towards optimal nutrition and thereby preventing malnutrition from becoming worse.
Conclusion
LNS supplementation provided through the national health system during the lean season to underweight children, the majority of whom were only slightly wasted, was associated with a modest increase in weight gain compared with no supplementation. The effect size was however lower than previously reported in more controlled research settings. There is need to verify the findings through a study with longer supplementation duration that covers both seasons to determine the effects of supplement duration and season on the outcomes.
Acknowledgements
This work was supported by the Academy of Finland (grant 109796). C.M.T. was supported by a personal stipend from Nestlé Foundation and the St. Louis Nutrition Project. There are no conflicts to declare. All authors designed the trial; P.A. wrote the protocol and raised funds for the trial; C.M.T. and M.J. were responsible for data collection; Y.B.C. designed the details of the statistical analysis; C.M.T. and J.C.P. did the analysis; C.M.T. wrote the first draft of the manuscript under the supervision of K.M., P.A. and Y.B.C. All authors commented on the analysis and participated in revising the manuscript. K.M.M. had full access to all data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis. The authors thank the study participants and the staff at Jalasi, Koche, Kukalanga and Katema health centres in Mangochi District for their positive attitude, support and help in all stages of the study; Andre Briend and Taneli Puumalainen for commenting on the analysis and participating in the writing of the manuscript; the DSMB (Kamija Phiri, Paul Ndebele, Tom Heikens) for monitoring the trial; and Laszlo Csonka for designing the data entry program.










