Vitamin D is a pro-hormone whose activated metabolite regulates bone, Ca and P metabolism( Reference Holick 1 ). The main source of this vitamin is endogenous synthesis through the skin in response to solar UVB radiation. It may also be obtained from foods (dairy products, eggs, cold-water fish, mushrooms), supplements and food fortification( Reference Schmid and Walther 2 , Reference Holick and Chen 3 ).
During childhood, vitamin D effects on bone are especially obvious( Reference Cashman 4 ), but vitamin D is also linked to lower risk of infections and allergies( Reference Aryan, Rezaei and Camargo 5 ), type 1 diabetes mellitus( Reference Franchi, Piazza and Sandri 6 ), hypertension( Reference Petersen, Dalskov and Sørensen 7 ) and obesity( Reference Cunha, Magalhães and Loureiro 8 ). In 2011, the Institute of Medicine established the Estimated Average Requirement (EAR) of 10 μg vitamin D/d (400 UI/d) and the RDA of 15 μg vitamin D/d (600 UI/d) for ages 1 to 70 years( 9 ). These recommendations consider only the maintenance of bone health and do not address the prevention of cardiometabolic and autoimmune illnesses( 9 ).
Vitamin D deficiency is considered a global public health problem, with high prevalence in different age groups, even in low-latitude countries where more UVB radiation is available( Reference Palacios and Gonzalez 10 ). Studies in Brazil, a country close to the equator, have shown that the prevalence of vitamin D insufficiency/deficiency varies between 67·9 %( Reference Santos, Mascarenhas and Satler 11 ) and 90·6 %( Reference Lourenço, Qi and Willett 12 ) in children and adolescents. This scenario, observed already in childhood, may be due to the little time spent in outdoor activities, use of sunscreens and low consumption of foods rich in vitamin D( Reference Voortman, van den Hooven and Heijboer 13 , Reference Bezrati, Fradj and Ouerghi 14 ).
Insufficient vitamin D intake is common in the child population. Studies from Europe and North America have shown high prevalence of consumption below the EAR( Reference Soininen, Eloranta and Lindi 15 , Reference Munasinghe, Willows and Yuan 16 ). The identification of the main food sources of vitamin D in Brazil may contribute to specific public health interventions for children and their families. Despite knowing the importance of consuming these food sources, there is no consensus on the association between vitamin D intake and serum 25-hydroxyvitamin D (25(OH)D) concentration( Reference Voortman, van den Hooven and Heijboer 13 , Reference Soininen, Eloranta and Lindi 15 , Reference Julián, González-Gross and Breidenassel 17 ).
As the prevalence of vitamin D insufficiency/deficiency has increased, the prevalence of cardiometabolic risk factors such as alterations in lipid profile has increased in the paediatric population( Reference Gupta, Shah and Nayyar 18 ). In Brazilian children and adolescents, the prevalence of dyslipidaemia varies between 29·7 %( Reference Franca and Alves 19 ) and 66·7 %( Reference Carvalho, Paiva and Melo 20 ). Studies with children and adolescents have shown associations between serum 25(OH)D concentrations and lipid profile markers( Reference Rusconi, De Cosmi and Gianluca 21 , Reference Al-Daghri, Sabico and Al-Saleh 22 ). Nevertheless, the relationship between vitamin D intake and these markers has not been confirmed in the literature, especially in children( Reference Moreira, Moreira and Abreu 23 ).
In this context, the aim of the present study was to evaluate the association of vitamin D intake with lipid profile markers and serum 25(OH)D concentration in Brazilian children, as well as to identify main groups of food sources for vitamin D in this population. Our hypothesis is that low vitamin D intake is associated with a worse lipid profile and lower serum 25(OH)D concentration already in childhood.
Methods
Study and population design
The present study was a cross-sectional study of a representative sample of children between 8 and 9 years of age enrolled in public and private schools in the urban area of Viçosa, Minas Gerais, Brazil. The city is in the Zona da Mata region of Minas Gerais (latitude 20°45′14″S, longitude 42°52′55″W), with a population of 72 220, of whom 93·2 % live in the urban area, and a Human Development Index of 0·775( 24 ). In 2015, the city had seventeen public schools and seven private schools for children aged from 8 to 9 years.
The 8–9-year-old age group comprises prepubescent children, wherein it is likely already to find cardiometabolic alterations that extend into adulthood( Reference Barker, Osmond and Forsen 25 ).
The study is part of the Survey of Health Assessment of Schoolchildren (PASE), which aimed to evaluate cardiovascular health of children in the city of Viçosa, Minas Gerais, Brazil.
Sampling
The sample size was calculated using the software Epi Info version 7.2 from the total number of children aged 8 and 9 years (n 1464) enrolled in all urban schools in 2015. Considering the analysis of multiple outcomes, the sample was calculated on the basis of 50 % prevalence, 5 % error tolerated, 95 % CI, 5 % significance level and 20 % dropout rate, resulting in the sample size of 366 children.
The schoolchildren were selected by stratified random sampling. The sample from each school met the proportionality ratio of students enrolled by age and sex. The selection of children was done by random simple draw until the necessary number for each school was completed.
After selection, the children’s guardians were contacted by telephone and invited to participate in the study. The objectives and methodology of the study were then explained to the guardians and a first meeting was arranged. At this time, the steps were discussed and those who would like to participate signed an informed consent form.
Children were excluded from the study if they had health problems which affected their nutritional state or body composition; chronic use of medication that affected the metabolism of vitamin D (corticoids, anticonvulsants, antifungals), glucose and/or lipids; and use of vitamin and mineral supplements for the last three months. When the guardians could not be contacted after three attempts, they were also excluded.
A pilot study was made with 10 % of the sample (n 37), including children aged 8–9 years enrolled in a school selected randomly. These children did not participate in the final sample. The pilot study was carried out to test the questionnaires.
Covariables
A semi-structured questionnaire eliciting socio-economic and demographic information, such as age, sex, skin colour, area of residence, school type, income per capita, mother’s age and education, was applied. Income per capita and mother’s age and education were classified according to the sample median. The guardians declared their children’s ethnicity according to the race/skin colour categories used by the Brazilian Institute of Geography and Statistics( 26 ). The presence of maternal and paternal dyslipidaemia was self-reported in a semi-structured questionnaire.
A questionnaire about lifestyle, which was developed in another study( Reference Andaki 27 ) with the same study population as ours, was applied to estimate screen time per day (hours spent watching television, playing video games, using cell phones, computers, etc.). Sedentary behaviour was classified as screen time more than 2 h/d( 28 ).
The season of study was that of blood sampling. Sun exposure was evaluated by using reports of time the child spent in outdoor activities like walking to school or playing in the backyard or on the street.
Anthropometry and body composition evaluation
Weight and height were measured by a standard and regularly standardized methodology( 29 ) using respectively a digital electronic scale (Tanita® model BC 553, Arlington Heights, IL, USA), with 150 kg capacity and 100 g accuracy, and a 2 m portable vertical stadiometer (Alturexata®, Belo Horizonte, MG, Brazil) scaled in millimetres. These values were used to calculate the children’s BMI (=[(body mass (kg)]/[height (m)]2).
The body composition of the children was evaluated by dual-energy X-ray absorptiometry (Lunar Prodigy Advance; GE Medical Systems Lunar, Milwaukee, WI, USA) to obtain body fat. These examinations were carried out in the morning after an overnight fast at the sector of Image Diagnosis of the Federal University of Viçosa’s Health Division by a specially trained technician. During the exam, the children were in a supine position until data were collected, wearing light clothes without any metal accessories.
Biochemical analysis
Blood tests were performed after 12 h of fasting at the Laboratory of Clinical Analysis of the Federal University of Viçosa. The samples were collected by venepuncture and the serum separated was stored in 1·5 ml Eppendorf tubes at −80°C for biochemical profile measurements, including lipid profile (total cholesterol (TC), HDL cholesterol (HDL-C), LDL cholesterol (LDL-C), TAG), apolipoproteins (apoB and apoA1), 25(OH)D, parathyroid hormone (PTH), glucose and insulin.
The lipid profile and glucose were determined with the enzymatic colorimetric method, using the commercial kit Bioclin® (Belo Horizonte, MG, Brazil) and measured using automatic analysing equipment (BS-200 Mindray®, Nanshan, China) at the Laboratory of Clinical Analysis at the Department of Nutrition and Health at the Federal University of Viçosa. The corresponding kits were used following the manufacturer’s instructions.
ApoB and apoA1 were determined using the kinetic nephelometry method (Beckman Coulter, Inc., Brea, CA, USA) and classified according to the 90th percentile of the sample because of the lack of specific cut-off points for children. Therefore, the apoB:apoA1 ratio was calculated using the 90th percentile of the sample as cut-off point.
25(OH)D, PTH and insulin were determined by the chemiluminescence immunoassay method at the Brazil Diagnosis Laboratory. 25(OH)D was obtained by the ARCHITECT® (Abbott Diagnostics, Lake Forest, IL, USA) 25-OH Vitamin D test with a correlation coefficient of ≥0·80 for serum samples when compared with the LIAISON® (DiaSorin, Inc., Stillwater, MN, USA) 25-OH Vitamin D Total test and with a cumulative within- and between-assay variance of ≤10 %. The Access® Intact PTH test (Beckman Coulter, Inc.) was used for PTH. Serum insulin was measured by the Elecsys Insulin® test (Roche Diagnostics, Indianapolis, IN, USA) with detection limit of 0·200–1·000 μU/ml.
The classification of lipid profile components was made according to the update of the Brazilian directives on dyslipidaemias and atherosclerosis prevention of the Brazilian Society of Cardiology( Reference Faludi, Izar and Saraiva 30 ).
The concentration of 25(OH)D was expressed in ng/ml (1 ng/ml=2·5 nmol/l) and defined as deficiency (<20 ng/ml), insufficiency (≥20–<30 ng/ml) and sufficiency (≥30 ng/ml)( Reference Holick, Binkley and Bischoff-Ferrari 31 ).
Fasting values of glucose and insulin were used to calculate the homeostatic model assessment of insulin resistance (HOMA-IR) index, obtained by the formula: {[fasting insulin (μU/ml) × fasting glucose (mmol/ml)]/22·5}( Reference Matthews, Hosker and Rudenski 32 ).
Dietary evaluation
Food consumption was evaluated by three 24 h dietary recalls on three non-consecutive days, one of these tests being made at the weekend. The children responded to the food questionnaire with their guardians, preferentially with the guardian directly involved with the child’s diet. For children who ate at school, researchers collected information from the child and completed it with data from the school when necessary. The number of daily meals was obtained with the 24 h dietary recall. In order to help children and guardians to determine portion sizes, we used household utensils and a photo album of utensils and different portions of foods( Reference Zabotto, Veanna and Gil 33 ).
The analysis of dietary data was carried out with Diet Pro® 5i software version 5.8. Intakes of vitamin D and energy were evaluated. Due to different preparation habits and addition of sugar, salt and soya oil, some foods had a chemical composition different from their nutritional facts. These new values were computed with recipes standardized by the team, nutrition fact labels and food database tables. The Food Database: Support for Nutritional Decisions( Reference Philippi 34 ) was used.
The adequacy of vitamin D intake was compared with the EAR of 10 µg vitamin D/d (400 UI/d)( 9 ). Adjustments were made for intra-individual variability as proposed by Willett( Reference Willett 35 ).
The main food sources of vitamin D were grouped into dairy products, eggs and fish, according to the Food Guide for the Brazilian Population (2006)( 36 ), and expressed as grams. The medians of consumption of these food groups were used for the analyses.
To control for the effect of energy consumption on the nutrients evaluated, we used the residual method proposed by Willett and Stampfer( Reference Willett and Stampfer 37 ).
Statistical analysis
Statistical analyses were carried out using the Stata statistical software package version 13.0. The continuous variables were tested for normality with the Shapiro–Wilk test.
Descriptive statistics were used to characterize the sample by socio-economic, demographic, lifestyle, food consumption, lipid profile and 25(OH)D status variables. Means and standard deviations were used for parametric variables, and median and interquartile ranges were used for non-parametric variables. Categorical variables were expressed as number and percentage.
The association between categorical variables was examined by Pearson’s χ 2 test or Fisher’s exact test. Differences between two independent groups were evaluated by Student’s t test or the Mann–Whitney test. Spearman’s correlation coefficients were obtained to evaluate the correlation of vitamin D intake with lipid profile markers and serum 25(OH)D concentration.
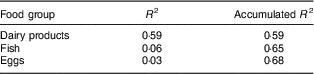
Stepwise multiple regression was applied to identify the main food groups that predicted the consumption of vitamin D( Reference Willett 35 ).
Bivariate analysis was carried out using Poisson regression with robust variance, with lipid profile and 25(OH)D status as dependent variables and dietary intake of vitamin D as the explanatory variable. The model that tested the association between vitamin D intake and lipid profile was adjusted for the following variables: age, sex, income per capita, BMI, maternal and paternal dyslipidaemia, sedentary behaviour and number of daily meals. On the other hand, the model that tested the association between vitamin D intake and 25(OH)D status was adjusted for age, sex, income per capita, skin colour, sun exposure, season, PTH, body fat percentage, HOMA-IR and maternal education. Using the backward procedure, the predictor variables that reached P value smaller than 20 % (P<0·20) were inserted into the Poisson regression with robust variance. From the complete model, the variable that contributed least (largest P value) was removed. The procedure was repeated until all the variables present in the model had statistical significance (P<0·05). The goodness-of-fit test was used to evaluate the adjustment of the final model. We considered the group with vitamin D intake ≥10 μg/d as reference. The prevalence ratio (PR) with 95 % CI was used as an effect value. For all tests performed, the significance was defined as P<0·05.
Ethical aspects
The study was conducted according to the guidelines established in the Declaration of Helsinki and all procedures involving human subjects were approved by the Ethics Committee in Research on Humans of the Federal University of Viçosa (protocol: 663.171/2014). It was also approved by the Municipal Secretariat of Education, by the Regional Education Superintendent and by the school boards.
Results
At the end of the data collection, 4·2 % of the survey respondents were excluded due to the non-accomplishment of all stages of the research. A sample of 378 children was assessed, most being girls (52·1 %), non-black (88·6 %), urban residents (98·4 %) and from public schools (70·9 %). A high prevalence of inadequate vitamin D intake was observed (91·3 %). A lower intake of vitamin D was found for girls, those who had fewer than five meals per day and those whose consumption of dairy products and fish was below the sample median (Table 1).
Table 1 Vitamin D intake according to sociodemographic, lifestyle and food consumption characteristics of urban schoolchildren aged 8–9 years (n 378), Viçosa, Minas Gerais, Brazil, 2015
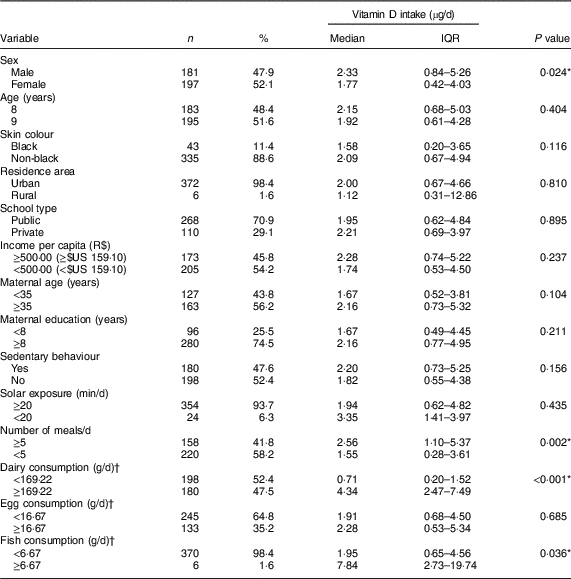
IQR, interquartile range.
* P<0·05 (Mann–Whitney test).
† Classification according to the median of average three-day consumption.
Vitamin D intake showed a direct correlation with serum 25(OH)D concentration (r=0·205; P<0·001) and HDL-C (r=0·135; P=0·047) but was not correlated with other lipid factors (data not shown).
In relation to the lipid profile, most children showed dyslipidaemia (72·8 %), while 12·8 and 43·4 % of the children had deficiency and insufficiency of vitamin D, respectively. Children with inadequate consumption of vitamin D had lower concentrations of HDL-C (P=0·018) and 25(OH)D (P=0·020; Table 2). This finding persisted after adjustment of the regression model, in which insufficient vitamin D intake increased the prevalence of low HDL-C (PR=2·51; 95 % CI 1·02, 6·18; P<0·05) and vitamin D insufficiency/deficiency (PR=1·61; 95 % CI 1·01, 2·58; P<0·05; Table 3).
Table 2 Alterations in lipid profile and serum 25-hydroxyvitamin D (25(OH)D) concentration according to vitamin D intake in urban schoolchildren aged 8–9 years (n 378), Viçosa, Minas Gerais, Brazil, 2015
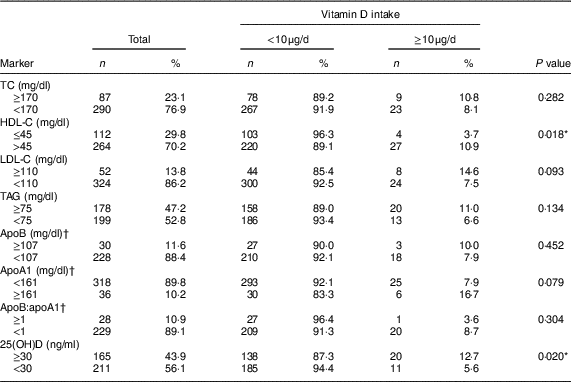
TC, total cholesterol; HDL-C, HDL cholesterol; LDL-C, LDL cholesterol.
* P<0·05 (Fisher’s exact test).
† Classification according to the 90th percentile of the sample.
Table 3 Association of vitamin D intake with lipid profile markers and serum 25-hydroxyvitamin D (25(OH)D) concentration in urban schoolchildren aged 8–9 years (n 378), Viçosa, Minas Gerais, Brazil, 2015
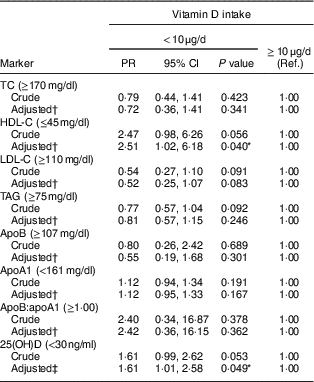
PR, prevalence ratio; Ref., reference category; TC, total cholesterol; HDL-C, HDL cholesterol; LDL-C, LDL cholesterol; PTH, parathyroid hormone; HOMA-IR, homeostatic model assessment of insulin resistance.
Goodness-of-fit test (used to evaluate the adjustment of the final model), P>0·05.
* P<0·05.
† Adjusted for age, sex, income per capita, BMI, maternal and paternal dyslipidaemia, sedentary behaviour and number of daily meals.
‡ Adjusted for age, sex, income per capita, skin colour, solar exposure, season, PTH, body fat percentage, HOMA-IR and maternal education.
Greater consumption of dairy products and fish contributed to adequacy of vitamin D intake (Table 4; Fig. 1). Between the sexes, the only difference was that boys had higher average consumption of dairy products (boys, 201·29 g; girls, 168·25 g; P<0·05; data not shown).
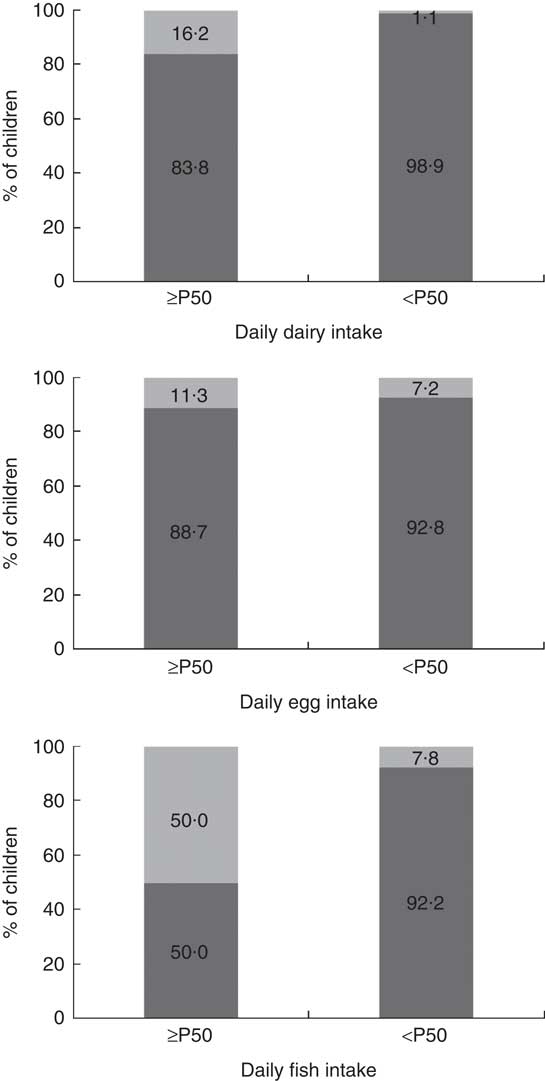
Fig. 1 Vitamin D intake (![]() , <10 µg/d;
, <10 µg/d; ![]() , ≥10 µg/d) according to the consumption of dairy products, eggs and fish (equal to or greater than the median, ≥P50; less than the median, <P50) by urban schoolchildren aged 8–9 years (n 378), Viçosa, Minas Gerais, Brazil, 2015. Dairy intake: P50=169·2 g/d (P<0·001*); egg intake: P50=16·7 g/d (P=0·184); fish intake: P50=6·7 g/d (P=0·009). *P<0·05 (Fisher’s exact test)
, ≥10 µg/d) according to the consumption of dairy products, eggs and fish (equal to or greater than the median, ≥P50; less than the median, <P50) by urban schoolchildren aged 8–9 years (n 378), Viçosa, Minas Gerais, Brazil, 2015. Dairy intake: P50=169·2 g/d (P<0·001*); egg intake: P50=16·7 g/d (P=0·184); fish intake: P50=6·7 g/d (P=0·009). *P<0·05 (Fisher’s exact test)
Table 4 Main food group sources of vitamin D consumed by urban schoolchildren aged 8–9 years (n 378), Viçosa, Minas Gerais, Brazil, 2015
Discussion
Vitamin D intake by most children was lower than that recommended by the Institute of Medicine, with dairy products and fish accounting for most of its intake. The inadequate vitamin D intake was associated with higher prevalence of low HDL-C and vitamin D insufficiency/deficiency. More than half of the children had dyslipidaemia and vitamin D insufficiency/deficiency.
Research in Europe has shown an inverse association between serum 25(OH)D concentration and adverse lipid profile during childhood and adolescence( Reference Rodríguez-Rodríguez, Ortega and González-Rodríguez 38 , Reference Pacifico, Anania and Osborn 39 ). Considering this association, it is important to know the effect of vitamin D intake on serum lipids, especially in children who have shown high levels of dyslipidaemia. So far, the present study has been one of few to evaluate the association between vitamin D intake and lipid profile in children. A study with Portuguese adolescents showed that low vitamin D intake was associated with a worse metabolic profile, including low HDL-C( Reference Moreira, Moreira and Abreu 23 ). In New Zealand, children fed milk fortified with vitamin D had serum concentrations of HDL-C improved( Reference Graham, Kira and Conaglen 40 ). However, a clinical trial carried out with children in Iran found no effects of vitamin D supplementation on serum concentrations of HDL-C and other lipid profile markers( Reference Kelishadi, Salek and Sakek 41 ). The results of the present study point out the necessity for experimental and interventional research to further investigate the effect of vitamin D intake on HDL-C concentration and other serum lipids.
One mechanism that could explain this relationship would be the participation of vitamin D in the regulation of reverse cholesterol transport through macrophages( Reference Matsuura, Wang and Chen 42 ). Reverse cholesterol transport carries cholesterol out of lipid-laden macrophage sponge cells in atherosclerotic plaque, as HDL-C, for clearance from the circulation( Reference Rye, Bursill and Lambert 43 ).
We found an association between vitamin D intake and serum 25(OH)D concentration in children. A longitudinal study in Iceland with children aged 1 to 6 years found similar results, showing that vitamin D intake was a predictor of serum 25(OH)D concentration( Reference Thorisdottir, Gunnarsdottir and Steingrimsdottir 44 ). Another study in Sweden reported that adequate vitamin D intake had greater influence on serum 25(OH)D concentration than regional latitude( Reference Åkeson, Lind and Hernell 45 ). Although sun exposure is responsible for most of the serum concentration of 1,25-dihydroxy-cholecalciferol (1,25-dihydroxyvitamin D3), vitamin D intake is also important, especially in the winter season, when there is a lower incidence of UVB rays( Reference Holick 1 ).
Other research in paediatric populations from different places corroborates our results, showing high prevalence of inadequate vitamin D intake( Reference Raimundo, Bueno and Moulin 46 , Reference Ortega Anta, González-Rodríguez and Jiménez Ortega 47 ). In this way, one can see the difficulty in reaching the daily recommendation for vitamin D, highlighting the necessity of better food and nutritional education as well as programmes that stimulate access to these foods.
In the present study population, the main sources of vitamin D were dairy products and fish. In Brazil, few foods are enriched with vitamin D, unlike other countries, where policies exist that stimulate food fortification with this nutrient( Reference Calvo and Whiting 48 , Reference Black, Walton and Flynn 49 ). Moreover, it is observed that there is a worldwide tendency of decreased consumption of milk products, replacing them by sugar-sweetened beverages( Reference Dror and Allen 50 ). In relation to fish, it is known that regular consumption is low, especially in Minas Gerais( Reference Jaime, Stopa and Oliveira 51 ). However, each region or country has its own main food sources of vitamin D. One study including a number of European countries identified fish as the main source for adolescents( Reference Julián, Mouratidou and Vicente-Rodriguez 52 ). Another study with Spanish schoolchildren found that eggs were the main source( Reference Aparicio Vizuete, López-Sobaler and López Plaza 53 ), unlike the present study, where eggs were not as important.
Differences in vitamin D intake between the sexes may be explained by the fact that boys consume more dairy products, as also shown by the National Research on School Health (PeNSE), where a larger proportion of boys (58·3 %) than girls (49·7 %) consumed milk( 54 ). The results of the present study are in line with other studies across the world showing that boys consume more vitamin D( Reference Boucher-Berry, Speiser and Carey 55 , Reference Salamoun, Kizirian and Tannous 56 ).
It is important to stimulate outdoor activities, as well as the consumption of food sources of vitamin D, because of the high prevalence of vitamin D insufficiency/deficiency in the children of our population. In another study with adolescents in Juiz de Fora, Minas Gerais, Brazil, deficiency and insufficiency of vitamin D was also found (1·25 and 70·6 %, respectively)( Reference Oliveira, Novaes and Azeredo 57 ). In the current study, the majority of children had sun exposure over 20 min/d (93·7 %) and it was conducted in a region with high incident UVB. Other studies with children in countries in low latitudes such as India or Lebanon have shown similar results( Reference Marwaha, Tandon and Reddy 58 , Reference El-Hajj Fuleihan, Nabulsi and Choucair 59 ).
Our results point out the necessity of planning and implementing public health policies that stimulate outdoor activities in schools and elsewhere. Other strategies may be adopted, such as food and nutritional education activities, stimulating the consumption of source foods, as well as facilitating access of families to these foods. Generalized supplementation of the population is not recommended by the Brazilian Society of Endocrinology and Metabology( Reference Maeda, Borba and Camargo 60 ). However, a policy of food fortification should be encouraged in foods that are part of Brazilian children’s eating habits, such as cereals and dairy products, as a proven strategy for reducing the prevalence of vitamin D insufficiency/deficiency.
We highlight that the present study is one of the few in the world to have evaluated the relationship between consumption of vitamin D and dyslipidaemia, as well as vitamin D insufficiency/deficiency in childhood. However, the study is limited by use of a cross-sectional design, not allowing us to establish causality. Moreover, there is still no consensus on the cut-off points for normal apoB and apoA1 for the paediatric population to infer its prevalence. Another point is that the nutritional fact labels of most foods include no information about their vitamin D content. Even though the 24 h dietary recall has its own limitations because it depends on the memory of the interviewee and does not predict habitual intake, adjustments were made( Reference Willett 35 , Reference Willett and Stampfer 37 ). Energy intake was adjusted for intra-individual variability to minimize possible biases, and photo albums were provided showing household utensils and examples of portion sizes.
Conclusion
In conclusion, the present study has found that Brazilian children have a high prevalence of inadequate vitamin D intake, dyslipidaemia and 25(OH)D insufficiency/deficiency. Inadequate intake was associated with low HDL-C and vitamin D insufficiency/deficiency. Dairy products and fish were the groups that provided the largest contribution to vitamin D intake. The importance of better food and nutritional education and programmes stimulating and facilitating access to source foods is also highlighted. Moreover, experimental research and interventional studies are needed to better understand the effect of vitamin D intake on serum concentrations of HDL-C and other lipids in childhood.
Acknowledgements
Acknowledgements: The authors are grateful to the National Council for Scientific and Technological Development (CNPq) for financial support; BIOCLIN® for providing material for biochemical analysis; the Research Support Foundation of State of Minas Gerais (FAPEMIG) for the granting of a doctor’s scholarship to M.S.F. and the Coordination for the Improvement of Higher Education Personnel (CAPES) for the scholarships granted to L.G.S., M.A.S. and N.P.R. They also thank all the children who participated in this work and their guardians. Financial support: This work was supported by the CNPq (grant number 478910/2013-4). The CNPq had no role in the design, analysis or writing of this article. Conflict of interest: The authors declare no conflict of interest. Authorship: M.S.F. assisted the conception and design of this work, assisted data collection, analysis and interpretation of the data, conducted the literature search, as well as wrote the manuscript. L.G.S. and M.A.S. revised and approved the final version to be published. N.P.R. assisted data collection, revised and approved the final version to be published. J.F.N. designed the study including the data collection, coordinated and supervised and approved the final version to be published. Ethics of human subject participation: This study was conducted according to the guidelines laid down in the Declaration of Helsinki and all the procedures involving human subjects were approved by the Ethics Committee on Human Research of the Federal University of Viçosa (case number 663.171/2014). Moreover, this project was presented to the Municipal Department of Education, the Regional Superintendent of Education and principals of schools. All participants, as well as their responsible parents/guardians, were informed about the objectives of the research and informed consent was obtained from all children’s parents.









