Whereas the effects of exposure to a single severe traumatic event are widely known, a growing body of evidence suggests that repeated and ongoing work-related trauma experiences may also have a detrimental impact on mental health.Reference Benedek, Fullerton and Ursano1 Only a minority of individuals who have experienced trauma tend to develop post-traumatic stress disorder (PTSD) due to a wide range of psychological coping strategies.Reference Agaibi and Wilson2 This suggests that there is a potential compensatory mechanism in the brain that responds to trauma in such a way that many are able to cope, reducing the risk of PTSD. In certain populations such as first responders (firefighters, rescue workers, police officers, etc.), exposure to repeated traumatic stress cannot be fully avoided due to the nature of the occupation. To further develop prevention strategies for PTSD in these vulnerable populations, it is important to investigate the underlying brain alterations in response to repeated and prolonged occupational trauma exposure. For instance, understanding the neural correlates of risk for – or resilience to – traumatic stress may provide insight with regards to potential biomarkers for screening individuals who are at risk, as well as potential target areas for early preventive intervention. A previous study showed that resting-state functional connectivity can predict future PTSD symptoms in people who experience acute trauma.Reference Lanius, Bluhm, Coupland, Hegadoren, Rowe and Theberge3 However, studies on the neurobiology of occupational exposure to repeated trauma are scarce and the majority have only focused on vulnerable populations who have already been diagnosed with PTSD.Reference Fragkaki, Thomaes and Sijbrandij4 Since individuals with PTSD may already have chronic deficits in maintaining physiological homeostasis when faced with trauma, it may be of value to examine the normal adaptive reactions of the brain in those who have been routinely exposed to occupation-related trauma, but did not develop PTSD. Furthermore, conducting an in vivo neuroimaging study on these vulnerable professionals without PTSD may provide unique opportunities to examine the potential neural pathways underlying the risk for, or resilience to, repeated traumatic stress. However, only a few studies have been performed in first responders without PTSD.Reference Hennig-Fast, Werner, Lermer, Latscha, Meister and Reiser5–Reference Shucard, Cox, Shucard, Fetter, Chung and Ramasamy9 Although some of these studies reported structural and functional alterations in the brain areas involved with fear processing,Reference Jung, Chang and Kim6, Reference Reynaud, Guedj, Trousselard, El Khoury-Malhame, Zendjidjian and Fakra8 the results are still limited and inconclusive.
Method
This study aimed to investigate the countering or compensatory role of the human brain in responding to ongoing occupation-related trauma using resting-state functional magnetic resonance imaging (fMRI). For this purpose, we recruited currently employed professional firefighters without any mental illness and non-firefighter healthy controls without any previous traumatic exposure. We assessed the functional connectivity within the fear circuit including the amygdala, hippocampus, dorsal anterior cingulate cortex (dACC), insula and ventromedial prefrontal cortex (vmPFC).Reference Shin and Liberzon10 Associations between alterations in functional connectivity and trauma-related symptoms were also evaluated.
Participants
Professional firefighters, who had been employed for at least 6 months and repeatedly exposed to aversive traumatic events in the course of their duties, were recruited as the ‘trauma-exposed group’. Non-firefighter, healthy individuals who had no history of traumatic experiences were recruited as the ‘trauma-unexposed group’. The following exclusion criteria were applied to both groups: (a) Axis-I mental disorders including major depressive disorder, PTSD, psychotic disorder, bipolar disorder or substance dependence; (b) major medical or neurological disorders; (c) history of mild traumatic brain injury; (d) current use of psychotropic medications; and (e) contraindications to MRI examinations such as claustrophobia or implants of ferromagnetic material. All participants provided written informed consent to participate in the study. The study protocol was approved by the Institutional Review Board of Ewha Womans University (53-10).
Clinical assessment
Physical examinations, vital signs, electrocardiograms and clinical laboratory tests were performed for screening. To assess medication use, participants were requested to bring a copy of the prescription or medications they were taking. No participant reported taking current psychotropic medication or having used illicit drugs. Evaluation of injuries showed that one firefighter was receiving treatment for a ruptured knee ligament. The absence of any mental disorders was confirmed for all participants using the Structured Clinical Interview for DSM-IV.Reference First, Spitzer, Gibbon and Williams11 Trauma-related symptoms were evaluated using the Impact of Event Scale – Revised (IES-R),Reference Eun, Kwon, Lee, Kim, Choi and Cho12 a 22-item self-report questionnaire that consists of three subscales: intrusion, avoidance and hyperarousal. Each item is rated on a 5-point Likert scale, ranging from 0 (not at all) to 4 (extremely). The total score of IES-R ranges from 0 to 88, with higher scores indicating higher levels of trauma-related symptoms. Social relationships were assessed with the Social Network Index which produces three outcomes:Reference Cohen, Doyle, Skoner, Rabin and Gwaltney13 (a) network diversity is the number of social roles in which the respondent has regular contact with at least one person, (b) network size is the total number of people with whom the respondent has regular contact and (c) embedded networks refer to the number of network domains in which a respondent had at least four high-contact people. Handedness was determined using the Edinburgh Handedness Inventory.Reference Oldfield14
Image acquisition
All MRI data were acquired using a Philips Achieva 3T scanner (Philips Medical Systems, Best, The Netherlands) at Ewha Brain Institute (Seoul, South Korea). Resting-state fMRI scans were obtained using a gradient-echo echo-planar imaging sequence (echo time (TE) 21 ms, repetition time (TR) 2000 ms, flip angle (FA) 76°, field of view (FOV) 22 × 22 cm2, matrix 64 × 64, voxel size 3.44 × 3.44 × 3.44 mm3, 38 slices). Two sessions were acquired from each participant and each session consisted of 200 volumes. Before each session, participants in the scanner were asked to press a button if they were awake and were then instructed to keep their eyes closed, not to fall asleep, think of nothing in particular and to let their mind wander freely. Afterwards, all participants confirmed that they did not fall asleep during the fMRI scans. Resting-state fMRI scans were always conducted after the T1-weighted scans to allow participants to get accustomed to the environment and noise inside the scanner. High-resolution, T1-weighted MPRAGE scans were performed using the following sequence: TE 3.4 ms, TR 7.4 ms, FA 8°, FOV 22 × 22 cm2, matrix 256 × 256, voxel size 0.86 × 0.86 × 0.86 mm3.
T2-weighted (TE 90 ms, TR 3000 ms, FA 90°, FOV 22 × 20.1 cm2, matrix 240 × 240, voxel size 0.92 × 0.92 × 0.92 mm3) and fluid-attenuated inversion recovery (FLAIR) images (TE 338 ms, TR 8000 ms, FOV 25 × 25 cm2, matrix 576 × 576, voxel size 0.43 × 0.43 × 0.43 mm3) were also obtained from all participants. T1-weighted, T2-weighted and FLAIR images were reviewed by an experienced radiologist (S.M.L.) to screen for the presence of any gross abnormalities in the brain.
Functional connectivity analysis
Resting-state fMRI images were processed using the CONN toolbox (https://www.nitrc.org/projects/conn)Reference Whitfield-Gabrieli and Nieto-Castanon15 and Statistical Parametric Mapping 12 (SPM12; Wellcome Department of Cognitive Neurology, Institute of Neurology, London, UK; http://www.fil.ion.ucl.ac.uk/spm). In the preprocessing steps, the functional images were realigned, unwarped, slice-timing corrected, spatially normalised into the Montreal Neurological Institute (MNI) space and resampled to 2 × 2 × 2 mm3 voxels. T1-weighted images were segmented into grey matter, white matter and cerebrospinal fluid, and then normalised into the MNI space.
To reduce the noise of the fMRI images, head motion parameters and motion outlier volumes were regressed out. Functional outlier detection and scrubbing were conducted by using the Artifact Detection Tools (ART; http://www.nitrc.org/projects/artifact_detect). After the average signal across the entire time series was calculated and z-transformed, volumes were defined as outliers if the combination of translational and rotational voxel displacements exceeded 0.9 mm from the previous volume or if the average intensity deviated more than 5 s.d. from the mean intensity of the session. The number of outlier volumes did not differ significantly between the two groups (t = 1.19, P = 0.23). In addition, both groups did not show any difference in the composite average (t = 1.12, P = 0.27) and maximum measures of head motion (t = 0.25, P = 0.81), both of which were estimated by using the ART. Signals from white matter and cerebrospinal fluid were removed by linear regression by using the anatomical component-based noise correction method (aCompCor) to remove physiological and other spurious sources of noise.Reference Behzadi, Restom, Liau and Liu16 Finally, a temporal band pass filter (0.008–0.09 Hz) was applied to the residual time series.
The cortical seed regions of interests (ROIs) were defined as 6 mm radius spheres centred on the following MNI coordinatesReference Fair, Cohen, Power, Dosenbach, Church and Miezin17 using the Wake Forest University PickAtlas (http://fmri.wfubmc.edu/software/pickatlas): dACC (−1, 10, 46), left anterior insula (−35, 14, 5), right anterior insula (36, 16, 4) and vmPFC (−3, 39, −2). For the exact coverage of subcortical structures, bilateral seed masks for the amygdala and hippocampus were taken from the Harvard–Oxford Atlas (Harvard Center for Morphometric Analysis, Charlestown, US; http://www.cma.mgh.harvard.edu) (Fig. 1). The time series of all voxels within each seed were averaged and the bivariate correlation coefficients between each pair of ROIs were then computed and converted to z-scores by using Fisher's transformation.
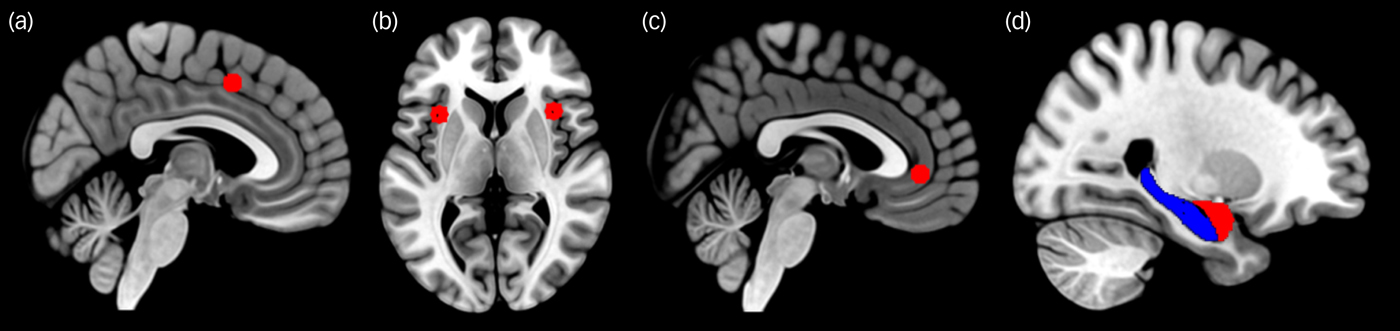
Fig. 1 Seed areas of the fear network. Shown are (a) dorsal anterior cingulate, (b) insula, (c) ventromedial prefrontal cortex, (d) amygdala (red) and hippocampus (blue). The cortical seeds (a, b, c) were defined as 6-mm radius spheres whereas the bilateral seed masks for the amygdala and hippocampus (d) were taken from the Harvard–Oxford Atlas.
Sample size estimation
The estimated effect size was based on a previous resting-state fMRI study that reported lower functional connectivity in the default mode network (Cohen's d = −0.90) of combat-exposed veterans without PTSD as compared with that of healthy civilians.Reference DiGangi, Tadayyon, Fitzgerald, Rabinak, Kennedy and Klumpp18 Considering that the typical traumatic situations experienced by firefighters may be less extreme than the battlefield experiences of combat-exposed veterans, we expected half of the effect size of the previous study (Cohen's d = −0.45). By selecting a power of 0.80 and α of 0.05, the estimated minimum sample size resulted in 79 individuals per group.
Statistical analysis
Differences in continuous and categorical demographic variables between the two groups were assessed by using an independent t-test, chi-squared test or Fisher's exact test.
Group differences in functional connections between all ROIs of the fear network were examined using analysis of covariance models with age and gender as covariates. The results were considered statistically significant at a false discovery rate (FDR)-corrected P-value threshold of less than 0.05.
To examine whether altered functional connectivity in the fear circuitry may be correlated with the severity of trauma-related symptoms in the firefighter group, multiple linear regression analysis was used with the total IES-R score as the dependent variable and with functional connectivity values as the independent variables. Age and gender were also included into the model as covariates. Functional connectivity values that demonstrated group differences (trauma-exposed group versus trauma-unexposed group) were selected and entered jointly into the multiple linear regression analysis model to estimate their degree of contribution. Multicollinearity among independent variables was also assessed by using the variance inflation factor.
When attending the scene of a fire, firefighters are frequently exposed to carbon monoxide and other chemical hazards that may have detrimental effects on the brain.Reference Guidotti and Clough19 Previous studies suggested that prolonged exposure to chemical by-products of combustion may increase the risk of Parkinson's disease in firefighters.Reference Ye, Kim, Jeong-Choi, Kim, Park and Lee20 To evaluate the potential confounding effects of chronic exposure to chemical hazards, we repeated multiple linear regression analysis by including the frequency of dispatches per week as an additional covariate.
A two-tailed P-value of less than 0.05 was considered statistically significant. All statistical analyses were conducted using Stata version 13.1 (StataCorp., College Station, US). The BrainNet Viewer was used for the visualisation of the neuroimaging results (https://www.nitrc.org/projects/bnv).
Results
Demographic and clinical characteristics
A total of 98 firefighters and 98 non-firefighter participants without any mental disorders were included in the analysis. The demographic and clinical characteristics of all the participants are presented in Table 1. All participants were East Asians. There were no significant differences in age (t = −0.26, P = 0.80), gender (χ 2 = 0.07, P = 0.79), education level (χ 2 = 2.09, P = 0.15), handedness (χ 2 = 1.09, P = 0.30), smoking (χ 2 = 1.83, P = 0.18) and alcohol use (χ 2 = 0.10, P = 0.75) between two groups. Although the firefighter group had a significantly higher body mass index than the non-firefighter group (24.6 (s.d. = 2.6) versus 23.7 (s.d. = 2.3), t = 2.54, P = 0.01), the proportion of obesity (body mass index ≥30) did not differ between the two groups (Fisher's exact test, P = 0.12). In addition, there were more firefighters who were married or living with a partner (75.5%) compared with non-firefighters (57.4%, χ 2 = 7.04, P = 0.01). However, scores of Social Network Index did not statistically differ between the two groups. Mean years employed as a firefighter was 13.7 years (s.d. = 8.1) and mean total score of the IES-R was 18.6 (s.d. = 18.4) in the firefighter group.
Table 1 Demographic and clinical characteristics of participants
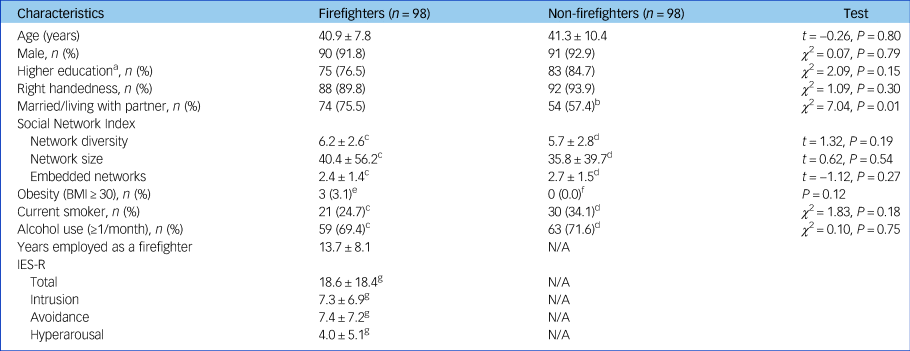
BMI, body mass index; N/A, not applicable; IES-R, Impact of Event Scale – Revised.
a. College or higher.
b. n = 94.
c. n = 85.
d. n = 88.
e. n = 96.
f. n = 97.
g. n = 84.
Group differences in functional connectivity of the fear network
Among the ROIs in the fear network, we found that the connectivity of the insula was altered in the trauma-exposed group. Specifically, stronger left insula connectivity was found with the left amygdala (t = 2.64, FDR-corrected P = 0.03), right amygdala (t = 3.20, FDR-corrected P = 0.01), left hippocampus (t = 2.32, FDR-corrected P = 0.03), right hippocampus (t = 2.37, FDR-corrected P = 0.03) and vmPFC (t = 2.38, FDR-corrected P = 0.03) (Fig. 2) among firefighters relative to the trauma-unexposed group. In addition, functional connectivity between the right insula and left amygdala was also higher in the trauma-exposed group (t = 3.02, FDR-corrected P = 0.02) as compared with the trauma-unexposed group. There were no between-group differences in functional connectivity among other ROIs of the fear network.
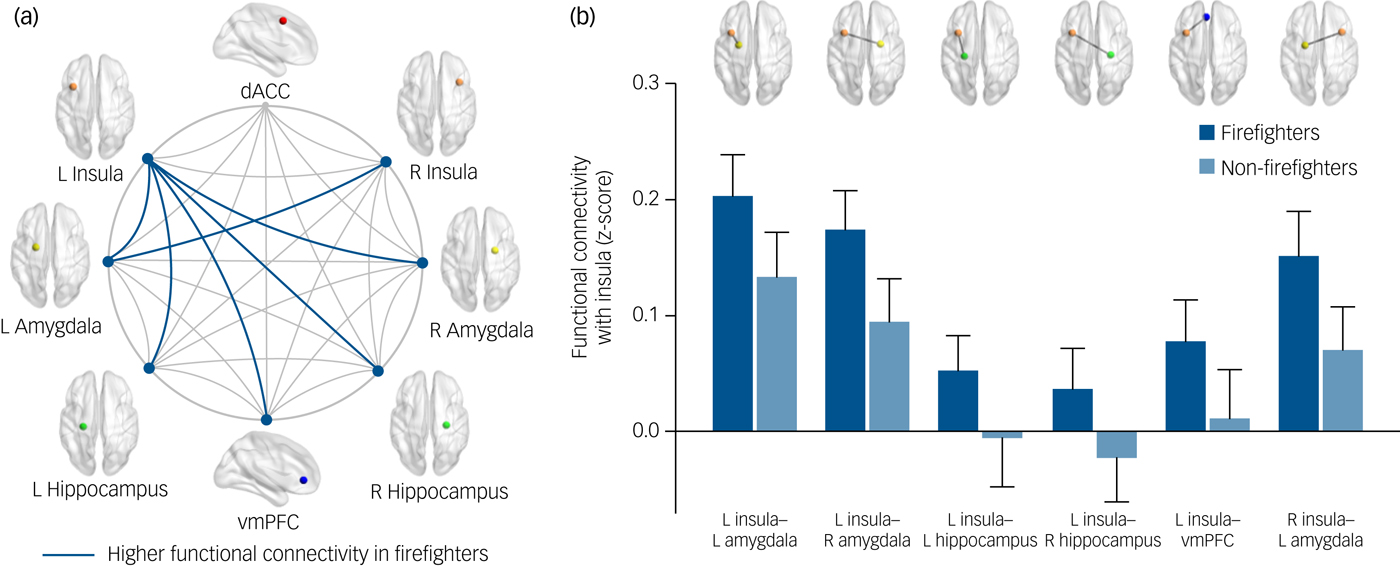
Fig. 2 Group comparisons of functional connectivity in the fear network between firefighters (n = 98) and non-firefighters without trauma experiences (n = 98). (a) The fear network consists of the dACC, insula, amygdala, hippocampus and vmPFC. Firefighters exhibited higher insular functional connectivity to bilateral amygdalae, bilateral hippocampi and vmPFC after adjusting for age and gender (false discovery rate-corrected P < 0.05). (b) Bar graph illustrates significant between-group differences in means and 95% confidence intervals of the insular functional connectivity. dACC, dorsal anterior cingulate cortex; L, left; R, right; vmPFC, ventromedial prefrontal cortex.
Associations of altered fear network connectivity with trauma-related symptoms
Functional connectivity values that showed between-group differences were entered into the multiple linear regression analysis. The averaged connectivity value between the right insula and the left amygdala as well as that between the left insula and the bilateral amygdalae, which represented the insular connections with the amygdala, were entered into the multiple regression model. Likewise, the left insula connectivity values in relation to the left and right hippocampi were averaged. The model included the IES-R score as the dependent variable and the three connectivity measures (insula–bilateral amygdalae, left insula–bilateral hippocampi and left insula–vmPFC) as independent variables, adjusting for age and gender. Functional connectivity between the insula and bilateral amygdalae (β = 0.36, P = 0.005) showed positive association with the IES-R scores, whereas connectivity between the left insula and vmPFC (β = −0.28, P = 0.01) was negatively correlated with the IES-R scores (Fig. 3).
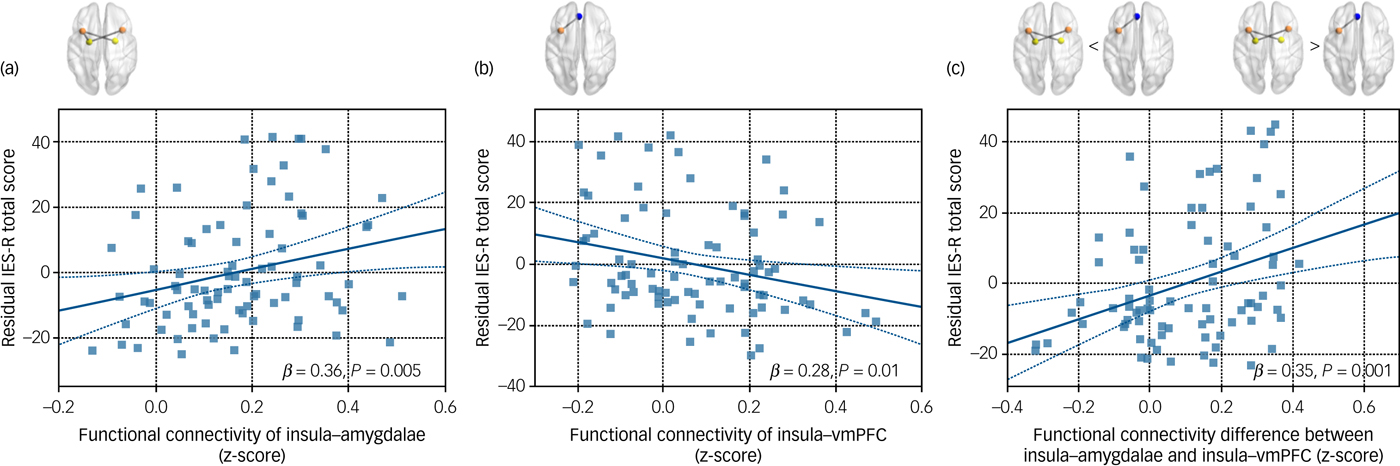
Fig. 3 Scatter plots and regression lines that indicated the relationships between the total IES-R score and insular functional connectivity in firefighters (n = 84). (a) The relationship between the total IES-R score and functional connectivity of insula–amygdalae. (b) The relationship between the total IES-R score and functional connectivity of left insula–vmPFC. (c) The relationship between the total IES-R score and functional connectivity difference between insula–amygdalae and left insula–vmPFC. Age and gender were included as covariates in all analyses. IES-R, Impact of Event Scale – Revised; vmPFC, ventromedial prefrontal cortex.
The functional connectivity value between the left insula and vmPFC was subtracted from that of the insula and amygdalae connection. This connectivity difference showed a positive correlation with the IES-R score after adjusting for age and gender (β = 0.35, P = 0.001) (Fig. 3).
The above-mentioned associations of altered fear network connectivity with trauma-related symptoms remained unchanged even when the frequency of dispatches per week was included as an additional covariate. Detailed results are shown in the Supplementary Material available at https://doi.org/10.1192/bjp.2018.260.
Discussion
To our knowledge, this study is the largest neuroimaging study on firefighters that examines resting-state functional connectivity in the fear network and its associations with trauma-related symptoms. We found stronger insular connectivity with the bilateral amygdalae, bilateral hippocampi and vmPFC in repeated trauma-exposed firefighters without PTSD as compared with non-firefighters. Furthermore, the insula–amygdala connectivity showed a positive correlation with trauma-related symptoms as measured with the IES-R, whereas the insula–vmPFC connection was negatively associated with trauma-related symptoms in the firefighter group. Taken together, our results may provide insight into the mechanisms involved in the response to repeated trauma in individuals without any mental disorder.
The most interesting finding of our study is that functional connections of the insula within the fear network were enhanced in the firefighter group. According to previous studies, insular hyperactivity has been consistently observed in people with PTSD as well as during fear conditioning in healthy individuals.Reference Etkin and Wager21 In addition, insular activity has been associated with resilience in healthy individuals exposed to threatening visual stimuli.Reference Waugh, Wager, Fredrickson, Noll and Taylor22 The insula also mediates complex social emotions such as empathyReference Singer, Critchley and Preuschoff23 and compassion for social, psychological and physical pain,Reference Immordino-Yang, McColl, Damasio and Damasio24 all of which may be experienced by firefighters in their line of duty. As such, increasing evidence suggests that the insula may be of significance in understanding the integrative processing of the external sensory information with emotional, cognitive and motivational signals.Reference Namkung, Kim and Sawa25 The insula has reciprocal connections to the prefrontal and limbic regions and plays a pivotal role as an integral hub in regulating dynamic interactions among other brain networks involved in internally oriented cognition and externally oriented attention. Specifically, the insula marks and prioritises salient stimuli based on one's subjective awareness of both positive and negative feelings, and initiates further cognitive processing to guide appropriate behavioural reactions.Reference Namkung, Kim and Sawa25, Reference Menon and Uddin26 In addition, the insula plays an important role in uncertainty, anticipation of aversive stimuli, threat detection and fear generalisation.Reference Dunsmoor, Prince, Murty, Kragel and LaBar27 In line with previous studies, our results may underline the central roles of the insula in response to repeated traumatic stress.
We found a positive correlation between the insula–amygdala functional connectivity and trauma-related symptoms in firefighters. The insula has direct anatomical connections with the amygdala, a key player of fear and threat processing.Reference Ohman28 Previous studies reported greater resting-state functional connectivity between the insula and amygdala in combat-related people with PTSDReference Rabinak, Angstadt, Welsh, Kenndy, Lyubkin and Martis29, Reference Brown, LaBar, Haswell, Gold, Mid-Atlantic and McCarthy30 and trauma-exposed individuals.Reference Brown, LaBar, Haswell, Gold, Mid-Atlantic and McCarthy30 In addition, functional coupling between the insula and amygdala is positively associated with perceived threat in combat-exposed soldiers.Reference van Wingen, Geuze, Vermetten and Fernandez31 Our results may reflect that excessive and sustained vigilance may be present in individuals who have been repeatedly exposed to trauma, even without the presence of an actual threat. Therefore, the current findings may suggest a potential risk for individuals exposed to occupation-related repeated trauma.
However, stronger functional connectivity between the insula and vmPFC was related with less severe trauma-related symptoms in firefighters. The vmPFC has been implicated in conditioned fear extinctionReference Milad and Quirk32 and is associated with the ability to alleviate negative emotions related to trauma.Reference Urry, van Reekum, Johnstone, Kalin, Thurow and Schaefer33 Furthermore, people with PTSD demonstrate hypoactivation of the vmPFC, indicating impaired regulation of fear responses.Reference Etkin and Wager21 Consistent with our results, compensatory increases in functional connectivity between the insula and vmPFC were found in healthy individuals with high trait anxiety after fear conditioning.Reference Feng, Feng, Chen and Lei34 As for the current study, the heightened insula–vmPFC connectivity in firefighters may be related to emotional coping and resilience mechanisms against repeated trauma experiences.
Here we calculated an arbitrary value to subtract the insula–vmPFC connectivity from the insula–amygdala connectivity. Positive values may indicate that the insular connections with the amygdala are more predominant as compared with those between the insula and vmPFC, whereas negative values may indicate that the insular connections with the vmPFC are stronger than those with the amygdala. Interestingly, these values, which represent the connectivity difference between the insula–amygdala and the insula–vmPFC, were positively associated with the severity of trauma-related symptoms in firefighters. This result additionally supports the neuroanatomical theory of PTSD that hypothesise a hyperresponsivity of the amygdala to trauma-related stimuli and a hyporesponsivity of the vmPFC that exerts top-down regulation.Reference Rauch, Shin and Phelps35 In the face of repeated exposure to occupation-related trauma, the balance or equilibrium within the fear network may be key to understanding the dynamic mechanism behind resilience or maladaptation to adverse stress.
It is also noteworthy that the insula–hippocampus connectivity was higher in the firefighter group as compared with the trauma-unexposed group. Previous studies of people with PTSD have consistently reported structural and functional deficits of the hippocampus, which is involved in explicit memory processing and encoding of contextual information during fear conditioning.Reference Shin, Rauch and Pitman36 In addition, enhanced insula–hippocampus functional coupling was found in healthy individuals during fear memory consolidation.Reference Feng, Feng, Chen and Lei34 Although the association between insula–hippocampus connectivity and the severity of trauma-related symptoms has yet to be concluded, we speculate that our results are in tandem with such previous studies, and may further indicate the presence of facilitated processing and excessive rumination of traumatic memories.
Resting-state fMRI shows potential for a broader range of clinical applications compared with task-based fMRI due to some notable advantages such as a better signal to noise ratio and avoidance of task-related confounds.Reference Fox and Greicius37 Previous studies suggested that resting-state functional connectivity is useful in predicting disease prognosis and monitoring treatment effects in trauma-exposed individuals.Reference Lanius, Bluhm, Coupland, Hegadoren, Rowe and Theberge3, Reference Koch, van Zuiden, Nawijn, Frijling, Veltman and Olff38 Furthermore, resting-state fMRI can be used for identifying treatment targets and optimal protocols for brain stimulation in the case of various neuropsychiatric disorders.Reference Fox, Buckner, Liu, Chakravarty, Lozano and Pascual-Leone39 The current findings suggest that the insular functional connectivity may be a candidate for these areas of application in individuals exposed to repeated trauma. To facilitate the translation from research into clinical practice, further studies are required to evaluate the reproducibility of our results, along with an improvement and standardisation of image processing and analytical methods.Reference Fox and Greicius37
The current study is unique as the firefighters recruited are a population that consists of individuals who are healthy despite being exposed to repeated traumatic events. However, the generalisability of findings is limited, potentially due to the nature of their occupation such as undergoing rigorous training. Firefighters may have higher resilience to trauma than the average population. Moreover, most of the participants recruited in the current study were male. As such, our sample represented only a subgroup of the overall population and may not be fully representative. With the underlying assumption that the brain's response to trauma would be dependent on the demographic characteristics of the study population as well as the nature of trauma, further studies with different populations are required to generalise our findings. Another potential limitation is that the current cross-sectional design only provides correlational findings and is therefore limited in terms of causal inferences. Future longitudinal investigations may provide further insight into the nature of these relationships and disentangle trauma-induced brain deficits from pre-existing vulnerability factors. Finally, although the additional analyses demonstrated that the findings were not attributed to the weekly number of dispatches, the biological damage due to carbon monoxide or other hazardous substances still may have influenced the results. Future studies using other imaging modalities may provide further insight with regards to the underlying neural mechanisms to repeated and ongoing trauma.
Supplementary material
Supplementary material is available online at https://doi.org/10.1192/bjp.2018.260.
Funding
This research was supported by the Brain Research Program through the National Research Foundation of Korea funded by the Ministry of Science and ICT (2015M3C7A1028373, 2015M3C7A1028376) and by the Field-oriented Support of Fire Fighting Technology Research and Development Program funded by the National Fire Agency (MPSS-Fire Fighting Safety-2016-86).






eLetters
No eLetters have been published for this article.