The course of a mental disorder is likely to be affected by psychological stress caused by the patient's family members, and many studies of patients with schizophrenia have reported an association between the level of expressed emotion (EE) of family members and patients’ relapse. Reference Leff and Vaughn1,Reference Bebbington and Kuipers2 Intervention studies have supported this causative association, as family psychoeducation aimed at reducing the EE level has been shown to prevent relapses in schizophrenia. Reference Falloon, Boyd, McGill, Razani, Moss and Gilderman3–Reference Shimodera, Inoue, Mino, Tanaka, Kii and Motoki5 The association between bipolar affective disorder and EE has also been reported. Reference Miklowitz, Goldstein, Nuechterlein, Snyder and Mintz6,Reference Kim and Miklowitz7 Several intervention studies employing family psychoeducation for bipolar affective disorder have also been performed, and a relapse-preventive effect has been confirmed. Reference Reinares, Colom, Sanchez-Moreno, Torrent, Martinez-Aran and Comes8–Reference Rea, Tompson, Miklowitz, Goldstein, Hwang and Mintz10 There have also been a number of reports describing an association between EE and relapse of depression. Reference Vaughn and Leff11–Reference Mino, Shimodera, Inoue, Fujita, Tanaka and Kanazawa16 Our prospective study suggested that the association of EE with relapse might be even stronger in depression than in schizophrenia. Reference Mino, Shimodera, Inoue, Fujita, Tanaka and Kanazawa16
Depression is a common disease, with a lifetime prevalence of about 5–12% for men and 10–25% for women. Reference Kessler, Demler, Frank, Olfson, Pincus and Walters17 It is also known to be highly recurrent. Reference Kanai, Takeuchi, Furukawa, Yoshimura, Imaizumi and Kitamura18,Reference Ramana, Paykel, Cooper, Hayhurst, Saxty and Surtees19 It causes large economic losses to society as it markedly reduces the ability of people to work, and is associated with increased medical service use and with suicide. Reference Greenberg, Kessler, Birnbaum, Leong, Lowe and Berglund20 As relapse and recurrence are closely related to the family environment, Reference Vaughn and Leff11–Reference Mino, Shimodera, Inoue, Fujita, Tanaka and Kanazawa16 family psychoeducation may offer an effective measure to tackle the many problems involving the familial environment surrounding the patient and thereby reduce relapses or recurrences of major depression. We therefore launched a randomised controlled trial to examine the effectiveness of family psychoeducation in the maintenance treatment of major depression, and the influence of EE on its effectiveness.
Method
The participants were patients who satisfied the eligibility criteria below, and their primary family members.
-
(a) Age 18–85 years.
-
(b) Diagnosis of major depressive disorder according to DSM–IV. 21
-
(c) Expectation of patient receiving continuation/maintenance antidepressant therapy for the duration of the trial after responding to acute-phase antidepressant therapy, and being in partial or full remission (i.e. no longer fulfilling the diagnostic threshold for major depressive episode).
-
(d) Patient not having undergone electroconvulsive therapy (ECT), or not having ECT already planned for the index episode.
-
(e) Patient living with the family for 3 months or longer before participating in this study and being expected to live with the family during the investigation period.
-
(f) Patient having at least one family member living with the patient who was available for family interviews (the relative aged 18 years or over who had had contact with the patient for the longest time was regarded as his or her primary family member).
Participants were recruited at the Department of Psychiatry, Kochi Medical School, Japan, or its affiliated hospital, Doujin Hospital, between April 2004 and April 2006. Patients were screened with the Mini-Mental State Examination when dementia was clinically suspected and those scoring 23 or below were excluded. Reference Folstein, Folstein and McHugh22 Patients suspected of having organic disease were examined by head magnetic resonance imaging, and those diagnosed with organic disease were excluded. Of the 103 patients who met the eligibility criteria, 57 provided written informed consent to participate after full disclosure of the purposes and procedures of the study. The major reason for refusing consent was that the primary family members were unable to attend the psychoeducation sessions because of their work. The 57 patients who gave consent were randomly allocated to intervention and control groups. The random sequence was generated by use of a random number table and was kept by anindependent clerk who allocated the intervention to the consecutive sample. No stratification was used.
Evaluation of EE
Expressed emotion was evaluated using Five-Minute Speech Samples (FMSS) Reference Magaña, Goldstein, Karno, Miklowitz, Jenkins and Falloon23,Reference Shimodera, Mino, Inoue, Izumoto, Kishi and Tanaka24 and the Family Attitude Scale (FAS). Reference Kavanagh, O'Halloran, Manicavasagar, Clark, Piatkowska and Tennant25,Reference Fujita, Shimodera, Izumoto, Tanaka, Kii and Mino26 In the interview for the FMSS, a family member was instructed to speak freely about the patient's character and their relationships without disturbance from the interviewer for 5 min. This speech sample was then evaluated by two qualified judges who had been certified through official training for the FMSS from the University of California at Los Angeles School of Medicine Family Project according to an evaluation manual of the FMSS. The interrater reliability for FMSS was excellent (κ = 0.86). We previously reported that the FMSS agreed well with the Camberwell Family Interview, which is a recognised evaluation method for EE, in Japanese participants with mood disorder. Reference Shimodera, Mino, Fujita, Izumoto, Kamimura and Inoue27 Ratings on the FMSS consisted of the four categories of initial statement, relationship, critical comments and dissatisfaction, which were used to assess criticism, and the six categories of self-sacrificing/overprotection, lack of objectivity, emotional display, statement of attitude, positive remarks and excessive detail, to assess emotional overinvolvement (EOI). The determinations of EE status were based on these categories, and family members were classified as high or low in EE. Low-EE participants were further classified into pure low EE, and those on the borderline between high and low EE. Participants with any one of the categories of initial statement, relationship or criticism assessed as satisfying the rating criteria for ‘critical’ were classified as ‘high critical’. Similarly, anyone fulfilling the rating criteria for any of the categories self-sacrifice/overprotection, lack of objectivity or emotional display was classified as ‘high EOI’. Participants assessed as satisfying criteria for more than two of the three categories of statement of attitude, positive remarks or excessive detail were also rated as high EOI. If only one category was present, the participants were classified as borderline EOI/low EE. If only dissatisfaction was present, they were classified as borderline critical/low EE. When dichotomising, it has been proposed to include borderline low-EE families in the high-EE category, as a means of compensating for the diminished sensitivity of the FMSS to high EE in schizophrenia. Reference Shimodera, Mino, Inoue, Izumoto, Kishi and Tanaka24,Reference Uehara, Yokoyama, Goto and Ihda28 The sensitivity also tended to increase in the study of mood disorders when borderline low-EE families were included in the high-EE category. Reference Shimodera, Mino, Fujita, Izumoto, Kamimura and Inoue27
For self-rated EE evaluation the FAS was used. This is a self-rating scale attaching a greater importance to evaluation of the two EE elements of criticism and hostility, and its validity in schizophrenia has been confirmed in Japan. Reference Fujita, Shimodera, Izumoto, Tanaka, Kii and Mino26 The FAS contains 30 questions such as ‘I wish he were not here’, ‘He is a real burden’ and ‘He is hard to get close to’. Respondents reported how often each statement was true on a scale ranging from ‘every day’ (4) to ‘never’ (0). Responses were summed to give a score that ranged from 0 to 120, with higher scores indicating higher levels of burden or criticism.
Evaluation of psychiatric symptoms
To evaluate the depressive state we administered the Hamilton Rating Scale for Depression (HRSD) and the Beck Depression Inventory (BDI) before intervention and after 9 months. Reference Hamilton29,Reference Beck, Ward, Mendelson, Mock and Erbaugh30 When the treating psychiatrist masked to the allocated intervention or EE status recognised re-emergence of a major depressive episode according to DSM–IV criteria in the course of the bi-weekly visits constituting treatment as usual, the patient was referred to an independent psychiatrist, also masked to the patient's allocation, who administered the HRSD and BDI. Relapse was declared when the diagnostic threshold for a major depressive episode as specified in DSM–IV was met according to the interview by this independent psychiatrist. Remission was defined as an HRSD score of 6 or lower.
Family psychoeducation
Family psychoeducation took the form of courses attended by up to five family members, without the participation of the patients. Only one family member per patient attended. Sessions took place once every 2 weeks, and the full course comprised four sessions: ‘Epidemiology and causes’, ‘Symptoms’, ‘Treatment and course’ and ‘Coping with the patient’. Each session lasted 90–120 min: the first 30 min were devoted to providing information regarding depression and its treatment, and the subsequent 60–90 min were devoted to group discussion and problem-solving for high-EE situations experienced by the participating families. A videotape and a textbook explaining depression and its treatment were prepared for this study and were used as teaching materials. In the group problem-solving exercises, family members were asked to collaborate on compiling a list of possible solutions, discussing their advantages and disadvantages, and arriving at the best possible coping solution in response to high-EE situations suggested by family members. The therapists tried to minimise their intervention in order to respect the families’ autonomy and to empower them maximally.
The number of participants was limited to five to encourage them to contribute to the group discussion. Participating staff consisted of two psychiatrists (S.S. and H.F.) and one clinical psychologist. S.S. had over 10 years of clinical experience as a psychiatrist and over 10 years of experience in conducting psychoeducation mainly for people with schizophrenia and their families. H.F. had 10 years of clinical experience as a general psychiatrist and 7 years of experience in psychoeducation. The psychologist had 7 years of experience in conducting psychoeducational groups. The whole programme was supervised by S.I., who had 30 years of experience in psychoeducation for people with severe mental illness. Sessions were videotaped and the treatment team discussed their performance after the session was over. In order to avoid increasing tension in the participants, only the first session was videotaped. Lectures were given by the psychiatrists, and group meetings were led jointly by the clinical psychologist and the psychiatrists. None of the participating staff was aware of the EE status of the patients or the families.
Out-patient treatment
Both the intervention and control group received standard out-patient treatment, which was provided by psychiatrists unaware of the treatment allocation of the patients or the EE level of patients’ families. This treatment as usual consisted of evaluation of psychiatric symptoms, assessment and management of drug treatment, and supportive psychotherapy on a bi-weekly basis.
Statistical analysis
For analysis, SPSS for Windows version 17.0 was used. Parametric and non-parametric analyses were employed for continuous and categorical/ordinal data respectively. The time to relapse was compared between the two groups using Kaplan–Meier survival analysis. The influence of withdrawals was examined in a sensitivity analysis using the ‘worst-case scenario’ whereby we assumed that those withdrawing from the intervention group relapsed whereas those from the control group did not. Cox proportional hazard analysis was performed to control for the effects of potential confounding factors, including the age and gender of the patient, illness duration, HRSD score on entry, and high or low level of EE according to FMSS on entry. The influence of EE on the effectiveness of the intervention was explored through entering the interaction term (intervention EE status) in the Cox proportional hazard model. The influence of the intervention on the EE status of the families was examined by comparing the FMSS and FAS scores at 9-month follow-up between the two groups while controlling for their baseline scores.
Results
Of the 57 dyads originally giving their consent and being randomised, 1 withdrew consent after randomisation in the intervention group (refusal to undergo FMSS) and 2 withdrew in the control group (death of the patient from physical illness and rejection of FMSS respectively), resulting in 24 and 30 patients respectively for whom there were data available for analysis (Fig. 1).Table 1 shows the baseline demographic and clinical characteristics of the 54 patients. There was no statistically significant or clinically meaningful difference between the intervention and control groups. The average patient profile based on the above findings was that of a person in late middle age with a course of mild to moderate depression lasting about a decade and with one related hospital admission, which is a type frequently encountered in routine psychiatric practice in Japan. All the patients were out-patients at the time of study entry.Table 1 also shows the characteristics of the family members; again, there was no statistically significant or clinically meaningful difference in any of the baseline attributes between the two groups.
Table 1 Comparison of the intervention and control groups at baseline

| Intervention group (n = 24) | Control group (n = 30) | |
|---|---|---|
| Patients | ||
| Gender, n male:female | 15:9 | 15:15 |
| Age, years: mean (s.d.) | 59.2 (14.6) | 60.9 (13.0) |
| Illness duration, years: mean (s.d.) | 11.6 (2.7) | 11.0 (2.0) |
| Number of admissions, mean (s.d.) | 0.8 (1.2) | 0.8 (1.9) |
| Antidepressant dosage, mg: mean (s.d.) | 100.3 (71.5) | 88.1 (60.9) |
| HRSD score, mean (s.d.) | 13.4 (8.3) | 13.7 (10.5) |
| HRSD score ≤6, n (%) | 5 (21) | 9 (30) |
| BDI score, mean (s.d.) | 12.4 (6.8) | 12.0 (7.9) |
| Family members | ||
| Relatives, n | ||
| Father | 2 | 0 |
| Mother | 0 | 3 |
| Husband | 7 | 13 |
| Wife | 14 | 12 |
| Son | 1 | 1 |
| Daughter | 0 | 1 |
| Age, years: mean (s.d.) | 59.0 (11.4) | 61.8 (10.7) |
| Education, years: mean (s.d.) | 12.0 (2.9) | 10.7 (3.4) |
| FAS total score, mean (s.d.) | 28.1 (18.3) | 33.5 (20.7) |
| High EE in FMSS, n (%) | 7 (23.3) |
Including the cases of borderline EE on FMSS in the high-EE category, high EE was detected in 6 (25%) and 10 (33%) families in the intervention and control groups respectively. The category of high EE was high critical comments (CC) in 3, high EOI in 1 and borderline in 2 in the intervention group, and high CC in 3, high EOI in 3, high CC/EOI in 1 and borderline in 3 in the control group, showing no significant difference in the FMSS findings between the two groups; nor was there a significant difference in the mean FAS scores between the groups (28.1 v. 33.5).
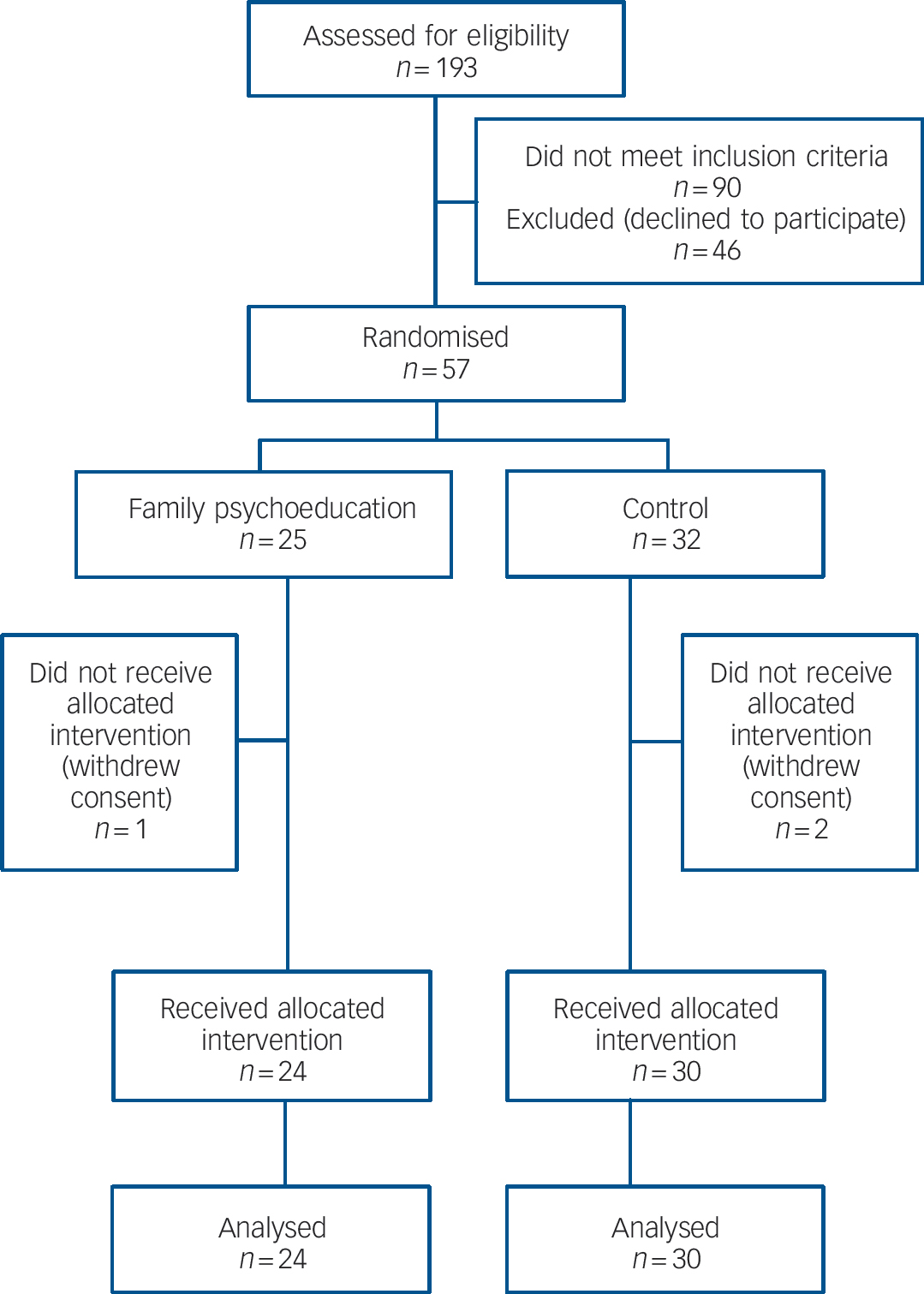
Fig. 1 Flow of participants through the trial.
Relapse and outcome at 9 months
All the 54 patients continued the treatment for 9 months, and were assessed at follow-up. All families allocated to the intervention group participated in the family class. Four family members missed one session each: two came to the hospital later to receive an individual session, and we visited the remaining two to provide the psychoeducation that they had missed. For these four sessions, individual discussion of coping with high-EE situations replaced group discussion. The mean daily doses of antidepressants at 9 months were 101 mg and 94 mg (medians 75 mg and 55 mg) in the intervention and control groups respectively. One patient in each group had stopped taking medication by the time of the 9-month follow-up.
Relapse occurred before the completion of the 9-month follow-up assessment in 2 patients (8%) in the intervention group and 15 (50%) in the control group. Kaplan–Meier survival analysis revealed that time to relapse was statistically significantly longer in the intervention group than in the control group (χ2 = 9.57, d.f. = 1, P = 0.002) (Fig. 2). The worst-case scenario sensitivity analysis did not change the results (χ2 = 6.63, d.f. = 1, P = 0.01). The hazard ratio (HR) of relapse by 9 months was 0.17 (95% CI 0.04–0.75; Fisher's exact test, P = 0.002). At the time of the recurrence the mean HRSD scores were 22.5 and 29.1 and the mean BDI scores were 26.5 and 25.2 in the intervention and control groups respectively. The remission rates at 9 months were 83% and 33% respectively, showing a significant difference between the two groups (Fisher's exact test, P = 0.001). When gender and age of the patient, illness duration, HRSD score and EE status at baseline were entered into Cox proportional hazard analysis, only HRSD score emerged as a significant predictor (OR = 1.08, 95% CI 1.03–1.14, P = 0.003) and the effect of the intervention remained statistically significant (OR = 0.17, 95% CI 0.04–0.75, P = 0.02) (Table 2).
Table 2 Cox proportional hazard analysis of baseline predictors

| β | s.e. | Wald | d.f. | P | HR | 95% CI | |
|---|---|---|---|---|---|---|---|
| Intervention | – 1.800 | 0.768 | 5.484 | 1 | 0.019 | 0.165 | 0.037–0.746 |
| Gender | – 0.350 | 0.544 | 0.413 | 1 | 0.520 | 0.705 | 0.243–2.047 |
| Age | 0.005 | 0.019 | 0.065 | 1 | 0.799 | 1.005 | 0.967–1.044 |
| Illness duration | – 0.011 | 0.032 | 0.117 | 1 | 0.732 | 0.989 | 0.929–1.053 |
| HRSD score | 0.081 | 0.027 | 9.059 | 1 | 0.003 | 1.084 | 1.029–1.143 |
| EE status | 0.256 | 0.573 | 0.199 | 1 | 0.655 | 1.291 | 0.420–3.967 |
Associations among intervention, EE and outcomes
The influence of baseline EE status on the effectiveness of the intervention was examined by entering the interaction term (EE status intervention) in the Cox proportional hazard model. The interaction term was not statistically significant, suggesting that the baseline EE status did not moderate the effectiveness of the intervention (Table 3). However, this analysis may have been underpowered because our sample was too small to examine an interaction effect. Second, the mediating effect of EE was examined by investigating the influence of the intervention on EE. Both FMSS and FAS could be measured at 9-month follow-up for 52 families. In the intervention group, neither EE status according to FMSS nor FAS score decreased significantly from baseline to follow-up. Nor did EE status or FAS scores at 9-month follow-up differ significantly between the intervention and control groups when controlled for respective baseline values.
Table 3 Cox proportional hazard analysis examining interaction (intervention × EE status)

| β | s.e. | Wald | d.f. | P | HR | 95% CI | |
|---|---|---|---|---|---|---|---|
| Intervention | – 2.144 | 1.061 | 4.084 | 1 | 0.043 | 0.117 | 0.015–0.937 |
| EE status | 0.736 | 0.522 | 1.990 | 1 | 0.158 | 2.088 | 0.751–5.806 |
| Intervention × EE status | 0.448 | 1.507 | 0.088 | 1 | 0.766 | 1.565 | 0.082–30.02 |
Discussion
Family psychoeducation consisting of four sessions significantly reduced relapse of major depression for up to 9 months in comparison with treatment as usual (RR = 0.17, number needed to treat 2.4, 95% CI 1.6–4.9). The intervention was acceptable to the family members as all the participants allocated to the intervention completed four sessions. This effectiveness, however, was not moderated by baseline EE status, nor was there a statistically significant reduction in EE measured with FMSS or FAS after the family psychoeducation.
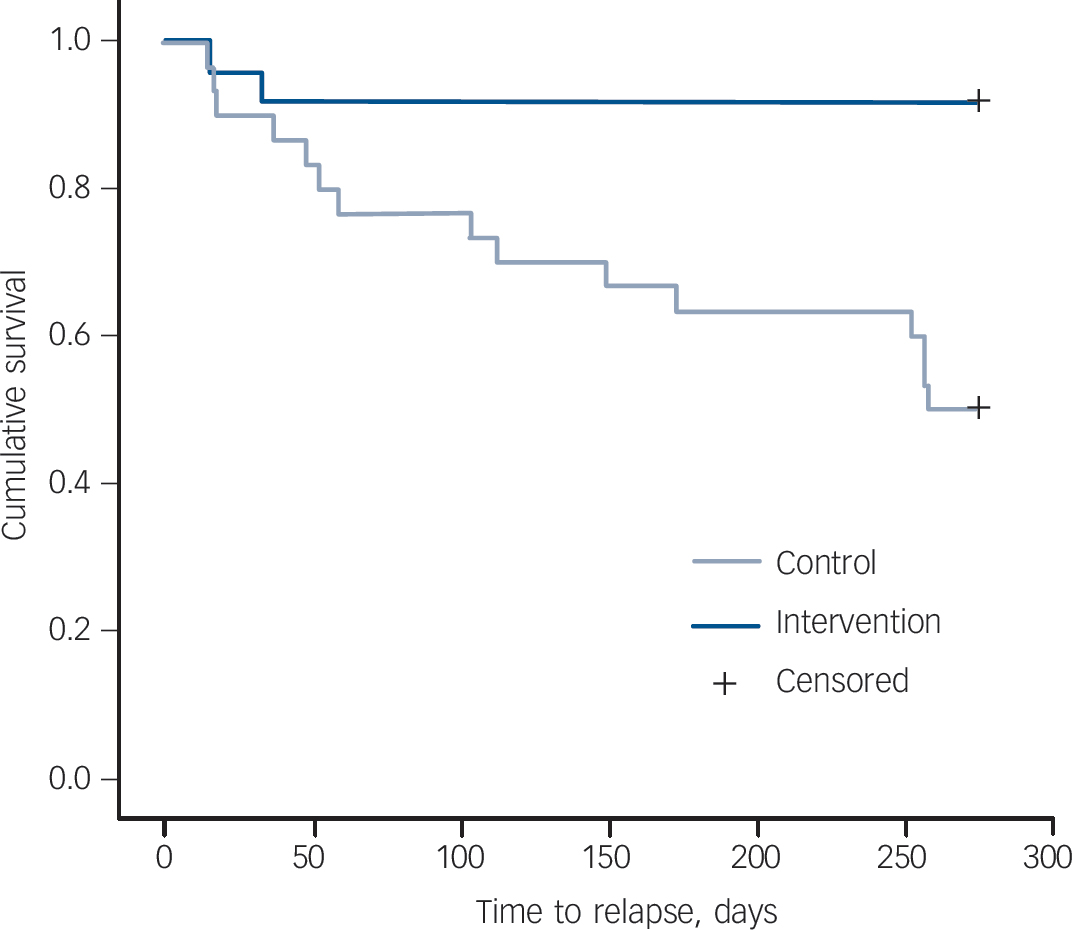
Fig. 2 Time to relapse in the intervention and control groups.
High effectiveness of family psychoeducation is in accordance with the strong predictive power of family environment previously demonstrated in observational studies. Reference Vaughn and Leff11–Reference Mino, Shimodera, Inoue, Fujita, Tanaka and Kanazawa16 As was the case with patients with schizophrenia, working on the predictors improved the outcome. However, further analyses were unable to detect the significant involvement of the family's EE in this change process. There are several possible reasons why we could not detect a significant reduction in EE or a moderating interaction effect by EE in the relapse prevention. First, it is likely that the FMSS and FAS are not sensitive measures of EE, especially in depression. All the studies that have established high EE as a risk factor for depression relapse had used the Camberwell Family Interview. Reference Vaughn and Leff11–Reference Mino, Shimodera, Inoue, Fujita, Tanaka and Kanazawa16 Second, we measured EE at baseline, i.e. as the patients were entering the continuation/maintenance treatment phase. The family's EE is usually most conspicuous at acute phases such as on admission of the patient to hospital. These limitations and the small sample size may also explain the non-significant difference in the relapse rates between high-EE v. low-EE groups, although the relapse rate was numerically higher among the high-EE patients than among the low-EE patients in both the control and intervention groups.
It is also possible that our family psychoeducation, although focusing on remedying high EE, might have exerted its influence through routes other than EE. The families of patients with mental disease are often markedly distressed themselves, and they are likely to be socially isolated. Psychoeducation can provide needed information to such families. Meeting other families in a similar situation in a group setting may also reduce their mental distress. Reducing the family's burden may have created a more supportive environment to the patient at home. Our study design comparing psychoeducation against treatment as usual does not allow for analyses in any greater detail. The exact mechanism of family psychoeducation in the prevention of relapse therefore remains unclear. In this connection it is interesting to note that couple therapy aimed at people with depression living with a critical partner was as effective as standard antidepressant therapy both in the acute phase and continuation/maintenance phases of treatment. Reference Leff, Vearnals, Wolff, Alexander, Chisholm and Everitt31 There may be different ways to influence the family and the patient and their interactions.
Family psychoeducation can be performed with or without the patient being present. Although it is impossible to know the differential effects of the two approaches in major depression (because ours is the only published study on this topic), two studies of bipolar affective disorder have shown interesting differences. Miklowitz et al, using a family and patient approach, found prophylactic efficacy for depression but not mania, Reference Miklowitz, George, Richards, Simoneau and Suddath9 whereas Reinares et al, using a family group psychoeducation approach (groups of relatives without patients), found prophylactic efficacy for mania but not depression. Reference Reinares, Colom, Sanchez-Moreno, Torrent, Martinez-Aran and Comes8 Whether and how the conjoint psychoeducation involving both family members and patients might differ from our family-only approach in depression needs to be explored in future studies.
There are several possible weaknesses in our study. First, inclusion of patients up to age 85 years may have been too broad and could have included families for whom the educational objectives could sensibly differ. We adopted this age range because depression in old age represents a clinically important problem. There were seven patients aged 75 years or over (including one patient aged 83): four in the intervention group and three in the control group. Their family members seemed to share common themes with younger family members such as lack of knowledge about depression and misattributing depression to lack of willpower. Second, we excluded patients who received ECT for the index episode, because the course of the illness of these patients after the acute phase of treatment might be different from those who recovered on pharmacotherapy only, Reference Sackeim32 and also because the contents of psychoeducation regarding treatment would be different. This decision may have biased our sample towards a less severe population. Lastly, a major shortcoming in the study design is that it was a comparison between family education in addition to treatment as usual v. treatment as usual only. It can therefore not be ruled out that it was not psychoeducation per se but rather non-specific factors such as time spent with the therapist, sense of belonging to a group and support by the group that could explain the differences we observed between our experimental and control groups. We adopted this design because it could answer the pragmatic clinical question we faced, namely whether it was of value to add family psychoeducation to treatment as usual or not. It must also be remembered that our programme involved family members only and therefore could not have provided non-specific support directly to the patients themselves.
Our study was the first to show that psychoeducation limited to patients’ families was effective in preventing relapse in the patients. Although individual psychotherapies have demonstrated effectiveness for patients with depression, 33 it can be stressful for them in the presence of many residual depressive and other symptoms. Intervention limited to families has an advantage in that it does not burden the patients. It must also be emphasised that our family psychoeducation – consisting of four sessions and using videotapes and booklets specifically prepared for this programme – was brief and easy to disseminate.
Given the great number of people affected by depression – both patients and their families – we believe that our study has paved a new way to their effective care. A replication study with a larger sample is warranted in order to confirm its effectiveness and to elucidate its mechanisms.
Funding
This study was supported by a Grant-in-Aid for Scientific Research, Ministry of Health, Labour and Welfare, 2004 (Comprehensive Research Project on Science of Longevity).










eLetters
No eLetters have been published for this article.