Psychological theories suggest that individuals with major depression have a distorted and negative view of their surroundings, Reference Beck1 and there is relatively consistent evidence that in comparison with a healthy control group they interpret ambiguous or neutral faces as being sad. Reference Bouhuys, Geerts and Gordijn2,Reference Leppanen, Milders, Bell, Terriere and Hietanen3 Deficits in social interactions also have a central role in the development and maintenance of major depression, Reference Joormann and Gotlib4 and impairment in the processing of emotional facial information may contribute to these social deficits. However, there is a lack of research examining the recognition accuracy of individual facial expressions of emotion in severe depression. Our aims were to replicate the findings of negative misinterpretation biases towards neutral faces in major depression, and also to examine facial expression recognition performance on five basic emotions in individuals receiving in-patient treatment for severe depression and a matched group of healthy controls.
Method
Individuals with a primary diagnosis of major depression (unipolar or bipolar) disorder according to DSM–IV criteria, consecutively admitted to Hillmorton Hospital, Christchurch, were approached to take part in the study over a 2-year period. Exclusion criteria included current significant alcohol or substance misuse or dependence; endocrinological, neurological or chronic medical conditions; pregnancy; previous serious head injury; or electroconvulsive therapy in the 12 months prior to admission. A total of 68 patients were eligible, gave consent and completed the study, which was approved by the National Health and Disability Ethics Committee. The control group consisted of 50 healthy individuals recruited from the general population in Christchurch, subject to the same exclusion criteria plus the additional criterion of no personal or immediate family history of major mental illness.
Neuropsychological and clinical assessment was conducted within 5 days of the patients' admission. The Montgomery–Åsberg Depression Rating Scale (MADRS) was used to rate depression severity, and the Structured Clinical Interview for DSM–IV Axis I Disorders (SCID–I) was used to assess comorbidity within the depression sample. Reference Montgomery and Åsberg5,Reference First, Spitzer, Gibbon and Williams6 The National Adult Reading Test (NART) was used to estimate verbal IQ in both groups. Reference Nelson7 For all participants, assessments were conducted between 11.00 h and 15.00 h, to reduce the effects of diurnal variation in mood and neuropsychological functioning.
All participants performed a modified version of the Facial Expression Recognition Task. Reference Harmer, Bhagwagar, Perrett, Vollm, Cowen and Goodwin8 Faces displaying five basic emotions (16 images each of angry, happy, sad, fearful and disgusted expressions) were presented successively on a computer screen for 500 ms, followed immediately by a blank screen. The faces had been morphed into variable intensities of each emotion from 50% full emotion (50% full emotion and 50% neutral) to 100% full emotion, in 10% steps. Sixteen neutral facial expressions were also presented, giving a total of 96 facial presentations. Participants were instructed to press one of six labelled buttons on a response pad (the five emotions and neutral) as quickly and as accurately as possible.
Statistical procedures were conducted using the Statistical Package for the Social Sciences (SPSS) version 13 for Windows. For demographic and clinical data, categorical variables were analysed with chi-squared tests and continuous measures with independent sample t-tests. Facial expression recognition accuracy and interpretation bias were analysed using repeated measures analysis of variance (ANOVA), with group (depression v. control) as a between-individual factor and ‘emotion’ (angry, happy, sad, fearful, disgusted, neutral) or ‘misinterpreted emotion’ (the emotion incorrectly ascribed to a neutral face) as a within-individual factor. Post hoc analyses used independent sample t-tests.
Results
Participants in the depression and control groups did not differ significantly in mean age (39.5 years, s.d. = 10.2 v. 38.5 years, s.d. = 9.8; t 116 = 0.45, P = 0.7), gender (59% female v. 63% female; χ2 1 = 0.14, P = 0.7), NART score (106.6, s.d. = 7.8 v. 107.4, s.d. = 6.3; t 116 = –0.42, P = 0.7), secondary education (4.2 years, s.d. = 0.7 v. 4.5 years, s.d. = 0.6; t 116 = –1.47, P = 0.1) or tertiary education (1.7 years, s.d. = 1.2 v. 2.0 years, s.d. = 0.8; t 116 = 0.43, P = 0.7).
In the depression group the mean MADRS score was 35.6 (s.d. = 8.4), the age at depression onset was 29.6 years (s.d. = 12.2) and the time elapsed since depression onset was 11.2 years (s.d. = 9.3). The ratio of single episode to recurrent depression was 21: 47, that of unipolar depression to bipolar depression was 60: 8 and that of non-psychotic to psychotic depression was 59: 9. Twenty-two individuals were unmedicated at testing. The remainder were taking selective serotonin reuptake inhibitors (n = 21), serotonin–noradrenaline reuptake inhibitors (n = 17), tricyclic antidepressants (n = 5) or monoamine oxidase inhibitors (n = 3). The most prevalent comorbid psychiatric disorders in the depression group were panic disorder with agoraphobia (13%), post-traumatic stress disorder (16%) and alcohol misuse (9%).
Repeated measures ANOVA showed a significant effect of group (F 1,116 = 10.3, P = 0.002) and a group×emotion interaction (F 5,580 = 2.4, P = 0.03). Post hoc analysis showed that the control group was significantly better than the depression group at recognising facial expressions of disgust (Fig. 1; t 116 = –3.7, P = 0.0001, effect size 0.70). When the analysis was repeated in a more homogeneous sample comprising only patients with non-psychotic major depressive disorder (n = 53), the significant difference in the recognition of disgusted facial expressions between the depression and control groups remained (t 101 = –3.5, P = 0.001, effect size 0.71). The difference in the recognition of disgusted facial expressions between the control group and unmedicated patients with depression (n = 22) was also significant (t 70 = –2.5, P = 0.01, effect size 0.68).
Neutral faces were interpreted differently between the two groups, with repeated measures ANOVA revealing a significant group×interpreted emotion interaction (F 4,464 = 3.8, P = 0.01). Post hoc analysis showed that participants with depression were more likely to interpret neutral faces as sad (15.1%, s.e.m. = 1.5 v. 10.3%, s.e.m. = 1.6; t 116 = 2.1, P = 0.03, effect size 0.41) and less likely to interpret neutral faces as happy (15.4%, s.e.m. = 1.4 v. 20.5%, s.e.m. = 1.9; t 116 = –2.2, P = 0.03, effect size 0.40) compared with controls.
Analysis restricted to cases of non-psychotic major depressive disorder showed a similar pattern, with this depression subgroup being significantly more likely to interpret neutral faces as sad (t 101 = 2.2, P = 0.03, effect size 0.44) and less likely to interpret neutral faces as happy (t 101 = –2.3, P = 0.02, effect size 0.46). Analysis of neutral misinterpretation bias with the unmedicated patient group and controls did not find significant differences for the misinterpretation of neutral faces as sad (t 70 = 1.6, P = 0.1, effect size 0.40) or happy (t 70 = –1.3, P = 0.2, effect size 0.33).
Repeated measures ANOVA of the percentage of disgusted faces incorrectly classified as each of the other emotions showed no interaction between group and emotion (F 4,436 = 1.2, P = 0.3), suggesting there was no specific bias in the emotion selected when a disgusted expression was misclassified.
No difference in reaction time on the Facial Expression Recognition Task was found between the depression and control groups.
Discussion
Our findings are consistent with previous research in which negative biases have been observed for neutral facial expressions in individuals with depression. Reference Leppanen, Milders, Bell, Terriere and Hietanen3 It is of interest that our sample with severe depression showed a negative interpretation bias, since it might have been predicted that such subtle psychological processes would be present only in people with milder depression. The specific impairment found in the recognition of disgusted facial expressions has not previously been reported in populations with depression. Disgust recognition has, however, been shown to be impaired in unmedicated patients with Parkinson's disease, Reference Sprengelmeyer, Young, Mahn, Schroeder, Woitalla and Buttner9 suggesting an association with dopamine dysfunction, particularly in the basal ganglia. Dopamine dysfunction may be especially important in severe depression. Reference Dunlop and Nemeroff10 Accuracy of disgust recognition has previously been found to be reduced after the repeated administration of citalopram in healthy volunteers. Reference Harmer, Shelley, Cowen and Goodwin11 This finding is of importance since approximately half of the patients in the depression sample were taking serotonergic antidepressants at testing. However, analysis of data for the unmedicated patients compared with controls also found a deficit in disgust recognition.
Other factors may have contributed to the results observed. Admission to a psychiatric hospital is stressful and involves removal of patients from their usual social environment. The resulting close proximity to many other distressed individuals could also affect emotional processing. The patients with depression also had comorbid psychiatric disorders, most commonly anxiety and substance use disorders; however, rates were relatively low and unlikely to have affected the overall result. Future studies could examine changes in disgust recognition and negative interpretation bias with successful treatment, in order to determine whether these may reflect objective markers of treatment outcome in major depression.
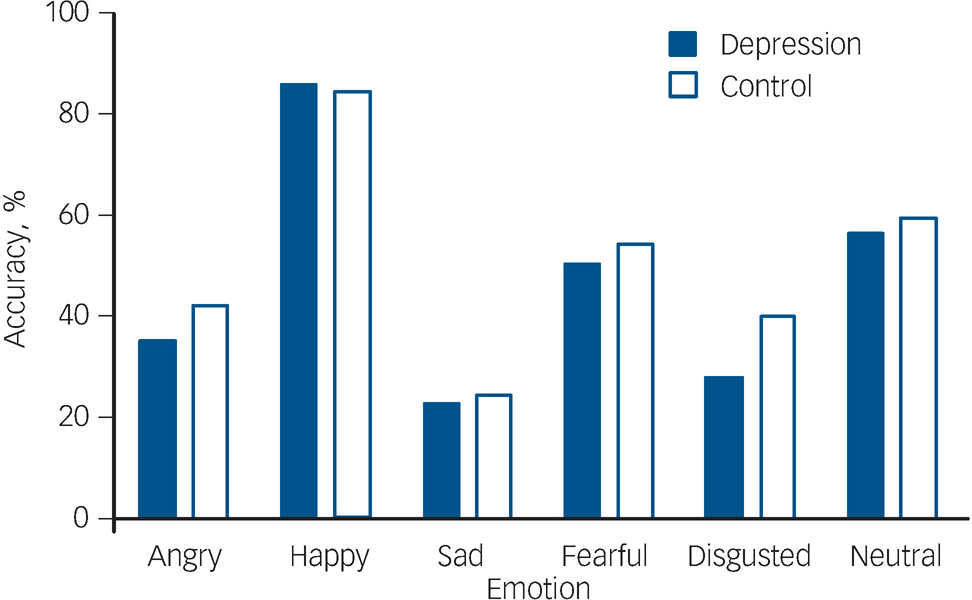
Fig. 1 Recognition accuracy (mean and s.e.m.) for the five facial expressions of emotion and neutral expressions on the Facial Expression Recognition Task, in the depression group (n = 68) and the healthy control group (n = 50). Difference is significant (P<0.001) for the disgusted facial expression.
Acknowledgements
We thank Dr Catherine Harmer for supplying the Facial Expression Recognition Task used in the study. Thanks also to the New Zealand Tertiary Education Commission for providing K.M.D. with a Bright Future Doctoral Scholarship.






eLetters
No eLetters have been published for this article.