Glyphosate-resistant (GR) Palmer amaranth is an economically devastating weed for producers throughout the southeastern United States. Palmer amaranth’s competitive ability is largely attributed to prolific seed production (Keeley et al. Reference Keeley, Carter and Thullen1987), extended emergence throughout the growing season (Jha et al. Reference Jha, Norsworthy, Riley and Bridges2010), shoot heights of 2 m or more (Horak and Loughin Reference Horak and Loughin2000), high photosynthetic capacity (Ehleringer Reference Ehleringer1983), and the capacity to evolve resistance to multiple herbicides (Heap Reference Heap2017). Previous studies have observed soybean [Glycine max (L.) Merr.] yield reductions as a direct result of Palmer amaranth. Klingeman and Oliver (Reference Klingeman and Oliver1994) reported soybean yield loss of 17% to 68% when Palmer amaranth densities were 0.33 to 10 plants per meter of row. In Kansas, Bensch et al. (Reference Bensch, Horak and Peterson2003) reported yield losses as high as 78% from a single Palmer amaranth plant per 0.125 meter of row. Consequently, these authors also observed Palmer amaranth had accumulated greater plant biomass and increased seed production and had a greater impact on soybean yield when compared to redroot pigweed (Amaranthus retroflexus L.) and tall waterhemp (Amaranthus tuberculatus (Moq.) Sauer). Palmer amaranth resistant to glyphosate has presented a challenge in GR cropping systems. To have successful control, producers have implemented the use of soil-residual herbicides in major row crops such as soybean and cotton (Gossypium hirsutum L.).
Flumioxazin is one herbicide that has been adopted for use to control GR Palmer amaranth. Flumioxazin is used in many crops including soybean, cotton, corn (Zea mays L.), peanut (Arachis hypoeaea L.), and other row crops (Anonymous 2017a; Senseman Reference Senseman2007a). Flumioxazin has a wide window of application that can be used in an early preplant burndown program or PRE and can be tank-mixed with other PRE herbicides. Flumioxazin controls many broadleaf species including common lambsquarters (Chenopodium album L.), common ragweed (Ambrosia artemisiifolia L.), Palmer amaranth, and various species of morningglory (Ipomoea) (Askew et al. Reference Askew, Wilcut and Cranmer2002; Clewis et al. Reference Clewis, Everman, Jordan and Wilcut2007; Niekamp et al. Reference Niekamp, Johnson and Smeda1999). In one study, authors evaluated the control of GR Palmer amaranth with two standalone herbicides, flumioxazin and fomesafen, and a tank-mix treatment of metribuzin plus chlorimuron applied as a PRE (Whitaker et al. Reference Whitaker, York, Jordan and Culpepper2010). They reported GR Palmer amaranth control with flumioxazin at 94%, 84%, and 70% control at 10, 30, and 90 DAT, respectively. Other positive attributes of flumioxazin include a low use rate, flexible crop rotations, and the ability to control weed species with acetolactate synthase and triazine resistance (Taylor-Lovell et al. Reference Taylor-Lovell, Wax and Bollero2002).
Fomesafen is a diphenylether herbicide labeled for use in soybean (Anonymous 2017b; Senseman Reference Senseman2007b). Fomesafen controls various broadleaf weeds such as common cocklebur (Xanthium strumarium L.), common lambsquarters, common ragweed, Palmer amaranth, and other problematic weeds. Nandula et al. (Reference Nandula, Ray, Ribeiro, Pan and Reddy2013) conducted a study that observed alternative soil-applied herbicides to control two GR and one glyphosate-susceptible Palmer amaranth biotypes. These authors concluded that fomesafen along with S-metolachlor and pendimethalin, provided 100% control of the Palmer amaranth biotypes at 4 weeks after treatment. Previous studies have demonstrated fomesafen control of Palmer amaranth resistant to dinitroaniline and sulfonylurea herbicides when applied PRE (Lundsford et al. Reference Lundsford, Harrison and Smith1998; Troxler et al. Reference Troxler, Askew, Wilcut, Smith and Paulsgrove2002). In addition to controlling Palmer amaranth and other broadleaf weed species, fomesafen can be applied for suppression of yellow nutsedge (Cyperus esculentus L.) and certain grass weeds.
Saflufenacil, belonging to the pyrimidinedione chemical family, is a protoporphyrinogen oxidase (PPO)-inhibiting herbicide labeled for use as a burndown, for soil residual control of broadleaf weeds, and as a harvest aid for crop desiccation in major crops (Grossmann et al. Reference Grossman, Hutzler, Caspar, Kwiatkowski and Brommer2011). Saflufenacil encompasses both residual and contact activity on susceptible broadleaf weeds including Palmer amaranth, multiple species of morningglory, and various weeds (Anonymous 2017c). One study documented a reduction in biomass of Palmer amaranth and two other Amaranthus species, ranging from 82% to 98% control, when saflufenacil was applied PRE at rates of 6 to 30 g ha−1, respectively (Geier et al. Reference Geier, Stahlman and Charvat2009). Saflufenacil has been adopted by producers primarily because of its low use rates, environmental soundness, and control of weeds resistant to other herbicide modes of action (Soltani et al. Reference Soltani, Shropshire and Sikkema2009).
Sulfentrazone is a member of the phenyl triazolinone herbicide family (Senseman Reference Senseman2007c). One advantage of incorporating sulfentrazone in a weed management program is its flexible window of application as a PPI or PRE in soybean (Anonymous 2017d). Sulfentrazone provides residual control of a wide range of weeds. Several studies have observed sulfentrazone PRE control of Palmer amaranth in various cropping systems, such as soybean (Sweat et al. Reference Sweat, Horak, Peterson, Lloyd and Boyer1998) and sunflower (Helianthus annuus L.) (Reddy et al. Reference Reddy, Stahlman, Geier and Thompson2012). Sulfentrazone can be used as an effective tool for soybean producers for managing GR weeds (Knezevic et al Reference Knezevic, Darta, Scott, Klein and Golus2009).
Several authors have reported PPO-inhibitor resistance in Palmer amaranth in the United States. Resistance was confirmed in Arkansas (Salas et al. Reference Salas, Burgos, Tranel, Singh, Glasgow, Scott and Nichols2016; Schwatrz-Lazaro et al. Reference Schwartz-Lazaro, Norsworthy, Scott and Barber2017), Indiana (Legleiter and Johnson Reference Legleiter and Johnson2016), and Missouri (Geist Reference Geist2016). In these areas, PPO-inhibitor resistance has been confirmed on Palmer amaranth plants surviving the normal labeled dosage rate from POST application. However, limited information is available on the efficacy of soil-residual PPO-inhibiting herbicides. Schwartz-Lazaro et al. (Reference Schwartz-Lazaro, Norsworthy, Scott and Barber2017) published a related paper dealing with PRE and POST Palmer amaranth control with these herbicides. Our results differ from those that they report. The Schwarz-Lazaro paper took seedling counts at 10 DAT, and our data indicate that maximum germination had not yet occurred at this time. It is possible that 10 DAT is an insufficient duration to measure the total herbicidal effects of soil-applied products. The methods also differed because they took their PRE-treated experiment and sprayed them at some “later” date when the largest plants were at the three-leaf stage. Thus, their biomass data measured the PRE herbicide effect as well as that of the POST application. Our entire study used different populations, but clearly all the tested herbicides still provided at least some level of activity.
Given the limited choices farmers have to control GR Palmer amaranth, soil applied products may still have utility. For many weeds, the most sensitive time for herbicidal control is when they are germinating from the soil. Therefore, the objective of this study was to evaluate the efficacy of four soil-applied PPO-inhibiting herbicides on PPO-resistant and PPO-susceptible Palmer amaranth biotypes.
Materials and Methods
Greenhouse experiments were conducted in Knoxville, Tennessee, in 2016, consisting of a dose-response study with two temporal replications. The Palmer amaranth seeds used were a known PPO inhibitor–resistant (R) biotype (data not shown) collected the previous year from a producer’s field located in Shelby County, Tennessee, and a susceptible (S) biotype purchased from Azlin Seed Co. in Leland, Mississippi. The R population had survived POST applications of the labeled dosages of fomesafen in 4 of the last 5 years (data not shown). Seed from the R population was collected from approximately 20 female plants and air-dried in paper bags prior to seed cleaning. The specific mutation that imparted the resistance was not characterized, although other research on this topic is ongoing (Giacomini et al. Reference Giacomini, Umphres, Nie, Mueller, Steckel, Young, Scott and Tranel2017). Seeds from both sources were subjected to scarification that involved a 70% sulfuric acid bath for 30 s, rinsed thoroughly with deionized water, and allowed to air dry for 24 h. Styrofoam cups of 500 ml size were used. Each cup had four small holes poked in the bottom prior to filling with sifted (2 mm screen size) soil of a silt loam (Sequatchie; 6 pH, 1.9% organic matter) that had no previous history of herbicide use or Palmer amaranth growth. The day prior to planting, pots were set onto trays filled with water and allowed to subirrigate overnight to ensure adequate moisture throughout the soil profile. The following day, pots were removed from trays to drain excess water, then an indention (3 cm wide and 3 mm in depth) was made at the soil surface for seeds to be planted in in order to reduce seed movement. Seeds of each biotype were planted into separate pots at a rate of 100 seeds per pot. Each pot contained only one biotype. Preliminary studies were conducted to determine the amount of seeds to place into each cup, and the final amount was based on a volumetric approach using a small lab scoopula. The use of seed with minimal foreign matter also was deemed to be important to make sure all cups were seeded uniformly.
Immediately after planting, pots were sprayed with a one-nozzle boom CO2 backpack sprayer equipped with a 8002 EVS nozzle (TeeJet Technologies, P.O. Box 7900, Wheaton, IL 60187) calibrated to deliver 190 L ha−1 at 210 kPa. Several preliminary studies were conducted that showed that excessive watering could leach herbicides from the soil surface, but insufficient watering precluded seed germination. One challenging aspect of this research was to determine an amount of irrigation that would activate the PRE herbicides and allow the seeds to germinate, but would not leach the herbicide out of the target zone.
Herbicide treatments consisted of four different herbicides applied at a rate structure of 26 to 420 g ha−1, 35 to 560 g ha−1, 6.3 to 100 g ha−1, and 26 to 420 g ha−1 for flumioxazin (Valor SX, Valent U.S.A. Corporation, Walnut Creek, CA), fomesafen (Flexstar, Syngenta Crop Protection, LLC, Greensboro, NC), saflufenacil (Sharpen, BASF Corporation, Research Triangle Park, NC), and sulfentrazone (Spartan, FMC Corporation, Philadelphia, PA), respectively. Herbicide rates were 12.5%, 25%, 50%, 100%, and 200% of the normal labeled use rate for flumioxazin (210 g ai ha−1), fomesafen (280 g ai ha−1), saflufenacil (50 g ai ha−1) and sulfentrazone (210 g ai ha−1). The 1× rate for saflufenacil used the higher labeled rate in this study due to previous research findings using this soil type. A greater saflufenacil rate would also be consistent with that in some products used PRE that contain this herbicide. To validate herbicide doses, filter paper (12.5 cm diameter, 1 mm thickness) was used to capture the sprayed treatments consisting of two duplicates placed between cups prior to treatment. After each treatment was applied, the filter papers were collected and placed into individual 250 ml Nalgene bottles and stored in a freezer (−20 C) for later lab analysis (Mueller et al. Reference Mueller, Boswell, Mueller and Steckel2014).
After spraying, cups were arranged in the greenhouse in a randomized complete block design with four replications and watered with approximately 0.8 cm to move herbicide into the soil. Pots were watered twice daily to maintain soil moisture and a complete fertilizer (15-30-15 MiracleGro®) was applied three times per week. Watering procedures were based on preliminary studies, where too much water leached the herbicide out of the surface zone and too little water did not provide for adequate seed germination (data not shown). Watering activities were carefully monitored and more frequent, light irrigations (approximately 2 mm) were used. Visual estimates of Palmer amaranth control, where 100% indicated complete control and 0% indicated no control, were collected at 3, 7, 14, 21, 28, and 35 days after treatment (DAT). Also at those times, the number of emerged Palmer amaranth was counted (data not shown). Seedlings with cotyledons fully expanded were considered to be emerged. At 35 DAT, plant aboveground fresh biomass was collected.
The design of this experiment was a completely crossed three-way factorial with two populations (R and S biotypes) by four herbicides (flumioxazin, fomesafen, saflufenacil, and sulfentrazone) by five rates with two runs and four replications in each run. Each run included nontreated or untreated controls (UTC) with a total of eight UTC cups per run. Sigma Plot version 12.5 was used to analyze data collected, and a simple first-order exponential decay model was used to describe the relationship between Palmer amaranth biomass (normalized to the amount in the UTC) and fomesafen rate. The model is represented by the following equation:
where Y is the biomass normalized as a percentage of the untreated control in that experiment, x is the herbicide dosage as a percentage of the normal labelled rate, k is the first-order rate constant, and a is the regression parameter at 0 dosage. The GR50 was then calculated by the relationship of 0.693 divided by k (Mueller and Senseman 2015):
The GR50 for each respective curve was then used to calculate a ratio of R:S response for that individual herbicide, with a greater value indicating a higher level of relative resistance for that herbicide.
Results and Discussion
Herbicide Dosage
The herbicide concentrations were normalized to the 1× dosage (Table 1). While some variation was observed, in general the relative dosages were near the targeted concentration. Greater variation at the 200% (2×) dose may be due to concentrations being toward the upper linear range of the liquid chromatography–mass spectrometry instrument (Mueller et al. Reference Mueller, Boswell, Mueller and Steckel2014). Given the small surface area of the seed to soil interface, validating the initial relative herbicide dosage applied adds confidence to these findings.
Table 1 Summary of herbicide mean concentrations and standard error at 0 DAT.
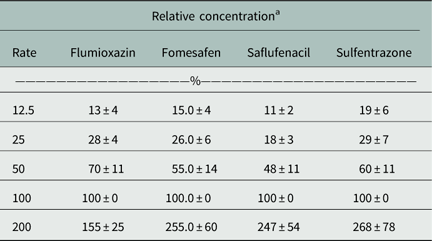
a Percent mean concentrations were normalized to the 1X labeled dose.
Palmer Amaranth Emergence
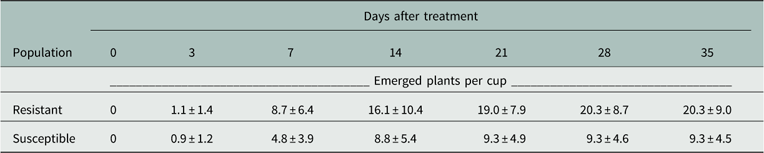
The germination profile in the nontreated cups over time showed similar temporal trends but different magnitudes of emergence (Table 2). Both populations reached maximum germination at approximately 14 DAT. The amount of germination in the S population was approximately 50% that of the R population in the nontreated cups. Although the S population had lower germination magnitude, the biomass data provided an objective measurement of herbicide response. The total germination percentage from the actual soil surface was 10% to 20% of the applied seeds, and was consistent over several preliminary runs and the two runs of this study.
Table 2 Germination profile of resistant and susceptible Palmer amaranth populations over time in nontreated cups. Data represent means plus or minus standard error of 16 replications.
Soil-Residual Control of Palmer Amaranth Biotypes
Significant differences were not detected at 3 DAT, largely because of the prolonged emergence of Palmer amaranth (Ward et al. Reference Ward, Webster and Steckel2012). However at 7 and 14 DAT, significant differences in control were observed between the R and S biotypes, as well as differences between herbicides within treatment rates (data not shown). Data indicated that at the 0.125× and 0.25× rates, control of the S biotype was >98% for flumioxazin, fomesafen, and saflufenacil treatments, with sulfentrazone providing <93% control at 7 and 14 DAT. Flumioxazin and fomesafen applied at 1× rate controlled 99% of the R biotype.
At 21 and 28 DAT, control of the S biotype was greater when compared to the R biotype (data not shown). Flumioxazin continued to show residual control at >90% and >94% for both R and S biotypes, respectively, across the five rates. Fomesafen control of the S biotype was >94%, but control of the R biotype resulted in <78% control. At 21 and 28 DAT, sulfentrazone provided 86% control of the S biotype at the 0.125× rate, but control was lower (62%) for the R biotype. At the 0.125× rate, control of the R population was in the order of flumioxazin (88%)=saflufenacil (86%)>fomesafen (64%)>sulfentrazone (59%). These results were consistent with the published literature on amaranth control by these herbicides (Geier et al. Reference Geier, Stahlman and Charvat2009; Nandula et al. Reference Nandula, Ray, Ribeiro, Pan and Reddy2013; Sweat et al. Reference Sweat, Horak, Peterson, Lloyd and Boyer1998; Whitaker et al. Reference Whitaker, York, Jordan and Culpepper2010).
Palmer Amaranth Biomass
Aboveground biomass was collected and weighed at 35 DAT for both R and S biotypes (Figures 1 and 2). The UTC R (10.3±6.1 g) and S (7.3±4.3 g) biotypes had similar biomass. Significant differences between biotypes were detected at 0.125×, 0.25×, and 0.5× herbicide dosages. At the 0.25× dose, flumioxazin (1.6 g) and saflufenacil (3.6 g) had less biomass of R biotype, whereas sulfentrazone (4.8 g) and fomesafen (4.2 g) resulted in greater biomass. As the dosage increased to 0.5×, the R biotype biomass was greater in sulfentrazone treatments, whereas no differences in biomass were observed for saflufenacil (0.6 g), fomesafen (0.3 g), and flumioxazin (0.08 g). No differences in biomass from any herbicide-treated pots were indicated for the S biotype. Overall, biomass in the susceptible biotypes was greatly reduced by all herbicide applications (Figure 1).
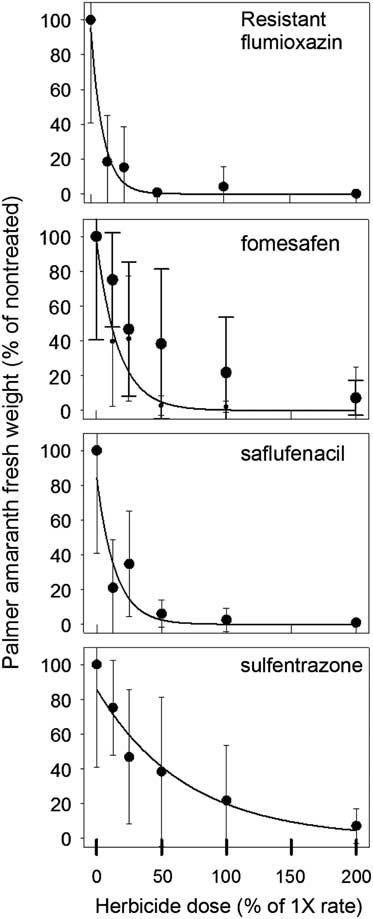
Figure 1 Palmer amaranth (Amaranthus palmeri) biomass normalized to percent of nontreated control for that experiment 35 days after treating resistant population with herbicide at rates ranging from 0% to 200% of normal 1× dosage. Means±standard error of eight measurements. The data were fit to the regression equation:
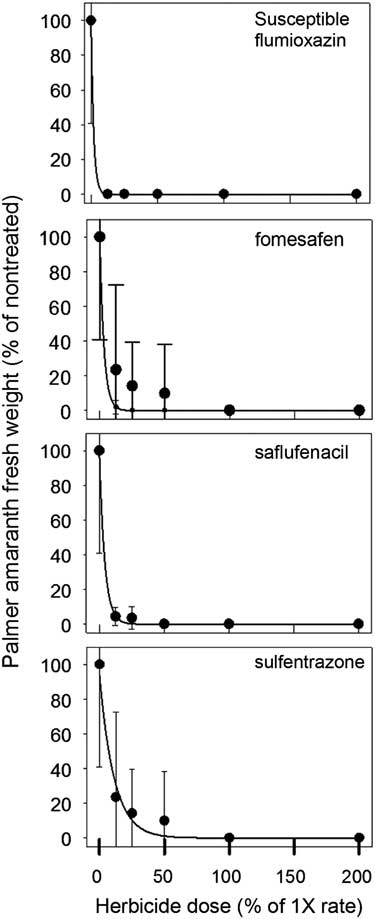
Figure 2 Palmer amaranth (Amaranthus palmeri) biomass normalized to percent of nontreated control for that experiment 35 days after treating susceptible population with herbicide at rates ranging from 0% to 200% of normal 1× dosage. Means±standard error of eight measurements. The data were fit to the regression equation:
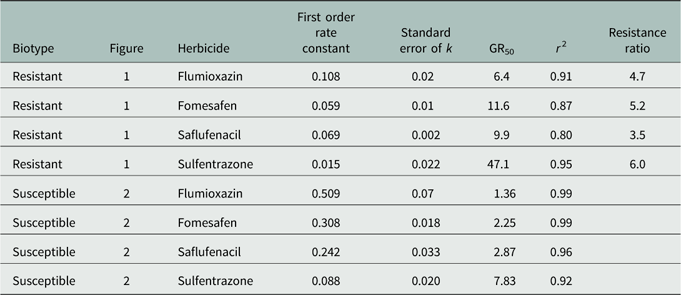
Regression results of the data indicated a good empirical fit of the model to the dose response curves (Table 3). GR50 values in the R population ranged from 6.4% to 47% of normal dosage and from 1.4% to 7.8% for the S population. The level of resistance, based on the calculated resistance factor, was similar for all four herbicides and was approximately 5×.
Table 3 Regression parameters (first order, exponential decay model) for Palmer amaranth biomass data 35 days after treatment, normalized to that of untreated control for each biotype, as affected by herbicides.
Flumioxazin was the most effective herbicide to reduce emergence and growth of the R Palmer amaranth biotype, followed by saflufenacil, fomesafen, and then sulfentrazone. Wuerffel et al. (Reference Wuerffel, Young, Tranel and Young2015) also concluded that flumioxazin had greater residual control on PPO-R tall waterhemp, another problematic weed in soybean systems in the midwestern region of the United States. Interestingly, Wuerffel concluded that control with fomesafen and sulfentrazone on PPO-R waterhemp was similar. Although Wuerffel’s results differed from those observed in our study, differences in herbicide rates used, and also resistant mechanisms, may differ between species. Several authors have concluded that differences in response to PPO herbicides vary greatly with chemical families (Falk et al. Reference Falk, Shoup, Al-Khatib and Peterson2006; Patzoldt et al. Reference Patzoldt, Tranel and Hager2005) and have reported that PPO-R waterhemp was more sensitive to flumioxazin. These authors also reported similar findings when sulfentrazone was applied. Although the data in this study did not agree with those of the authors on the results of sulfentrazone, differential herbicidal response could perhaps be attributed to differences between Amaranthus species.
In this study, fomesafen provided complete control (100%) of the S biotype; however, this was not observed for the R biotype when it was applied at the label rate. Shoup et al. (Reference Shoup, Al-Khatib and Peterson2003) concluded that diphenylether herbicides such as fomesafen may have the propensity for reduced response in PPO-R waterhemp. The reasoning these authors provided was that fomesafen is commonly used for POST control of waterhemp. In the southeast region of the United States, fomesafen is primarily used in GR soybean systems POST to control GR Palmer amaranth. The R biotype used in this study had four annual applications of POST fomesafen (Flexstar, Prefix) in the last five years (Steckel, personal communication). This further indicates that sensitivity to different PPO herbicide families can occur, and in this study, fomesafen and sulfentrazone were observed to have less activity on the R biotype.
Overall, this study further confirms that there is a differential herbicidal response in R and S Palmer amaranth. The herbicide treatments used in this study did not completely eliminate Palmer amaranth biomass in all replications of the R biotype (Figure 1). Clearly, all the PPO herbicides still provided some control, but less on the R versus S biotype. Therefore, data in this study demonstrates that GR Palmer amaranth in Tennessee has evolved to include PPO resistance. In previous years, Palmer amaranth control in a GR soybean system relied heavily on PPO-inhibiting herbicides to control GR Palmer amaranth. The data in this report suggest that changing PPO chemical families can in part improve Palmer amaranth control issues in this PPO-R population. However, a group 15 herbicide or metribuzin could be added to any of the PPO-inhibiting herbicides to provide adequate Palmer amaranth control.
Acknowledgments
This project was funded by the Tennessee Agricultural Experiment Station, the Tennessee Soybean Promotion Board, and Valent USA, Inc. Technical suggestions by Dr. John Pawlak and Dr. John Cranmer, both of Valent USA Corp., improved the conductance of this research. Technical assistance by Anna Ekene Davis Tharpe, Stephen Nassan, and Joseph Beeler is appreciated.









