Goosegrass is a summer annual weedy grass that is problematic throughout warm- and cool-season turfgrass (McCullough et al. Reference McCullough, Yu and Gomez de Barreda2013). Goosegrass infestations often occur at sites with compacted soils lacking in turf cover (Arrieta et al. Reference Arrieta, Busey and Daroub2009). Goosegrass germination is stimulated by fluctuating air temperature and light, with optimal germination observed following 16 h at 20°C and 8 h at 35°C with light (Nishimoto and McCarty Reference Nishimoto and McCarty1997). Dinitroaniline herbicides such as prodiamine and pendimethalin are frequently used PRE to control annual weeds such as goosegrass in managed turfgrass systems. These herbicides prevent microtubule polymerization in cells of susceptible species by binding to the globular protein tubulin (Vaughn et al. Reference Vaughn and Lehnen1991). This mechanism consequently prevents sister chromatids from segregating during the anaphase portion of mitosis, causing discontinuation of cell division (Senseman Reference Senseman2007).
Goosegrass resistance to dinitroaniline herbicides has been attributed to substitution of threonine for isoleucine at position 239 on α-tubulin (Yamamoto et al. Reference Yamamoto, Zeng and Baird1998; Anthony et al. Reference Anthony, Waldin, Ray, Bright and Hussey1998). Goosegrass phenotypes with resistance to dinitroaniline herbicides have been regularly reported throughout the southeastern United States. For example, Mudge et al. (Reference Mudge, Gossett and Murphy1984) identified goosegrass phenotypes resistant to benefin, ethalfluralin, fluchloralin, isopropalin, oryzalin, pendimethalin, and trifluralin in seven counties of northeastern South Carolina. Since then, there have been several reports of annual bluegrass (Poa annua L.) developing resistance to dinitroaniline herbicides following consecutive years of prodiamine applications for PRE weed control (Brosnan et al. Reference Brosnan, Reasor, Vargas, Breeden, Kopsell, Cutulle and Mueller2014; Cutulle et al. Reference Cutulle, McElroy, Millwood, Sorochan and Stewart2009; Isgrigg et al. Reference Isgrigg, Yelverton, Brownie and Warren2002). Recently, McCullough et al. (Reference McCullough, Yu and Gomez de Barreda2013) identified a goosegrass phenotype on a golf course in Georgia that was resistant to the dinitroaniline herbicide prodiamine but was effectively controlled with single and sequential PRE applications of indaziflam or oxadiazon. However, options for POST control of prodiamine-resistant (PR) goosegrass were not investigated.
Topramezone, a hydroxyphenyl-pyruvate-dioxygenase inhibitor, is a pyrazole herbicide labeled for POST goosegrass control in turf at rates of 12.3 to 36.8 g ai ha-1 (Anonymous 2015). Cox et al. (Reference Cox, Rana, Brewer and Askew2017) reported effective control of 8- to 18-tiller goosegrass with sequential topramezone applications at 6.1 or 12.3 g ai ha-1 on a 3-week interval. Foramsulfuron is an acetolactate synthase–inhibiting herbicide labeled for POST goosegrass control (Anonymous 2006). Busey (Reference Busey2004) reported effective control of mature (i.e., 3- to 10-tiller) goosegrass in south Florida with tank mixtures of foramsulfuron and metribuzin. Brosnan et al. (Reference Brosnan, Nishimoto and DeFrank2008) reported that foramsulfuron at 0.045 kg ai ha-1 controlled a population of goosegrass resistant to the photosystem II inhibitors metribuzin and simazine. Topramezone and foramsulfuron may provide turf managers with options for controlling dinitroaniline-resistant goosegrass POST. However, data on this subject are limited.
In the summer of 2013, poor goosegrass control (< 20%) was reported on several golf course roughs at Lambert Acres Golf Course in Maryville, TN (35.747307°N, −83.884067°W) following prodiamine treatment at 1,120 g ai ha–1. Course managers had applied prodiamine exclusively in this manner for residual weed control for >11 consecutive years at this location (J.D. Murr, personal communication). Additionally, populations of PR annual bluegrass have already been identified at this golf course (Brosnan et al. Reference Brosnan, Reasor, Vargas, Breeden, Kopsell, Cutulle and Mueller2014). A finding that goosegrass at this location is resistant to prodiamine would mark the first documented occurrence in Tennessee of goosegrass developing resistance to prodiamine. Thus, our objectives were to (1) determine the sensitivity of a putative PR goosegrass phenotype collected from this location, (2) elucidate the mechanism of resistance in this phenotype, and (3) identify options for effective POST control of this phenotype in the field.
Materials and Methods
Glasshouse Studies Confirming Resistance
Mature goosegrass plants suspected of being resistant to prodiamine (hereafter referred to as PR) were harvested from roughs at Lambert Acres Golf Club (Maryville, TN) during July 2013 with a tubular plugger (Turf Tec International, Tallahassee, FL 32303). Turf at this location was an unknown cultivar of common bermudagrass that had been treated with prodiamine at 1,120 g ha-1 during February 2013. A mature goosegrass phenotype known to be susceptible to prodiamine (hereafter referred to as S) was harvested from bermudagrass golf course roughs at Bay’s Mountain Golf (Seymour, TN; 35.51ºN, −83.49ºW). Individual tillers of harvested PR and S plants were removed and transplanted into 164-cm3 containers (SC10 Super Cell Conetainer. Steuwe & Sons. Tangent, OR 97389) filled with a peat moss growing medium (Growing Mix #2. Conrad Fafard, Inc., Agawam, MA 01001). A total of 700 single-tiller transplants (350 PR and 350 S) were propagated from field-harvested plants. These single-tiller transplants were maintained under controlled glasshouse conditions for several weeks before initiating research. During the acclimation period, plants were irrigated as needed to prevent moisture stress and clipped twice a week at a height of ~5 cm. By the time glasshouse experiments were initiated, these transplants had matured such that all plants had a minimum of two tillers.
Sensitivity of the PR and S phenotypes to prodiamine was confirmed using hydroponic methods of Brosnan et al. (Reference Brosnan, Reasor, Vargas, Breeden, Kopsell, Cutulle and Mueller2014) in a glasshouse at the University of Tennessee (Knoxville, TN; 35.564593°N, −83.561603°W). Polyethylene containers (Rubbermaid Roughneck, Rubbermaid Commercial Products LLC, Winchester, VA) containing 10 L of a full-strength Hoagland solution (Hoagland and Arnon Reference Hoagland and Arnon1950) were aerated with a blower (Model VB-007S, Sweetwater, Ft. Collins, CO), air stones (HAGEN Elite 1-inch Cube Air Stone, Rolf C. Hagen Corp., Mansfield, MA), and Tygon tubing (Saint-Gobain Performance Plastics Akron, OH). Into the lid of each container were drilled 10 holes of 0.4-cm diameter at 5.3-cm spacing. Thus, mean values for a single container were generated using 10 subsamples.
Goosegrass plants were cleansed of media and transplanted into each container such that root tissues were submerged in the aerated nutrient solution. All plants were trimmed to a uniform root length of 6 cm to facilitate assessments of root growth in response to herbicide treatment.
Prodiamine (Barricade 65 WG, Syngenta Professional Products, Greensboro, NC) was added to the nutrient solution in each container at concentrations of 0, 0.001, 0.01, 0.1, and 1.0 mM immediately after transplanting. The blower used to aerate each container provided enough agitation to prevent prodiamine from falling out of suspension in the container. Herbicides were placed into each container on August 19, 2013. During the entire first experimental run, maximum and minimum daily air temperatures were 30°C and 23°C, with a daily light integral of 34 mol m–2 d–1. At 10 days after treatment (DAT), goosegrass root length was measured on all PR and S plants. Root length data were used to determine effects of prodiamine concentration on root growth (as a percentage of the non-treated check) using the following equation:
Experiments were arranged in a randomized complete block design with three replications and repeated in time during 2013. The second experimental run was initiated on August 30, 2013. Observed maximum and minimum daily temperatures in the glasshouse during the second experimental run were also 30°C and 23°C, with a daily light integral of 37 mol m–2 d–1.
All root growth data were subjected to statistical analysis using R software (R version 3.3.1). Expected means squares of McIntosh (Reference McIntosh1983) determined that data could be combined over experimental runs before being subjected to ANOVA using the ‘ExpDes’ package in the R statistical platform. Root growth of the PR and S phenotypes in response to increasing concentrations of prodiamine was compared using nonlinear regression techniques in Prism (Prism 5 for Mac OS X. GraphPad Software. La Jolla, CA). Responses for each phenotype were fit to a one-phase exponential decay model and compared using a global sums of squares F-test at α=0.05.
Genetic Sequencing and Molecular Analysis
Genomic DNA was extracted with NucleoSpin® Plant II extraction kit (Macherey-Nagel, Düren, Germany) according to the manufacturer’s instructions. Leaves of S and PR goosegrass were harvested, lyophilized, and homogenized before DNA extraction. To amplify the α-tubulin gene (TUA1) at the position mediating dinitronaniline herbicide resistance (Thr-239-Ile; Yamamoto et al. Reference Yamamoto, Zeng and Baird1998; Anthony et al. Reference Anthony, Waldin, Ray, Bright and Hussey1998), PCR was carried out with KOD Hot Start DNA Polymerase (Merck Bioscience, Darmstadt, Germany) according to the manufacturer’s instructions. The forward PCR primer for TUA1 was 5’-GTCTGTTGACTACGGCAAGAA-3’ (Tre 460), and the reverse primer was 5’-CACTGGAGCGTAGGATGAAAG-3’ (Tre 461). PCR started with initial denaturation at 95°C for 2 min followed by 95°C denaturation for 20 s, annealing at 58°C for 10 s, and elongation at 70°C for 15 s. Denaturation, annealing, and elongation were repeated for 40 cycles. Sanger sequencing of PCR products was performed with a 3130xl Genetic Analyzer (Applied Biosystems, Thermo Fisher Scientific, Waltham, MA, USA) and BigDye® Terminator v3.1 cycle sequencing Kit (Thermo Fisher Scientific, Waltham, MA, USA) with DNA primers as mentioned above. DNA sequences for the PR and S phenotypes are available in GenBank (MF094447, MF094446; NCBI 2017).
To allow fast- and medium-throughput analyses of leaf samples from multiple plants, a Taqman®-based single-nucleotide polymorphism (SNP) genotyping assay was established. DNA oligos for SNP differentiation between the ACA (Thr) and ATA (Ile) codons were designed using custom Taqman® SNP genotyping service (Thermo Fisher Scientific, Waltham, MA, USA). The analyses were performed using a CFX Real-Time PCR detection system (Bio-Rad, Hercules, VA, USA). A total volume of 20 µL, consisting of 2.5-µL genomic DNA samples, 10 µL SsoAdvancedTM Universal Probes Supermix (Bio-Rad, Hercules, VA, USA), 0.5 µL flanking primers and probe mix, and 7 µL water was added to the PCR wells (Hard-Shell PCR Plates, 96-well; Bio-Rad, Hercules, VA, USA). Flanking primer sequences were the following: 5’-CCGTAACATGTGGTTGTTTCTTATGA-3’ (ELEIN_mut_F) and 5’-ACGTTCAGAGCACCATCGAA-3’ (ELEIN_mut_R). The following probes were designed to overlap the wild-type or Thr-239-Ile variation in TUA1, respectively: 5’-CAGAGAGGCTGTCAGTG-3’ (ELEIN_mut_VIC) and 5’-CAGAGAGGCTATCAGTG-3’ (ELEIN_mut_FAM) (Custom Taqman® SNP Genotyping Assay Service, Thermo Fisher Scientific, Waltham, MA, USA). Samples analyzed by Sanger sequencing were used as homozygous susceptible or resistant controls. Real-time PCR was run using FAM as the fluorescent dye for the resistant gene with the Thr-239-Ile variation and VIC as the fluorescent dye for the wild type. Cycling conditions were as follows: polymerase activation at 95°C for 2 min, denaturation at 95°C for 15 s, followed by annealing and extension at 64°C for 30 s, for a total of 70 cycles. Percentages of the resistant and wild-type variant were calculated based on the sum of fluorescence intensity VIC and FAM at PCR cycle 35.
Field Experiments
Field experiments were conducted at Lambert Acres Golf Course (Maryville, TN) evaluating the efficacy of topramezone and foramsulfuron for POST control of PR goosegrass. Trials were conducted in 2013 and 2015 in a common bermudagrass rough maintained at 4.5 cm. Soil was a Dewey silty clay loam (fine, kaolinitic, thermic typic Paleudults). The golf course rough site received no supplemental nutrition or irrigation aside from rainfall during the course of the study. This site had been treated with prodiamine at 1,120 g ha-1 for 11 consecutive years before initiating research, including applications made in February 2013 and March 2015.
Herbicide treatments included topramezone (12.3, 24.5, and 36.8 g ha-1) and foramsulfuron (29 and 43.6 g ha-1). Both topramezone and foramsulfuron are labeled for POST goosegrass control in turf (Anonymous 2006, 2013). A non-treated check was included for comparison. Topramezone treatments included a methylated seed oil adjuvant at 0.625% v/v. All treatments were applied POST in a water carrier to 1.2 by 1.8 m plots using a CO2-powered boom sprayer calibrated to deliver 281 L ha-1 through 8002 flat-fan nozzles. Applications at each site were made on July 25, 2013 and July 6, 2015 to goosegrass plants that contained a minimum of 10 tillers at application.
Goosegrass control was visually assessed by means of a 0% (i.e., no control) to 100% (i.e., complete control) scale relative to the non-treated check at 7, 14, 21, 28, 35, 42, and 50 DAT (Brown and Farmer Reference Brown and Farmer1991). Bermudagrass injury was assessed using a similar 0% to 100% scale relative to a non-treated check as well. Goosegrass plants present within a 1-m2 grid placed in the center of each plot were counted to quantitatively confirm visual evaluations of goosegrass control at 50 DAT. This grid contained 100 squares measuring 0.01 m2.
Experimental design each year was a randomized complete block with six replications. Visual assessments of goosegrass control and bermudagrass injury were arcsine transformed before being subjected to ANOVA in SAS using the expected means squares of McIntosh (Reference McIntosh1983). No treatment-by-year interactions were detected, thus allowing data from each year to be combined. Interpretations of ANOVA using transformed data were not different from non-transformed assessments; therefore, non-transformed means are presented for clarity. Fisher’s protected LSD test was used to separate treatment means at α=0.05.
Results and Discussion
Glasshouse Studies Confirming Resistance
Responses of the PR and S phenotypes to increasing concentrations of prodiamine were significantly different from one another (Figure 1). Exposure to prodiamine at 0.001 mM reduced root growth of the S phenotype to 11% of the non-treated check. Comparatively, exposure to 0.001 mM prodiamine had minimal effect on the PR phenotype, as root growth was 94% of the non-treated by 10 DAT (Figure 1). A similar response, although with a more severe impact on the PR phenotype, was observed following exposure to 0.01 mM prodiamine as well. Once prodiamine concentrations increased to 0.10 mM, no differences in root growth between the PR and S phenotypes were detected, as both measured≤9% of their respective non-treated check (Figure 1). These whole-plant phenotypic assessments support field observations that the PR goosegrass phenotype from Tennessee is resistant to the dinitroaniline herbicide prodiamine.
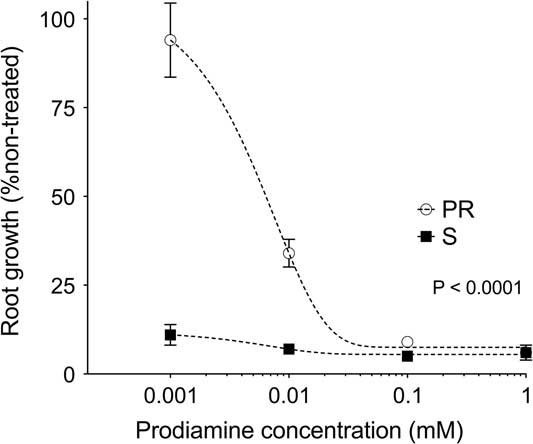
Figure 1 Root growth of prodiamine-resistant (PR) and susceptible (S) goosegrass (Eleusine indica L. Gaertn.) 10 d after treatment following exposure to increasing concentrations of prodiamine in hydroponic culture during glasshouse experiments in 2013. Root growth for the PR and S phenotypes was expressed as a percentage of a non-treated check (0 mM prodiamine), with means generated using 10 subsamples per concentration, replicated six times over the course of two repeated experiments (N=60).
To identify a possible target-site mutation causative for prodiamine resistance, part of the α-tubulin 1 gene (TUA1) known to encode the binding site of dinitronanilines (Yamamoto et. al. Reference Yamamoto, Zeng and Baird1998) was sequenced in both PR and S plants. The results showed that PR plants had a codon change from ACA to ATA, which corresponds to a threonine-to-isoleucine substitution at position 239 in TUA1. A genotyping assay successfully detected that all PR plants (R1 to R10) were either homozygous (7) or heterozygous (3) for Thr to Ile, whereas all S plants only encoded Thr, the wild-type form of TUA1 susceptible to dinitroaniline herbicides (Table 1). In combination with our whole-plant phenotyping and previously published results (Yamamoto et al. Reference Yamamoto, Zeng and Baird1998; Anthony et al. Reference Anthony, Waldin, Ray, Bright and Hussey1998), we conclude that a target-site mechanism is responsible for resistance to prodiamine in PR goosegrass studied herein. Our genotyping assay clearly distinguishes between S, heterozygous resistant, and homozygous resistant plants and therefore is a useful diagnostic assay to detect target-site resistance to dinitronaniline herbicides in goosegrass. Yamamoto et al. (Reference Yamamoto, Zeng and Baird1998) introduced a technique to genotype the ACA-to-ATA mutation (prompting an amino acid change from Thr to Ile at position 239) in goosegrass using PCR primer–introduced restriction analysis that requires (a) amplification of the target sequence, (b) use of a restriction enzyme to cut the amplified fragments, (c) samples to be run on an agarose gel, and (d) a visual inspection of the gel to determine which allele is present. The TaqMan assay developed herein performs these operations in one step, where amplification of each allele is monitored together with real-time PCR and genotype can be automatically assigned at the end of amplification. Our approach is much faster, requires less hands-on time, and is easier to automate. Future research should be conducted to expand this assay for use with other weed species and different herbicide target sites.
Table 1 Genotyping of prodiamine-resistant (PR) goosegrass plants for a threonine to isoleucine (Thr to Ile) substitution at position 239 on α-tubulin 1 (TUA1).
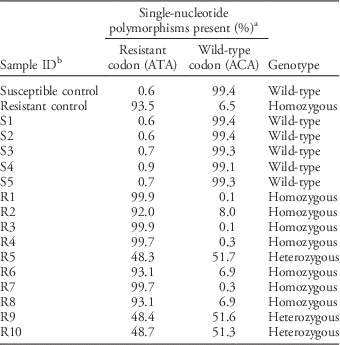
a Percentage value was calculated based on total fluorescence intensity of fluorochrome used for wild-type or mutated gene variant at PCR cycle 35. Based on fluorescence intensity for each allele, total being set at 100%, each sample taken individually.
b Control samples were confirmed by Sanger sequencing.
Field Experiments Evaluating POST Control
No significant treatment-by-year interactions were detected in goosegrass control or plant count data; therefore, data from each year were combined for analysis. Significant differences in goosegrass control due to herbicide treatment were detected 14, 28, and 50 DAT. Goosegrass control with topramezone was greater than foramsulfuron on all dates, with few significant differences detected among topramezone rates. By 50 DAT, topramezone controlled PR goosegrass 72% to 89% compared to only 22% to 23% for foramsulfuron (Table 2). Plant count data supported visual assessments of goosegrass control. Topramezone reduced goosegrass plant counts 60% to 83% compared to only 6% to 19% for foramsulfuron (Table 2). Cox et al. (Reference Cox, Rana, Brewer and Askew2017) reported similar responses with POST applications of topramezone to herbicide-susceptible goosegrass at four locations in Virginia, with sequential treatments at 12.3 g ai ha-1 resulting in an 86% reduction in goosegrass cover 56 d after initial treatment. Poor goosegrass control with foramsulfuron in this experiment supports previous reports by Busey (Reference Busey2004) that multiple POST applications of foramsulfuron are required to control goosegrass.
Table 2 Prodiamine-resistant (PR) goosegrass control and plant count reductions following topramezone and foramsulfuron applications at Lambert Acres Golf Club (Maryville, TN). Means were combined from separate trials conducted in 2013 and 2015.
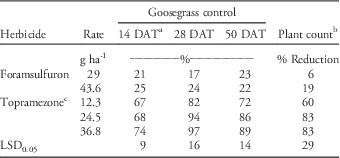
a Abbreviation: DAT, days after treatment.
b Plant count data were collected using a 1 m2 grid 50 DAT and expressed as a percent reduction relative to the non-treated check.
c All topramezone treatments included a methylated seed oil adjuvant at 0.625% v/v.
Whereas topramezone effectively controlled PR goosegrass in these studies, applications also caused significant bermudagrass injury 7 and 14 DAT (Table 3). During this time frame, topramezone injured bermudagrass 34% to 60% compared to only 0% to 4% for foramsulfuron. Injury with the 12.3 g ha-1 application rate was less than the 24.5 and 36.8 g ha-1 application rates at 14 DAT. Topramezone injury may be unacceptable to turf managers; however, this response was transient, as injury was≤6% 28 DAT and 0% by 50 DAT. These responses support previous findings by Elmore et al. (Reference Elmore, Brosnan, Kopsell, Breeden and Mueller2011), Brosnan et al. (Reference Brosnan, Kopsell, Elmore, Breeden and Armel2011), and Cox et al. (Reference Cox, Rana, Brewer and Askew2017) following topramezone applications to bermudagrass. By 21 DAT in a glasshouse, topramezone applications at 18 to 38 g ha-1 injured ‘Tifway’ hybrid bermudagrass 35% to 40% and ‘Riviera’ common bermudagrass 46% to 58% (Brosnan et al. Reference Brosnan, Kopsell, Elmore, Breeden and Armel2011; Elmore et al. Reference Elmore, Brosnan, Kopsell, Breeden and Mueller2011). This injury was characterized as visual bleaching and accompanied by reductions in chlorophyll and carotenoid pigments in both grasses. However, both researchers reported that bermudagrass injury following topramezone treatment was negligible by 35 DAT (Brosnan et al. Reference Brosnan, Kopsell, Elmore, Breeden and Armel2011; Elmore et al. Reference Elmore, Brosnan, Kopsell, Breeden and Mueller2011). Similarly, topramezone at 12.3 g ai ha-1 resulted in 31 bermudagrass varieties being injured≥30% for an average of 13.8 d (Cox et al. Reference Cox, Rana, Brewer and Askew2017); however, injury was transient.
Table 3 Bermudagrass injury following topramezone and foramsulfuron applications at Lambert Acres Golf Club (Maryville, TN). Means were combined from separate trials conducted in 2013 and 2015.
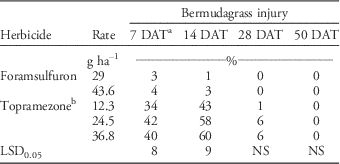
a Abbreviations: DAT, days after treatment; NS, nonsignificant at α=0.05.
b All topramezone treatments included a methylated seed oil adjuvant at 0.625% v/v.
Our results indicate the first incidence of a PR goosegrass phenotype in Tennessee. Resistance at this location probably developed after repeated use of prodiamine for residual weed control for >11 consecutive years. An amino acid substitution from Thr to Ile at position 239 in TUA1 was identified as the most likely mechanism of resistance in this phenotype. POST applications of topramezone at ≥ 24.5 g ha-1 effectively (86% to 89%) controlled this PR goosegrass phenotype; however, transient bermudagrass injury observed after application may be unacceptable to certain turf managers. The genotyping assay described herein clearly differentiated between S and PR plants and could be used as a diagnostic assay to detect target-site resistance to dinitronaniline herbicides in goosegrass populations from other locations.
Acknowledgments
The authors would like to thank the superintendent at Lambert Acres Golf Course, J.D. Murr, for his assistance with this project, as well as Tyler Campbell, Daniel Farnsworth, James Greenway, Eric Reasor, and Veronica Sublett. Drs. Thomas Mueller and Scott Senseman also contributed to this manuscript. Mention of trade names or commercial products in this publication is solely for the purpose of providing specific information and does not imply recommendation or endorsement by the University of Tennessee Institute of Agriculture.





