Introduction
In everyday life, beside cognitive performance per se, one extremely helpful capacity is the ability to infer the cognitive and emotional states of persons we come into contact with since this enables us to understand their behaviors and successfully interact with them. The capacity to deduce other persons’ mental states (thoughts, beliefs, feelings, etc.) is known as theory of mind (ToM) (Premack & Woodruff, Reference Premack and Woodruff1978), and it has two functional components: cognitive and affective (Brothers & Ring, Reference Brothers and Ring1992).
In recent years, interest has been growing in cognitive and affective ToM functioning in healthy older adults (see Henry, Phillips, Ruffman, and Bailey, Reference Henry, Phillips, Ruffman and Bailey2013, for a review) and in persons suffering from neurodegenerative disorders, such as Alzheimer’s disease (AD) (see Moran, Reference Moran2013, and Sandoz, Démonet, & Fossard, Reference Sandoz, Démonet and Fossard2014, for reviews). As far as affective ToM is concerned, this has been studied mostly with non-verbal tasks in which the emotional state has to be inferred on the basis of emotional facial cues, such as facial expression identification tasks and the Reading the Mind in the Eyes test and its different adaptations (RMET; Baron-Cohen, Wheelwright, Hill, Raste, & Plumb, Reference Baron-Cohen, Wheelwright, Hill, Raste and Plumb2001).In everyday life, however, other people’s emotional states are usually inferred within a context and not only on the basis of facial cues. Only a few studies have investigated affective ToM in healthy older adults and in persons with AD in tasks including contextual details. Thus, in the present study, we aimed to use non-verbal stimuli to explore the influence of healthy aging and AD pathology on affective ToM based on contextual cues. In addition, we were interested in whether the associated inferences would differ as a function of specific basic emotions.
The results of previous studies exploring affective ToM in healthy older adults on the basis of emotional faces presented without context are mixed. For example, several studies have shown that advancing age has a deleterious effect not only on the capacity to identify basic facial emotions, especially negative ones (Mahy et al., Reference Mahy, Vetter, Kühn-Popp, Löcher, Krautschuk and Kliegel2014; see Ruffman et al., Reference Ruffman, Henry, Livingstone and Phillips2008, for a review), but also on the capacity to infer emotional states from a person’s eyes (RMET, Fischer, O’Rourke, & Thornton, 2017; Slessor, Phillips, & Bull, Reference Slessor, Phillips and Bull2007) or from social scenes presented in short video clips (Slessor et al., Reference Slessor, Phillips and Bull2007). Nevertheless, certain other studies have shown preserved affective ToM abilities in RMET tasks (Castelli et al., Reference Castelli, Baglio, Blasi, Alberoni, Falini, Liverta-Sempio and Marchetti2010; Duval, Piolino, Bejanin, Eustache, & Desgranges, Reference Duval, Piolino, Bejanin, Eustache and Desgranges2011).
Previous results are also inconsistent concerning the identification of facial emotions in persons with mild to moderate AD (see Klein-Koerkamp, Beaudoin, Baciu, & Hot, Reference Klein-Koerkamp, Beaudoin, Baciu and Hot2012, for a review; Lavenu & Pasquier, Reference Lavenu and Pasquier2005). However, the identification of some basic emotions seems to be selectively preserved in persons with AD (e.g., joy, Maki, Yoshida, Yamaguchi, & Yamaguchi, Reference Maki, Yoshida, Yamaguchi and Yamaguchi2013, and disgust, Henry et al., Reference Henry, Ruffman, McDonald, O’Leary, Phillips, Brodaty and Rendell2008). Studies that have explored affective ToM in persons with AD using the RMET have also found inconsistent results (see Poletti, Enrici, & Adenzato, Reference Poletti, Enrichi and Adenzato2012, for a review). Preserved capacities were observed in persons with mild AD in both easier (i.e., in which participants had to choose between two word labels, Gregory et al., Reference Gregory, Lough, Stone, Erzinclioglu, Martin, Baron-Cohen and Hodges2002) and more difficult versions of this task (i.e., where participants had to choose between four word labels, Heitz et al., Reference Heitz, Noblet, Phillipps, Cretin, Vogt, Philippi and Blanc2016). However, other studies have reported impaired performance on the RMET in persons with mild AD (Laisney et al., Reference Laisney, Bon, Guiziou, Daluzeau, Eustache and Desgranges2013; Castelli et al., Reference Castelli, Pini, Alberoni, Liverta-Sempio, Baglio, Massaro and Nemni, R.2011).
Only a few studies have investigated affective ToM in AD by using non-verbal stimuli presented within a context. For example, Freedman, Binns, Black, Murphy, and Stuss (Reference Freedman, Binns, Black, Murphy and Stuss2013) showed preserved first-order affective ToM in persons with mild AD by presenting videos illustrating emotional situations involving three basic emotions (happiness, sadness, anger). In a task using drawings of social situations, Zaitchik, Koff, Brownell, Winner, and Albert (Reference Zaitchik, Koff, Brownell, Winner and Albert2006) also showed that first-order, but not second-order, affective ToM is preserved in persons with mild AD. However, in their study, the authors did not provide a detailed description of the emotional situations that were used. Thus, it is not clear if all basic emotions or only a few of them were used (the authors provide examples only of sad, angry, and happy situations).
In addition, the stimulus presentation included verbal and non-verbal modalities (the story was read out loud by the experimenter and illustrated by simple line drawings). Moreover, Shimokawa et al. (Reference Shimokawa, Yatomi, Anamizu, Ashikari, Kohno, Maki and Matsuno2000, Reference Shimokawa, Yatomi, Anamizu, Torii, Isono, Sugai and Kohno2001, Reference Shimokawa, Yatomi, Anamizu, Torii, Isono and Sugai2003) showed that persons suffering from mild to severe AD outperformed persons suffering from vascular dementia on first-order affective ToM in a task in which participants had to infer the emotion felt by characters presented in drawings of social situations involving four basic emotions (joy, anger, sadness, and surprise; e.g., a father receiving a gift from his children, two boys fighting), even though the two groups of persons did not differ in terms of general cognitive performance or visuoperceptual abilities. In addition, the performance of the persons with AD was not significantly correlated with the level of general cognitive impairment (Mini-Mental State Examination [MMSE] scores; Folstein, Folstein, & McHugh, Reference Folstein, Folstein and McHugh1975), suggesting that the capacity to comprehend the emotional nature of a situation and to infer someone’s emotional state does not decrease as the disease progresses. Nevertheless, in a longitudinal study, Torres et al. (Reference Torres, Santos, Barroso De Sousa, Simoes Neto, Lima Nogueira, Belfort and Nascimento Dourado2015) found that the performance of persons with mild to moderate AD in such tasks diminishes after six months. The main limitation of the studies by Shimokawa et al. (Reference Shimokawa, Yatomi, Anamizu, Ashikari, Kohno, Maki and Matsuno2000, Reference Shimokawa, Yatomi, Anamizu, Torii, Isono, Sugai and Kohno2001, Reference Shimokawa, Yatomi, Anamizu, Torii, Isono and Sugai2003) and Torres et al. (Reference Torres, Santos, Barroso De Sousa, Simoes Neto, Lima Nogueira, Belfort and Nascimento Dourado2015) is that they did not compare the performance of persons with AD with the performance of a control group of healthy older adults, thus making it impossible to draw any conclusion concerning the capacity to infer affective states from the context in AD.
Thus, given the mixed results and the methodological issues observed in previous studies, further attention must be paid to the question of affective ToM abilities in contextualized tasks using visual stimuli conducted in healthy older adults and in persons with AD. Among the methodological issues, the emotional states that the participants had to infer have differed across studies, and not all the basic emotions have always been included. Furthermore, in some of the studies, the participants had access to the facial expressions of the characters when they inferred their emotional states (Fischer et al., Reference Fischer, O’Rourke and Thornton2017; Heitz et al., Reference Heitz, Noblet, Phillipps, Cretin, Vogt, Philippi and Blanc2016), whereas in certain other studies they did not (Shimokawa et al., Reference Shimokawa, Yatomi, Anamizu, Ashikari, Kohno, Maki and Matsuno2000, Reference Shimokawa, Yatomi, Anamizu, Torii, Isono, Sugai and Kohno2001, Reference Shimokawa, Yatomi, Anamizu, Torii, Isono and Sugai2003; Torres et al., Reference Torres, Santos, Barroso De Sousa, Simoes Neto, Lima Nogueira, Belfort and Nascimento Dourado2015).
These two conditions are processed differently, and only the latter requires the affective state to be inferred on the basis of an intentional analysis of the context. In situations in which the facial expression of the characters is presented, it is possible to infer the emotional state simply through the automatic decoding of the facial expression (Sabbagh, Reference Sabbagh2004). Thus, in the present study, our aim was to further explore the influence of age and AD pathology on the capacity to infer affective states based on situational context and as a function of emotion type. To this end, we included all the basic emotions (joy, anger, disgust, fear, sadness, and surprise). We compared the performances of persons with AD and healthy older and younger adults on a task in which the participants were asked to infer the first-order emotional ToM of characters presented without facial details in drawings depicting emotional situations. We expected to observe poorer overall performances in healthy older adults than in healthy younger adults and poorer overall performance in persons with AD than in healthy older adults. Given that previous studies have suggested that the identification of joy (Maki et al., Reference Maki, Yoshida, Yamaguchi and Yamaguchi2013) and disgust (Henry et al., Reference Henry, Ruffman, McDonald, O’Leary, Phillips, Brodaty and Rendell2008) is preserved in persons with AD, we wondered if this observation could be generalized to the capacity to infer emotional states in situations in which facial cues are not provided.
Methods
Participants
A total of 63 participants, subdivided into three experimental groups, took part in our study after giving their signed informed consent. Our study was approved by the local ethics committee and conducted according to the standards of the 1964 Helsinki Declaration. The first group consisted of 17 persons with mild AD (7 women and 10 men) recruited from the Hospital of Charpennes, Lyon, France, and from the Saint-Louis Lariboisière – Fernand Widal Hospital, APHP, Paris. They had been diagnosed with Alzheimer’s-related dementia following a standardized clinical investigation that included a neurological and neuropsychological assessment (see Table 1 for results), as well as brain morphology imaging (NIA-AA, McKhann et al., 2011). All the persons with AD included in our study were suffering from mild dementia (MMSE > 20, Folstein et al., Reference Folstein, Folstein and McHugh1975). The second group consisted of 21 healthy older adults matched with the persons with AD for age and education (14 women and 7 men). To ensure that the healthy older adults did not have any cognitive deficits prior to performing the experimental task, they underwent a short battery of tests to permit their cognitive assessment (Table 1).
Table 1: Demographic data and neuropsychological tests’ results of the persons with Alzheimer’s disease and of the healthy older adults included in our study
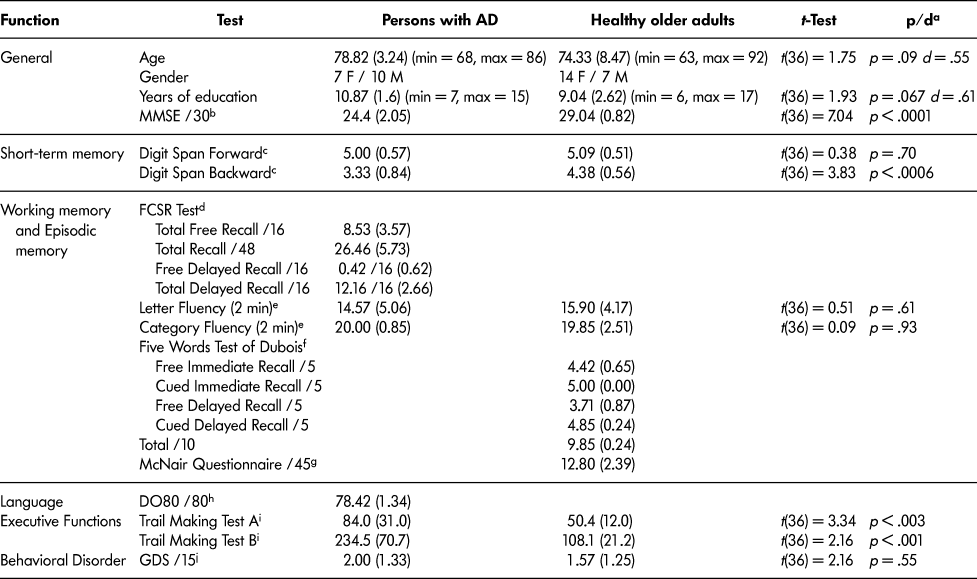
a p value and Cohen’s d (Cohen, Reference Cohen2007) derived from t-tests (unequal variances assumed; small effect size: d < 0.2; medium effect size: d > 0.5; large effect size: d > 0.8).
b MMSE; Folstein et al., Reference Folstein, Folstein and McHugh1975.
c Digit span forward and backward; Wechsler, Reference Wechsler1981.
d French version of the Free and Cued Selective Reminding Test (FCSR), Grober, Buschke, Crystal, Bang, & Dresner, Reference Grober, Buschke, Crystal, Bang and Dresner1988.
e Letter and category fluency tests, Cardebat, Doyon, Puel, Goulet, & Joanette, Reference Cardebat, Doyon, Puel, Goulet and Joanette1990.
f Dubois’ 5-words test, Dubois, Reference Dubois2001.
g McNair and Kahn Questionnaire of memory complaints, McNair & Kahn, Reference McNair, Kahn, Crook, Ferris and Bartus1983.
h Oral Naming (DO80), Deloche & Hannequin, Reference Deloche and Hannequin1997.
i Trail Making test, Reitan, Reference Reitan1958. For Trail Making Test A and B, the scores presented in table represent the realization time in seconds. Lower values indicate better performance, and higher scores indicate lower performance in these tasks.
j Short Geriatric Depression Scale (GDS), Sheikh & Yesavage, Reference Sheikh and Yesavage1986.
Finally, the third group consisted of 25 healthy younger adults (23 women and 2 men) – students at the University of Lyon II – who differed from those who had evaluated the visual complexity of the stimuli in the pretest (mean age = 19.84 years, SD = 2.03; mean education = 12.0 years, SD = 0.0). All the participants met the following inclusion criteria: they were native French speakers, had a minimum of 6 years of education, and had normal or corrected-to-normal vision and hearing. Participants were ineligible if they had a history of diagnosed cognitive impairment or a history of neurological (except for our group of persons with AD) or psychiatric disease.
Stimuli
The stimuli consisted of 32 black-and-white A4 landscape paper drawingsFootnote 1 inspired by the work of Kolb, Wilson, and Taylor (Reference Kolb, Wilson and Taylor1992). The first drawing depicted six faces with emotional expressions of anger, disgust, fear, joy, sadness, and surprise. The other 31 drawings depicted scenes with one, two, or three characters represented without facial details. One of these characters was the central character. There were five drawings for each emotional situation and one practice drawing depicting joy. The six faces with emotional expressions from the first drawing were presented below each scene and were used for the attribution of emotions (see Figure 1 and Appendix 1).

Figure 1: Experimental design and procedure of the present study
As the numbers of characters depicted in each scene were not identical and as this factor might have created variations in the visual complexity of the scenes, a group of 52 healthy younger participants evaluated the visual complexity of the scenes prior to the experimental tasks. Detailed information on this evaluation is provided in Appendix 2. Scenes depicting disgust had the lowest level of complexity; scenes depicting anger had the highest level of complexity.
Procedure
The participants were tested individually for approximately 20 minutes each. They first saw the drawing representing the six faces with emotional expressions and were asked to indicate the face that expressed joy, disgust, sadness, anger, surprise, and fear respectively. If the answer was incorrect, the experimenter gave the correct answer and the operation was repeated until the participant correctly associated each face with the correct verbal label. Eighty per cent of the persons with AD, 71 per cent of the healthy older adults, and 48 per cent of the healthy younger adults incorrectly identified at least one of the basic emotions in the first trial. In the second trial, all the participants correctly identified all the basic emotions. The participants were then told that they were going to see a succession of scenes and that, in each of these, they would have to indicate the affective state of the character pointed out by the experimenter by choosing the face with the corresponding emotional expression from the six faces presented below the scene.
The experimental task began with the presentation of the practice scene (see Figure 1). If the participant gave a wrong answer for the practice scene, the experimenter repeated the instructions and explained the meaning of the first scene before moving on to the second scene. All the participants answered correctly for the first scene. They were asked to describe each scene in order to make sure that they could perceive all the elements present in the drawing. All the participants correctly described the main elements in the drawings. The 30 scenes were presented in the same pseudorandom order.
Statistical Analyses
Our aim was to explore differences in performance between our experimental groups for each basic emotion and identify the pattern of interference between emotions in each experimental group. Statistical analyses were performed on the mean numbers of correct answers for each emotion in each experimental group. We first performed a normality test and a variance homogeneity test (Kolmogorov-Smirnov and Brown-Forsythe tests respectively). As the normality of distribution (K–S d = .149, p > .15) and the homogeneity of variance between groups (F[2, 60] = 2.49, p > .09) were respected, we conducted a mixed analysis of variance (ANOVA)Footnote 2 with between-subject factor Group (AD persons vs. older controls vs. young controls) and repeated factor Emotion (anger vs. disgust vs. fear vs. joy vs. sadness vs. surprise). Neuwman-Keuls post-hoc tests were performed at an alpha level of .05.
Several studies (Shimokawa et al., Reference Shimokawa, Yatomi, Anamizu, Ashikari, Kohno, Maki and Matsuno2000, Reference Shimokawa, Yatomi, Anamizu, Torii, Isono, Sugai and Kohno2001, Reference Shimokawa, Yatomi, Anamizu, Torii, Isono and Sugai2003) have shown that the performance of persons with AD in an affective ToM task does not significantly correlate with the level of general cognitive impairment (MMSE scores; Folstein et al., Reference Folstein, Folstein and McHugh1975), while others have shown that the performance of persons with mild to moderate AD in such tasks diminishes after six months (Torres et al., Reference Torres, Santos, Barroso De Sousa, Simoes Neto, Lima Nogueira, Belfort and Nascimento Dourado2015). We therefore also used Pearson’s correlation coefficient to examine the relationship between the MMSE scores (Folstein et al., Reference Folstein, Folstein and McHugh1975) and the number of correct answers in the experimental task in persons with AD. Correlations were also performed between the mean ratings of visual complexity of the scenes and the number of correct answers in the experimental task in each group (persons with AD, and healthy younger and older adults).
We performed the statistical analyses with Statistica 8 software.
Results
There was a main effect of group, F(2, 60) = 24.53, MSE = 1.53, p < .0001, ηFootnote 2 = .45. Post-hoc tests showed significantly lower numbers of correct answers for the persons with AD than for the younger and older adults (both p values < .001). No significant difference was observed between younger and older adults (p = .338). There was also a main effect of emotion, F(5, 300) = 28.10, MSE = 0.675, p < .0001, η2 = .32. Pairwise post-hoc tests between the numbers of correct answers for the six emotions are presented in Table 2a.
Table 2a: Mean numbers and standard deviations of the mean of correct answers and pairwise Newman-Keuls post-hoc comparisons between the numbers of correct answers for the six emotions in the Whole sample of participants, Healthy younger adults, Healthy older adults and persons with Alzheimer’s disease. Significant differences are indicated by * (p < .05)
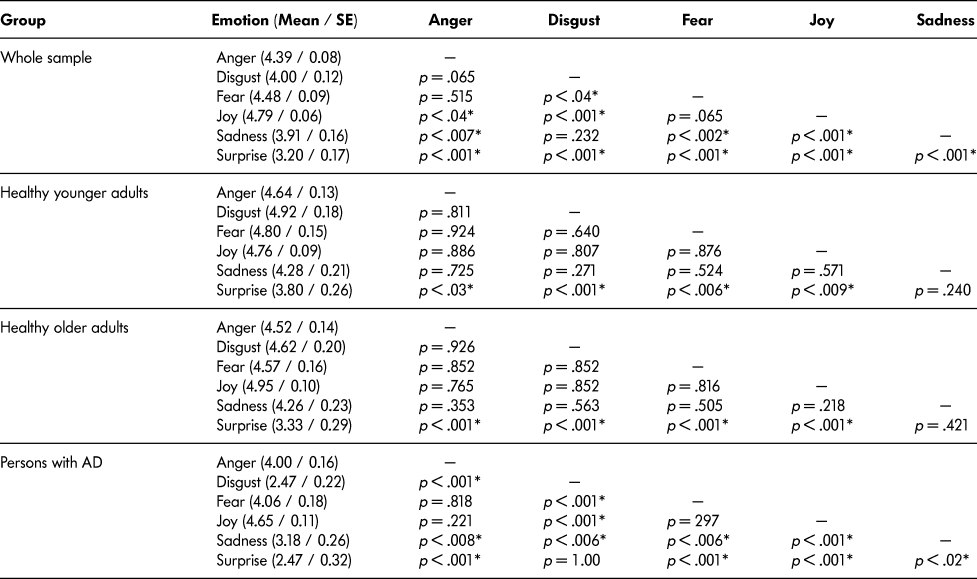
The interaction between group and emotion was also significant, F(10, 300) = 5.40, MSE = 0.675, p < .0001, ηFootnote 2 = .15. Post-hoc tests revealed that there was no significant difference between the persons with AD, the younger adults, and the older adults for anger, fear, and joy (all p values > .2). For disgust, sadness, and surprise, the persons with AD gave significantly fewer correct answers than the younger and older adults (all p values < .02). By contrast, there was no significant difference between the younger and older adults for anger, disgust, fear, sadness, or surprise (all p values > .09) (see Figure 2). The results of the pairwise post-hoc tests between the scores of the persons with AD and the healthy younger and older adults for each emotion are presented in Table 2b. In addition, the patterns of correct answers for the six emotions (see Figure 2) were different across groups. The results of the pairwise post-hoc tests between the numbers of correct answers for the six emotions in each experimental group are presented in Table 2a.
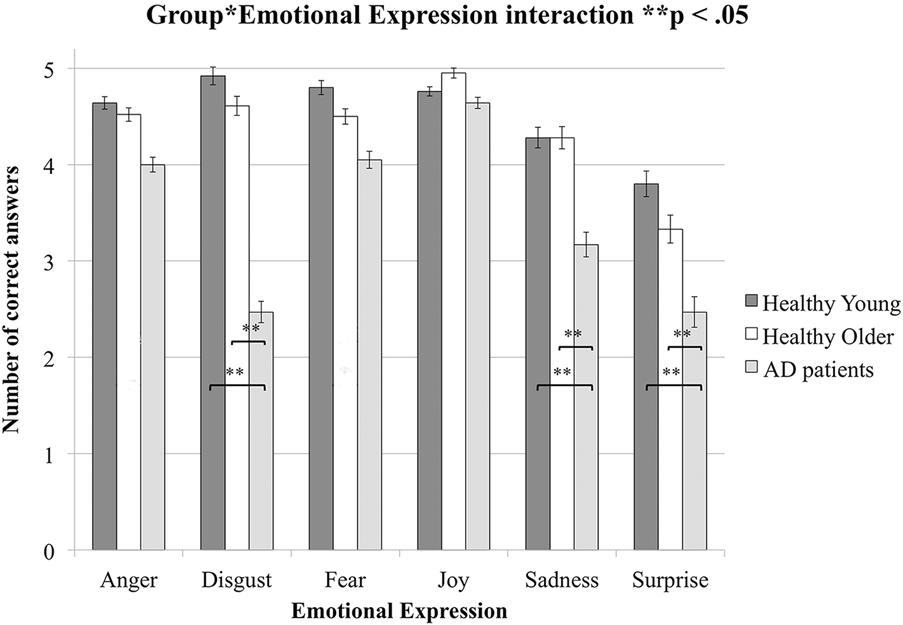
Figure 2: Mean numbers of correct answers (max = 5) as a function of emotion (anger, disgust, fear, joy, sadness, surprise) for healthy younger and older adults and for persons with Alzheimer’s disease. Error bars represent the standard error of the mean. Significant inter-group differences are indicated by ** p < .05.
Table 2b: Pairwise Newman-Keuls post-hoc comparisons between the numbers of correct answers of healthy younger adults, healthy older adults, and persons with AD (Alzheimer’s disease) for the six basic emotions (anger, disgust, fear, joy, sadness, and surprise). Significant differences are indicated by * (p < .05).

No significant correlation was observed between the MMSE score and the number of correct answers in the persons with AD (r = 0.19, p > .05) or in the healthy older adults (r = 0.23, p > .05). Significant negative correlations were observed between the number of correct answers and the mean ratings of visual complexity for the scenes in the healthy younger (r = –.38, p < .05) and older adults (r = –.37, p < .05), but not in the persons with AD (r = –.19, p > .05). Thus, the lower the visual complexity of a drawing, the more correct answers were given by the healthy younger and older adults.
One important aspect that needs to be mentioned is that a considerable number of our participants, especially the healthy younger and older adults, reached the maximum level of performance for one or more emotions. Globally, 20 per cent of the healthy younger adults, 10 per cent of the healthy older adults, and none of the persons with AD gave correct answers for all the stimuli presented in the experimental task. More precisely, the percentages of healthy younger adults who reached the maximum level of performance were as follows: 68 per cent for anger, 92 per cent for disgust, 80 per cent for joy, 84 per cent for fear, 32 per cent for surprise, and 52 per cent for sadness. The percentages of healthy older adults who reached the maximum level of performance were as follows: 61 per cent for anger, 71 per cent for disgust, 95 per cent for joy, 57 per cent for fear, 23 per cent for surprise, and 42 per cent for sadness. The percentages of persons with AD who reached the maximum level of performance were as follows: 23 per cent for anger, 17 per cent for disgust, 70 per cent for joy, 52 per cent for fear, 17 per cent for surprise, and 17 per cent for sadness. The implications of these effects for the interpretation of our results, especially with regard to the comparisons between older and younger adults, are addressed in detail in the next section.
Discussion
In this study, we were interested in the influence of the type of emotion on affective ToM in healthy older adults and in persons with mild AD. We therefore compared the performances of healthy older and younger adults and persons with mild AD in an affective ToM task in which the participants had to infer first-order affective states of characters from drawings depicting situations in which no cues were provided by the facial expressions of the characters. Contrary to our hypothesis, the performances of our healthy older and younger adults were not significantly different. As many of the healthy younger and older adults reached the maximum level of performance in the experimental task, our results do not allow us to interpret the lack of significant differences in terms of preserved affective ToM with advancing age in tasks in which the facial expressions of characters are absent.
Future studies, with larger samples and more difficult tasks (to avoid ceiling effects), are necessary in order to verify if this capacity is preserved, as suggested by our preliminary results, or if it declines with advancing age, as observed in studies that have found impaired first-order affective ToM in healthy older adults in tasks in which the emotional states had to be inferred based on facial expressions (Duval et al., Reference Duval, Piolino, Bejanin, Eustache and Desgranges2011; Fischer et al., Reference Fischer, O’Rourke and Thornton2017; Slessor et al., Reference Slessor, Phillips and Bull2007). In addition, the visual complexity of the scenes should also be normalized between experimental stimuli because, as suggested by our findings, this factor might influence performance in affective ToM tasks. Indeed, we observed a negative correlation between the visual complexity of the drawings and the performance of healthy younger and older participants in the experimental task.
In line with our hypothesis, we observed significantly poorer general performance on our affective ToM task in persons with AD as compared with healthy younger and older adults. In addition, the performance level was not correlated with general cognitive impairment, as measured by MMSE. Our results contradict previous studies showing preserved first-order affective ToM in persons with mild to moderate AD in tasks in which the emotional states of characters had to be inferred from videos or drawings of emotional scenes (Freedman et al., Reference Freedman, Binns, Black, Murphy and Stuss2013; Zaitchik et al., Reference Zaitchik, Koff, Brownell, Winner and Albert2006) or from pictures of the eye region (Gregory et al., Reference Gregory, Lough, Stone, Erzinclioglu, Martin, Baron-Cohen and Hodges2002; Heitz et al., Reference Heitz, Noblet, Phillipps, Cretin, Vogt, Philippi and Blanc2016). One possible explanation for these contradictory results might be that our task (choosing between six emotional expressions) was more difficult. Indeed, in two of the studies cited above, the participants had to choose between two (Zaitchik et al., Reference Zaitchik, Koff, Brownell, Winner and Albert2006) or four (Freedman et al., Reference Freedman, Binns, Black, Murphy and Stuss2013) possible answers. However, this explanation seems insufficient, given that other studies that did not show any ToM impairment (Gregory et al., Reference Gregory, Lough, Stone, Erzinclioglu, Martin, Baron-Cohen and Hodges2002; Heitz et al., Reference Heitz, Noblet, Phillipps, Cretin, Vogt, Philippi and Blanc2016) used more difficult affective ToM tasks in which the participants had to infer complex emotions, whereas the participants in our study had to infer basic emotions. Moreover, a large number of persons with AD reached the maximum level of performance for at least some of the emotions, suggesting that our task was not particularly difficult.
To better understand affective ToM difficulties in AD, we analysed group differences for each basic emotion. The performances of the persons with AD were significantly poorer than those of the younger and older adults for disgust, sadness, and surprise, but not for anger, fear, and joy. As far as joy is concerned, the results confirmed our hypothesis and are also in line with those of previous studies showing that persons with mild AD perform normally when required to identify facial expressions of joy (Maki et al., Reference Maki, Yoshida, Yamaguchi and Yamaguchi2013; see Elferink et al., Reference Elferink, van Tilborg and Kessels2015, for a review). However, due to the ceiling effect observed for joy in many of our participants (see Results section), the interpretation of our findings should be considered with caution, and it will be necessary to replicate these preliminary results in future studies using larger samples and more difficult versions of the experimental task.
Contrary to our hypothesis, the identification of disgust was poorer in the persons with AD than in the older and younger adults. In addition, disgust was the emotion that was the least well identified by the persons with AD. This result is consistent with the study by Drapeau, Gosselin, Gagnon, Peretz, and Lorrain (Reference Drapeau, Gosselin, Gagnon, Peretz and Lorrain2009) who showed that the identification of disgust is impaired in persons with AD. However, it partially contradicts the study of Henry et al. (Reference Henry, Ruffman, McDonald, O’Leary, Phillips, Brodaty and Rendell2008) who observed that the identification of disgust was preserved in tasks using “static” stimuli (i.e., photographs of faces), but not in tasks using videos in which the character was seen in various contexts (for example, listening on the phone). It is possible, therefore, that the ability of persons with mild AD to identify facial expressions of disgust presented without context is preserved, whereas their ability to infer this emotion from the context when facial cues are not present is impaired. This might be particularly true when the scenes do not provide enough details to allow this emotion to be inferred correctly, which may have been the case for our stimuli. Indeed, in the pretest phase of our study, the situations depicting disgust were judged as being the least visually complex.
As far as the other emotions are concerned (i.e., anger, fear, sadness, and surprise), we did not have specific hypotheses regarding the selective impairment/preservation of affective ToM abilities in persons with AD for these emotions because no previous studies have explored this question. Our results showed similar performances for fear and anger and poorer performances for sadness and surprise in persons with mild AD as compared with healthy younger and older adults. It is difficult to interpret this pattern of results because the performance of many of our healthy participants was at ceiling. Nevertheless, it would be interesting to further explore any differences that might arise between the evolution of the capacity to infer anger, fear, sadness, and surprise from social contexts with both advancing age and advancing AD, given that several studies investigating facial emotion identification have found identical (Hargrave, Maddock, & Stone, Reference Hargrave, Maddock and Stone2002) or very similar (Drapeau et al., Reference Drapeau, Gosselin, Gagnon, Peretz and Lorrain2009; Maki et al., Reference Maki, Yoshida, Yamaguchi and Yamaguchi2013) patterns of results.
To summarize, our data suggest that when facial cues are not available, persons with mild AD may find it difficult to infer the emotional state of the characters from the contextual information. Nevertheless, this ability seems to be impaired for some basic emotions (i.e., disgust, sadness, and surprise) but not for others (i.e., anger, fear, and joy). Thus, future studies that explore the influence of AD on affective ToM should consider analyzing the results as a function of the type of emotion, and further studies are needed to confirm our results.
Limitations and Perspectives
There are some limitations to the present study. First, we carried out our study on persons suffering from mild AD, and it cannot be generalized to persons with more advanced stages of AD. Future research should, therefore, explore in more detail the influence of general cognitive impairment on affective ToM. Moreover, some other factors that were not taken into account in the present study should be systematically controlled in the future as they were found to be related to “cognitive” and affective ToM abilities (for example, vascular health: Fischer, Bernstein, & Thornton, Reference Fischer, Bernstein and Thornton2014; executive functions, semantic and episodic memory: Fischer et al., Reference Fischer, O’Rourke and Thornton2017) and could influence participants’ performances.
Second, the results of our study should be considered with caution due to the small sample sizes and the small effect sizes for main effects and interactions that were reported significant in our study. Moreover, our experimental task was probably not difficult enough for healthy young and older controls; consequently, numerous participants in these groups had ceiling scores for one or more emotional categories of stimuli. Future research should examine whether these results are generalizable to larger populations and should adapt the difficulty of the task to the different experimental groups.
Third, future research should explore the reliability and internal consistency of our first-order affective ToM task. To do so, larger samples of participants should be tested in order to evaluate its internal consistency, and the relationship between the scores obtained in our task and in already-validated affective ToM tasks (i.e., the Reading the Mind in the Eyes test, Baron-Cohen et al., Reference Baron-Cohen, Wheelwright, Hill, Raste and Plumb2001) should also be explored in order to test its reliability.
Supplementary Material
To view supplementary material for this article, please visit https://doi.org/10.1017/S0714980818000363









